Statins Induce Locomotion and Muscular Phenotypes in Drosophila melanogaster That Are Reminiscent of Human Myopathy: Evidence for the Role of the Chloride Channel Inhibition in the Muscular Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Maintenance
2.2. Setting Up the Crosses
2.3. Exposure Concentration
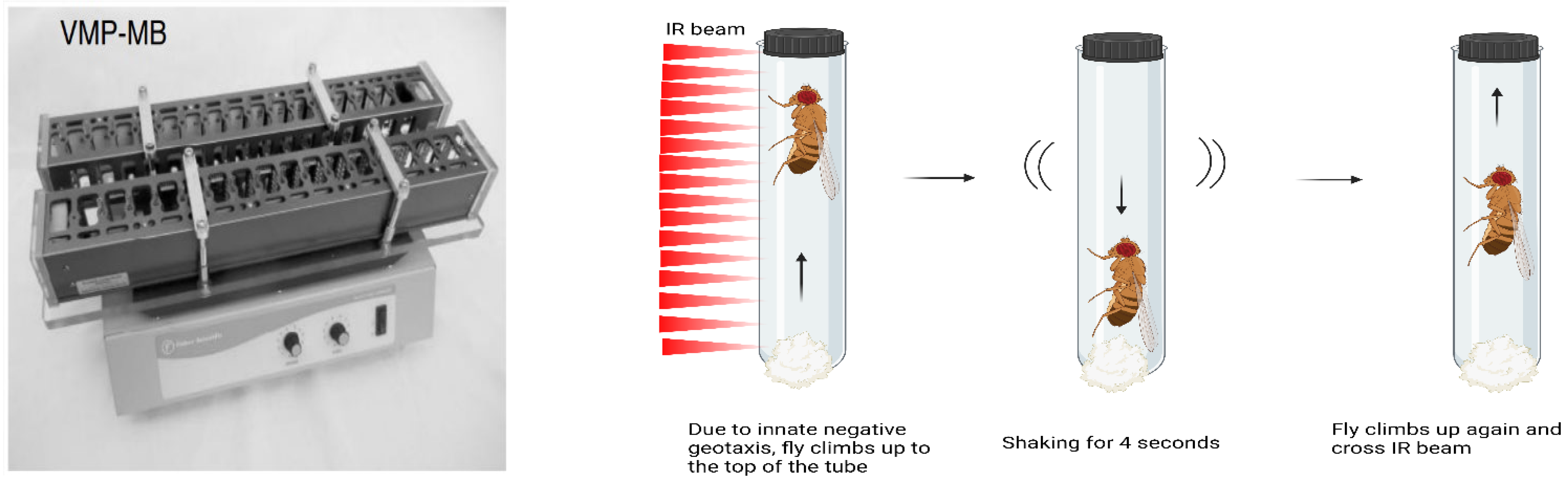
2.4. Quantification of Climbing Ability Using Forced-Climbing System
- I.
- Acute and chronic fluvastatin treatment
- II.
- Chronic fluvastatin 0.5 and 1 mM treatment
- III.
- Chelerythrine and fluvastatin treatment
2.5. Quantification of General Locomotion Activities Using Drosophila Activity Monitoring System (DAMS)
2.6. Transmission Electron Microscopy
- i.
- Morphology
- ii.
- Immunogold labelling
- iii.
- Chloride channel particles counting
- iv.
- Measuring the sarcomere length
- v.
- Measuring the mitochondria area
- vi.
- Counting the number of round-like shaped mitochondria
2.7. Quantification of Genes and Protein Expressions
- i.
- RNA purification and cDNA synthesis
- ii.
- Real-time qPCR
- iii.
- Western Blotting
3. Results
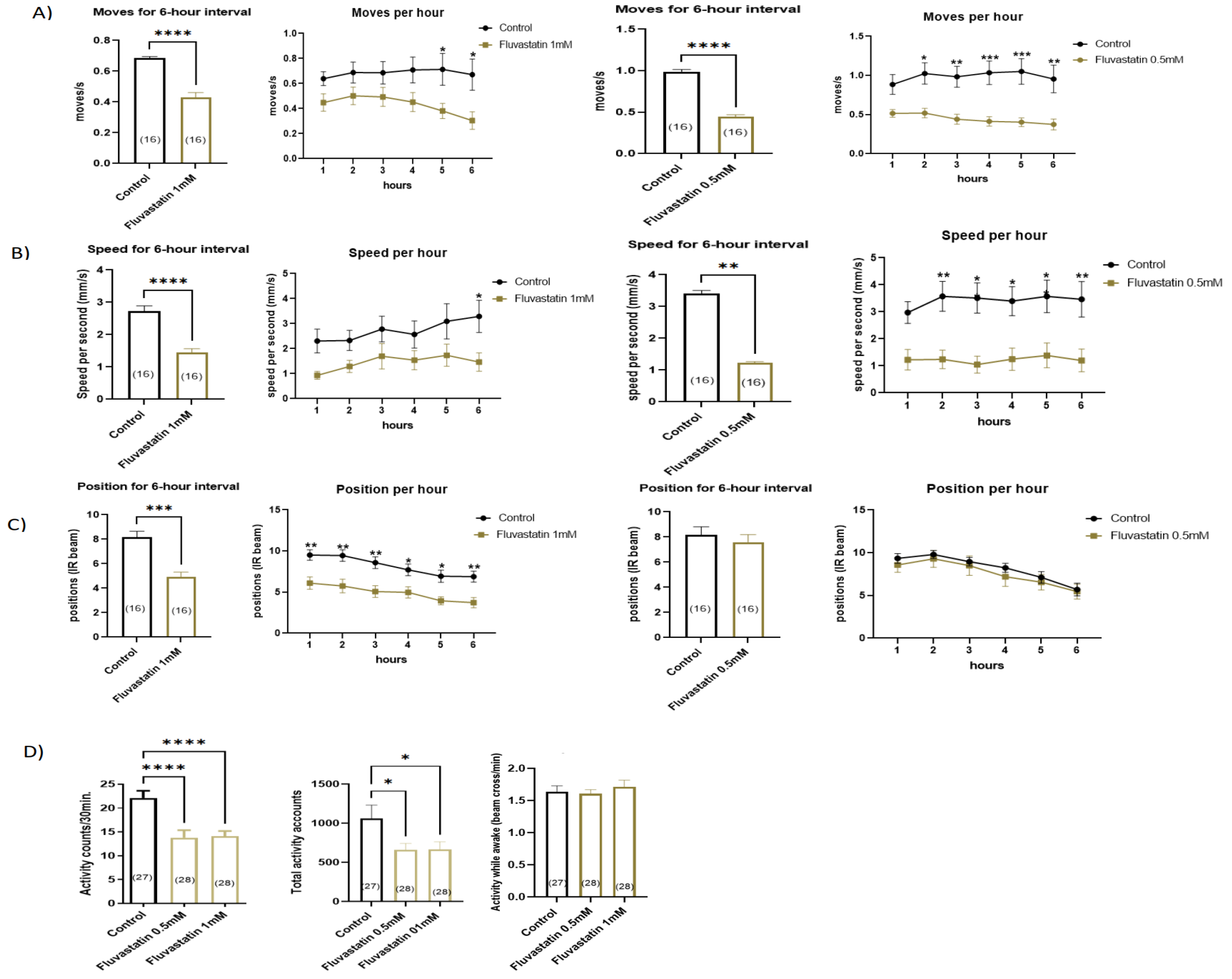
3.1. Chronic Fluvastatin Treatment Induces Lowered General Locomotion Activities and Climbing Ability
3.2. Chronic Fluvastatin Treatment Induces Phenotypic Changes in Myofibrils and Mitochondria and Is Associated with Reduced Muscle Regeneration
3.3. Chronic Fluvastatin Treatment Is Associated with Impaired Lipid Metabolism and Insulin Signalling
| Drosophila Gene/ Protein Name | Statin Effect | Human Homolog | Function | |
|---|---|---|---|---|
| 1 | Bmm/brummer | Upregulated Mean fold change (1.58) | PNPLA2/Patatin-like phospholipase domain-containing protein 2 [116,117] | Triglyceride lipase in adipose tissue and muscles [116,117] |
| 2 | Lip1/Lipase 1 | Downregulated Mean fold change (0.64) | LIPF/Gastric triacylglycerol lipase [116,117] | Triglyceride lipase and expressed in the digestive system, adipose tissue and muscles [116,117] |
| 3 | Lpin (Lipin)/ Phosphatidate phosphatase | Downregulated Mean fold change (0.55) | LPIN1/Phosphatidate phosphatase LPIN3 [116,117] | Conversion of phosphatidic acid to diacylglycerol during triglyceride biosynthesis and required for insulin signalling. Expressed in many tissues including adipose tissue and muscles [116,117] |
| 4 | mdy (midway)/ Diacylglycerol O-acyltransferase | Downregulated Mean fold change (0.74) | DGAT1/Diacylglycerol O-acyltransferase 1 [116,117] | Triglyceride biosynthesis in the muscles [116,117] |
| 5 | Dgat2/ Diacylglycerol O-acyltransferase 2 | Downregulated Mean fold change (0.43) | DGAT2/Diacylglycerol O-acyltransferase 2 [116,117] | Triglyceride biosynthesis and expressed in different tissues [116,117] |
| 6 | CG1941/ Diacylglycerol O-acyltransferase | No change | DGAT2/Diacylglycerol O-acyltransferase 2 [116,117] | Triglyceride biosynthesis [116,117] |
| 7 | CG1946/ Diacylglycerol O-acyltransferase | No change | DGAT2/Diacylglycerol O-acyltransferase 2 [116,117] | Triglyceride biosynthesis [116,117] |
| 8 | Hnf4 | Downregulated Mean fold change (0.43) | HNF4G/Hepatocyte nuclear factor 4-gamma [116,117] | A transcription factor involves in fatty acid oxidation, and lipid metabolism [118,119] |
| 9 | Chico/ encodes a substrate insulin receptor. | Downregulated Mean fold change (0.58) | IRS1/Insulin receptor substrate 1 [116,117] | Important for insulin signalling and action [116,117] |
| 10 | Thor/ encodes a eukaryotic translation initiation factor 4E binding protein | Downregulated Mean fold change (0.51) | EIF4EBP2/eukaryotic translation initiation factor 4E binding protein 2 [116,117] | Involved in insulin sensitivity [116,117] |
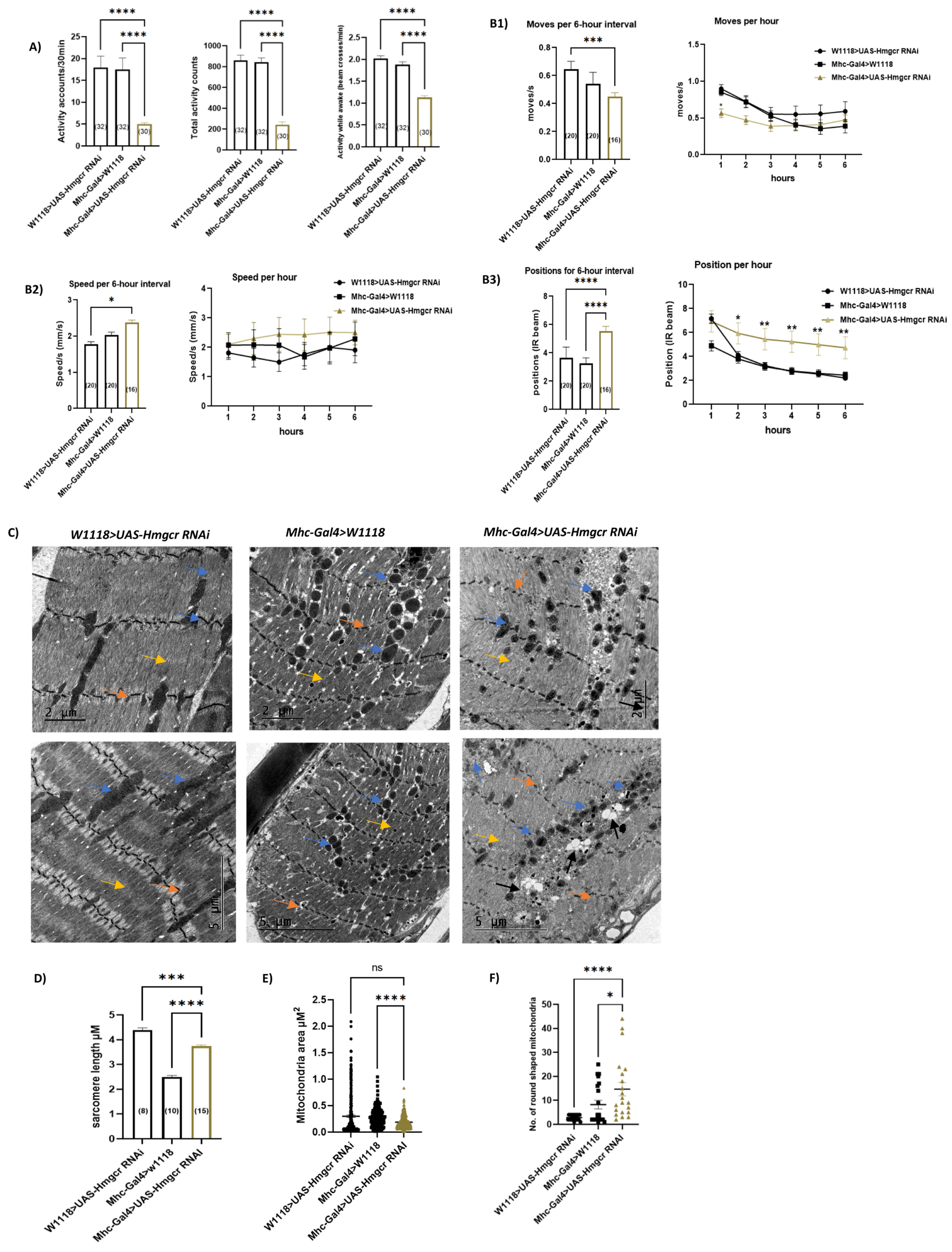
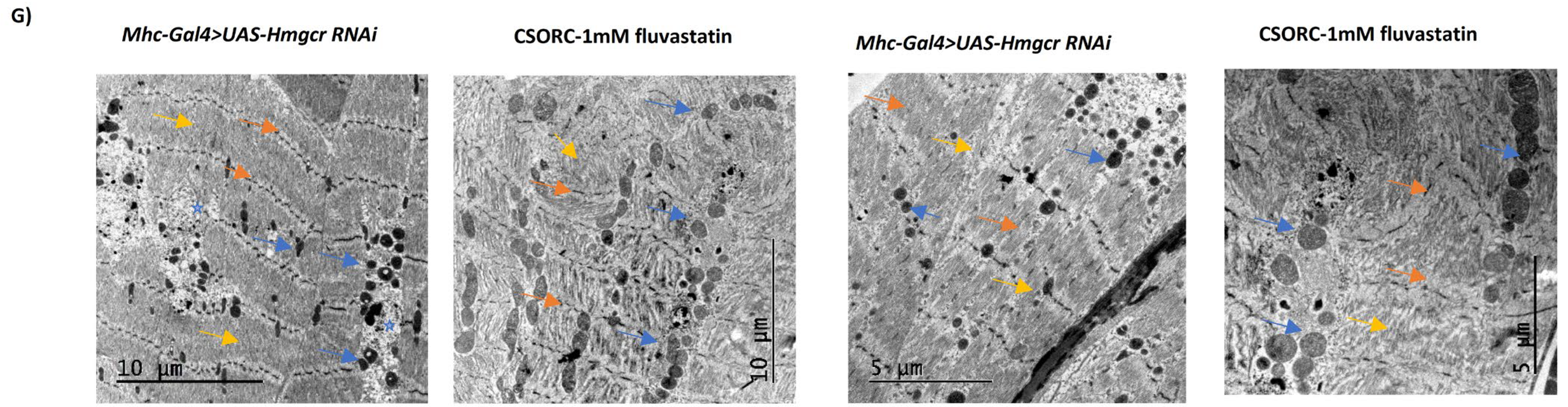
3.4. Loss of Hmgcr in Skeletal Muscles Results in Phenotypic Changes in Mitochondria and Reduced General Locomotion Activities without Affecting Myofibril Integrity or Climbing Moves and Speeds
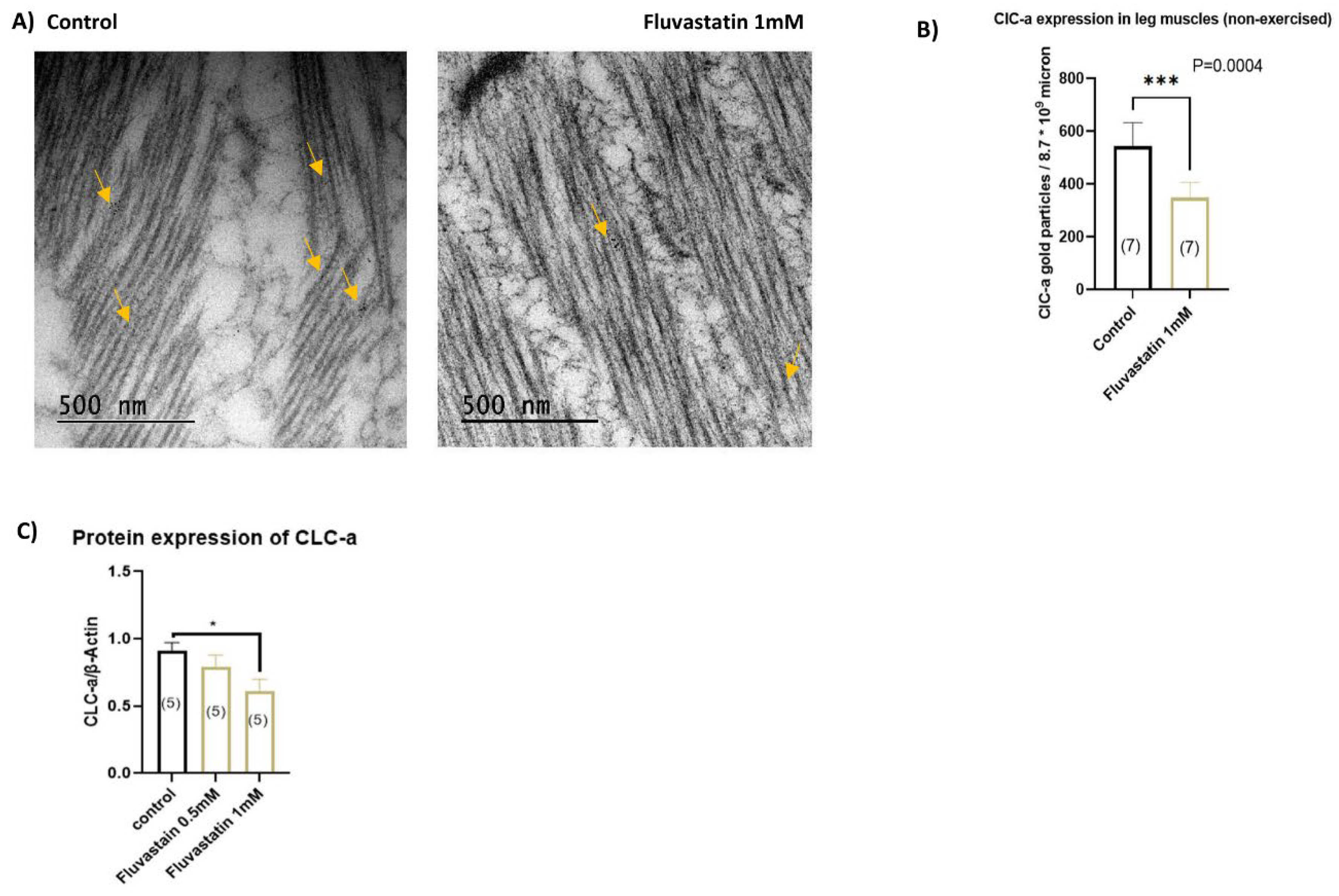
3.5. Chronic Fluvastatin Treatment Is Associated with Reduced Expression of the Skeletal Muscle Chloride Channel, ClC-a
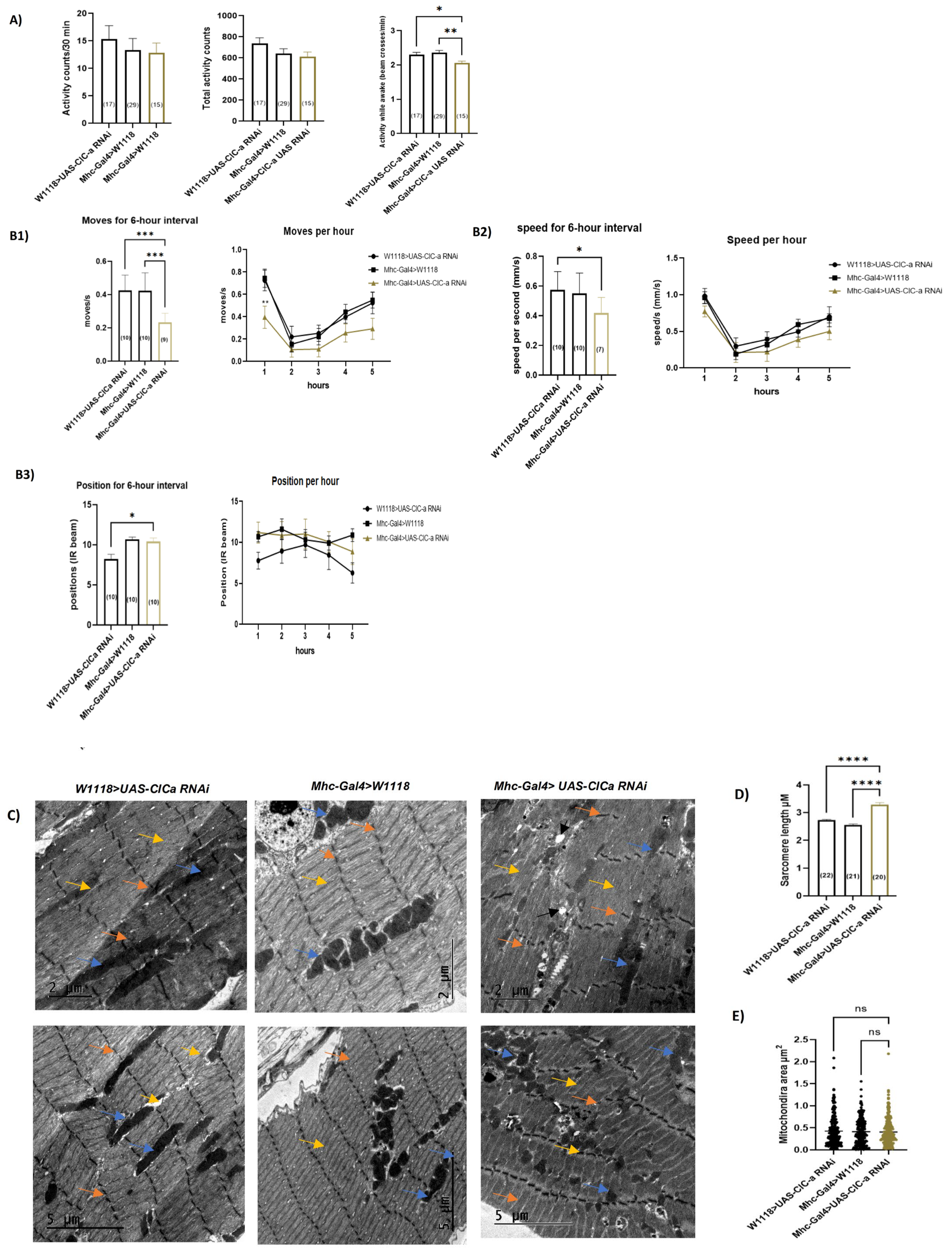
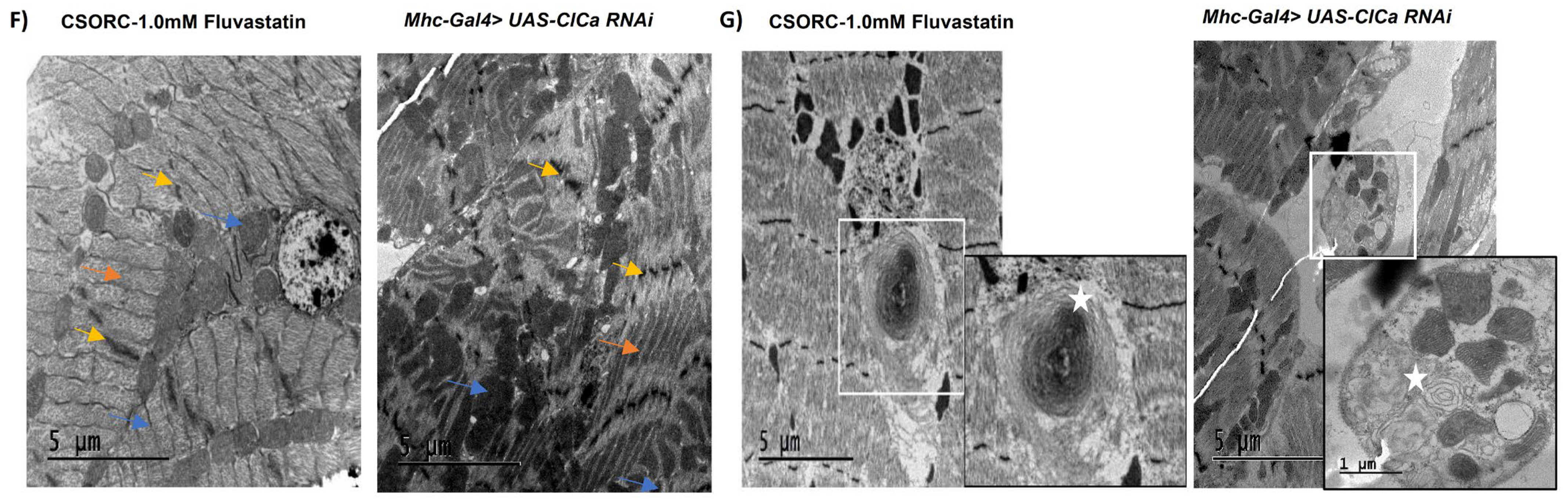
3.6. Loss of ClC-a in Skeletal Muscles Induces Myofibril Phenotypic Changes and Impairs the Climbing Ability
3.7. Chronic Fluvastatin Treatment Is Associated with an Upregulation of Pkcdelta and in Contrast to Fluvastatin, Inhibition of Skeletal Muscle Pkcdelta Improves General Locomotion Activities and Climbing Ability
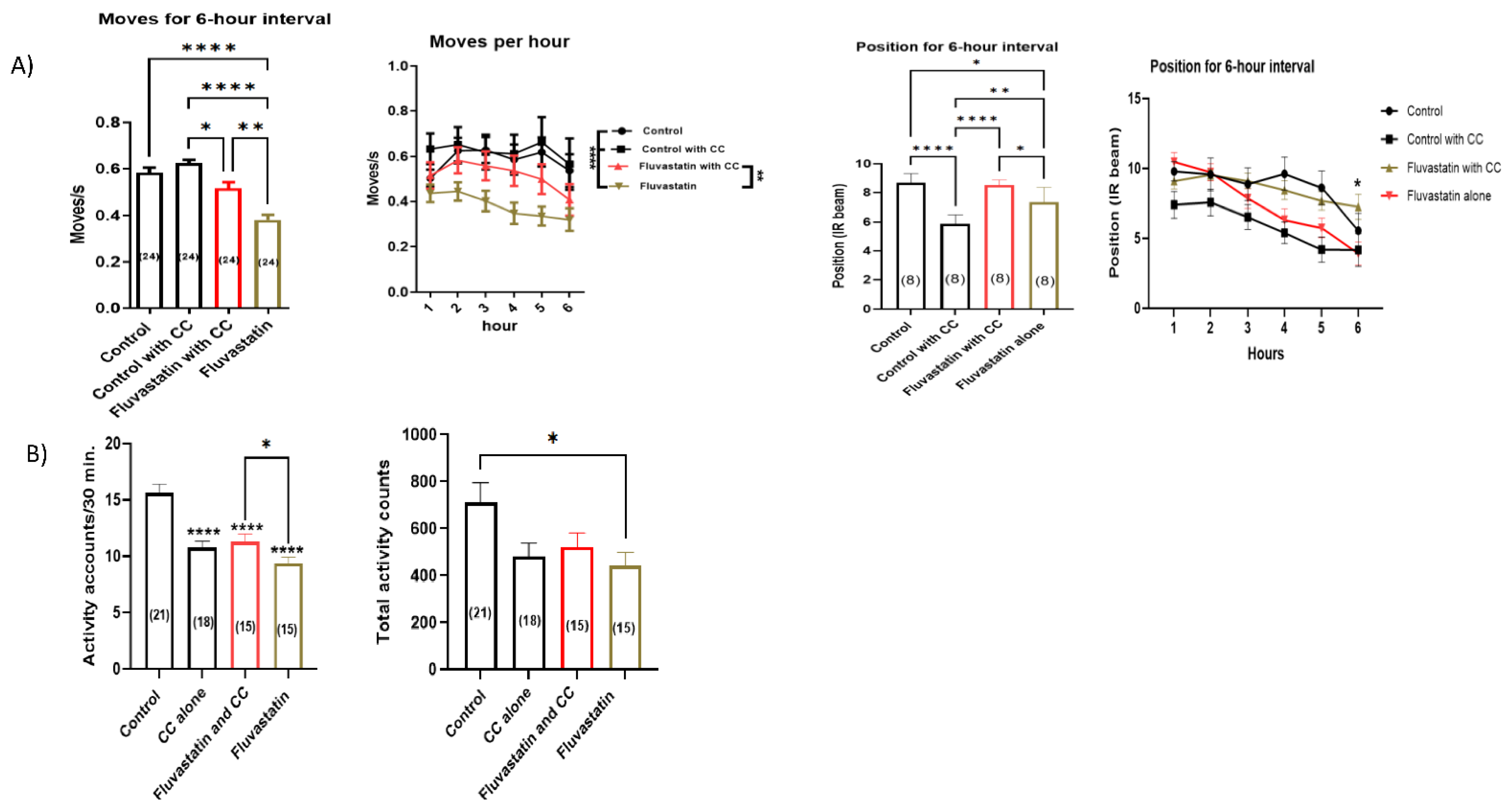
3.8. Chelerythrine Chloride, CC, a PKC Inhibitor, Rescues the Fluvastatin-Induced Lowered Locomotion and Climbing Ability
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Ward, K.; Moore, T.H.H.M.; Burke, M.; Smith, G.D.; Casas, J.P.; Ebrahim, S.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.H.M.; et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2011, CD004816. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Pleiotropic Effects of Statins—Basic Research and Clinical Perspectives. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef]
- Karmali, K.N.; Lloyd-Jones, D.M.; Berendsen, M.A.; Goff, D.C.; Sanghavi, D.M.; Brown, N.C.; Korenovska, L.; Huffman, M.D. Drugs for Primary Prevention of Atherosclerotic Cardiovascular Disease: An Overview of Systematic Reviews. JAMA Cardiol. 2016, 1, 341–349. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef]
- Clark, L.L.; Ikonomidis, J.S.; Crawford, F.A.; Crumbley, A.; Kratz, J.M.; Stroud, M.R.; Woolson, R.F.; Bruce, J.J.; Nicholas, J.S.; Lackland, D.T.; et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: An 8-year retrospective cohort study. J. Thorac. Cardiovasc. Surg. 2006, 131, 679–685. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G.; Mikhailidis, D.P. Statin discontinuation: An underestimated risk? Curr. Med. Res. Opin. 2008, 24, 3059–3062. [Google Scholar] [CrossRef]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-Associated Myopathy. J. Am. Med. Assoc. 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Cohen, J.D.; Brinton, E.A.; Ito, M.K.; Jacobson, T.A. Understanding Statin Use in America and Gaps in Patient Education (USAGE): An internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 2012, 6, 208–215. [Google Scholar] [CrossRef]
- Gomez Sandoval, Y.-H.; Braganza, M.V.; Daskalopoulou, S.S. Statin Discontinuation in High-Risk Patients: A Systematic Review of the Evidence. Curr. Pharm. Des. 2011, 17, 3669–3689. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, R.C.; Smith, S.C.; Merz, C.N.B.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation 2002, 106, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.; Stȩpień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef]
- Klopstock, T. Drug-induced myopathies. Curr. Opin. Neurol. 2008, 21, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Hilton-Jones, D. Statin-related myopathies. Pract. Neurol. 2018, 18, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Needham, M.; Fabian, V.; Knezevic, W.; Panegyres, P.; Zilko, P.; Mastaglia, F.L. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul. Disord. 2007, 17, 194–200. [Google Scholar] [CrossRef]
- Hadjiphilippou, S.; Ray, K.K. Cholesterol-Lowering Agents Statins—For Everyone? Circ. Res. 2019, 124, 354–363. [Google Scholar] [CrossRef]
- Shroufi, A.; Powles, J.W. Adherence and chemoprevention in major cardiovascular disease: A simulation study of the benefits of additional use of statins. J. Epidemiol. Community Health 2010, 64, 109–113. [Google Scholar] [CrossRef][Green Version]
- Lotteau, S.; Ivarsson, N.; Yang, Z.; Restagno, D.; Colyer, J.; Hopkins, P.; Weightman, A.; Himori, K.; Yamada, T.; Bruton, J.; et al. A Mechanism for Statin-Induced Susceptibility to Myopathy. JACC Basic Transl. Sci. 2019, 4, 509–523. [Google Scholar] [CrossRef]
- Draeger, A.; Monastyrskaya, K.; Mohaupt, M.; Hoppeler, H.; Savolainen, H.; Allemann, C.; Babiychuk, E. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. J. Pathol. 2006, 210, 94–102. [Google Scholar] [CrossRef]
- Flint, O.P.; Masters, B.A.; Gregg, R.E.; Durham, S.K. Inhibition of Cholesterol Synthesis by Squalene Synthase Inhibitors Does Not Induce Myotoxicity in Vitro. Toxicol. Appl. Pharmacol. 1997, 145, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.R.; Jacobson, T.A. Evidence-Based Management of Statin Myopathy. Curr. Atheroscler. Rep. 2010, 12, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dirks, A.J.; Jones, K.M. Statin-Induced Apoptosis and Skeletal Myopathy. Am. J. Physiol.-Cell Physiol. 2006, 291, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Matzno, S.; Yasuda, S.; Juman, S.; Yamamoto, Y.; Nagareya-Ishida, N.; Nakabayashi, T.; Matsuyama, K.; Tazuya-Murayama, K. Statin-induced apoptosis linked with membrane farnesylated Ras small G protein depletion, rather than geranylated Rho protein. J. Pharm. Pharmacol. 2010, 57, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Jokelainen, K.; Laakso, J.; Sahi, T.; Härkönen, M.; Tikkanen, M.J.; Himberg, J.J. The effect of Simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am. J. Cardiol. 1996, 77, 851–854. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Bookstaver, D.A.; Burkhalter, N.A.; Hatzigeorgiou, C. Effect of Coenzyme Q10 Supplementation on Statin-Induced Myalgias. Am. J. Cardiol. 2012, 110, 526–529. [Google Scholar] [CrossRef]
- Banach, M.; Serban, C.; Sahebkar, A.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; et al. Effects of Coenzyme Q10 on Statin-Induced Myopathy: A Meta-Analysis of Randomized Controlled Trials. Mayo Clin. Proc. 2015, 90, 24–34. [Google Scholar] [CrossRef]
- Hodel, C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol. Lett. 2002, 128, 159–168. [Google Scholar] [CrossRef]
- Marcoff, L.; Thompson, P.D. The Role of Coenzyme Q10 in Statin-Associated Myopathy: A Systematic Review. J. Am. Coll. Cardiol. 2007, 49, 2231–2237. [Google Scholar] [CrossRef]
- Dohlmann, T.L.; Morville, T.; Kuhlman, A.B.; Chrøis, K.M.; Helge, J.W.; Dela, F.; Larsen, S. Statin Treatment Decreases Mitochondrial Respiration But Muscle Coenzyme Q10 Levels Are Unaltered: The LIFESTAT Study. J. Clin. Endocrinol. Metab. 2019, 104, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Urbano, F.; Bugliani, M.; Filippello, A.; Scamporrino, A.; Di Mauro, S.; Di Pino, A.; Scicali, R.; Noto, D.; Rabuazzo, A.M.; Averna, M.; et al. Atorvastatin but Not Pravastatin Impairs Mitochondrial Function in Human Pancreatic Islets and Rat β-Cells. Direct Effect of Oxidative Stress. Sci. Rep. 2017, 7, 11863. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.A.; Lorson, L.; White, C.M.; Thompson, P.D. A randomized trial of coenzyme Q10 in patients with confirmed Statin Myopathy. Atherosclerosis 2015, 238, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Florkowski, C.M.; Molyneux, S.L.; McEwan, R.G.; Frampton, C.M.; George, P.M.; Scott, R.S. Effect of Coenzyme Q10 Supplementation on Simvastatin-Induced Myalgia. Am. J. Cardiol. 2007, 100, 1400–1403. [Google Scholar] [CrossRef]
- Schirris, T.J.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.M.; Brandt, U.; Koopman, W.J.H.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef]
- Schaefer, W.H.; Lawrence, J.W.; Loughlin, A.F.; Stoffregen, D.A.; Mixson, L.A.; Dean, D.C.; Raab, C.E.; Yu, N.X.; Lankas, G.R.; Frederick, C.B. Evaluation of ubiquinone concentration and mitochondrial function relative to cerivastatin-induced skeletal myopathy in rats. Toxicol. Appl. Pharmacol. 2004, 194, 10–23. [Google Scholar] [CrossRef]
- Lamperti, C.; Naini, A.B.; Lucchini, V.; Prelle, A.; Bresolin, N.; Moggio, M.; Sciacco, M.; Kaufmann, P.; DiMauro, S. Muscle Coenzyme Q10 Level in Statin-Related Myopathy. Arch. Neurol. 2005, 62, 1709–1712. [Google Scholar] [CrossRef]
- Bitzur, R.; Cohen, H.; Kamari, Y.; Harats, D. Intolerance to Statins: Mechanisms and Management. Diabetes Care 2013, 36, S325–S330. [Google Scholar] [CrossRef]
- Jones, S.P.; Teshima, Y.; Akao, M.; Marbán, E. Simvastatin Attenuates Oxidant-Induced Mitochondrial Dysfunction in Cardiac Myocytes. Circ. Res. 2003, 93, 697–699. [Google Scholar] [CrossRef]
- Sanvee, G.M.; Bouitbir, J.; Krähenbühl, S. Insulin prevents and reverts simvastatin-induced toxicity in C2C12 skeletal muscle cells. Sci. Rep. 2019, 9, 7409. [Google Scholar] [CrossRef]
- Agarwala, A.; Kulkarni, S.; Maddox, T. The Association of Statin Therapy with Incident Diabetes: Evidence, Mechanisms, and Recommendations. Curr. Cardiol. Rep. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J.; Carmena, R. The diabetogenic action of statins—Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2015, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zdebik, A.A. Statins and fibrate target ClC-1—From side effects to CLC pharmacology. Br. J. Pharmacol. 2009, 156, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Pierno, S.; Camerino, G.M.G.; Cippone, V.; Rolland, J.-F.F.; Desaphy, J.-F.F.; De Luca, A.; Liantonio, A.; Bianco, G.; Kunic, J.D.J.; George, A.G., Jr.; et al. Statins and fenofibrate affect skeletal muscle chloride conductance in rats by differently impairing ClC-1 channel regulation and expression. Br. J. Pharmacol. 2009, 156, 1206–1215. [Google Scholar] [CrossRef]
- Camerino, G.M.; Musumeci, O.; Conte, E.; Musaraj, K.; Fonzino, A.; Barca, E.; Marino, M.; Rodolico, C.; Tricarico, D.; Camerino, C.; et al. Risk of Myopathy in Patients in Therapy with Statins: Identification of Biological Markers in a Pilot Study. Front. Pharmacol. 2017, 8, 500. [Google Scholar] [CrossRef]
- Camerino, G.M.; Tarantino, N.; Canfora, I.; De Bellis, M.; Musumeci, O.; Pierno, S. Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 2070. [Google Scholar] [CrossRef]
- Charlet-B., N.; Savkur, R.S.; Singh, G.; Philips, A.V.; Grice, E.A.; Cooper, T.A. Loss of the Muscle-Specific Chloride Channel in Type 1 Myotonic Dystrophy Due to Misregulated Alternative Splicing. Mol. Cell 2002, 10, 45–53. [Google Scholar] [CrossRef]
- Koch, M.C.; Steinmeyer, K.; Lorenz, C.; Ricker, K.; Wolf, F.; Otto, M.; Zoll, B.; Lehmann-Horn, F.; Grzeschik, K.H.; Jentsch, T.J. The Skeletal Muscle Chloride Channel in Dominant and Recessive Human Myotonia. Science 1992, 257, 797–800. [Google Scholar] [CrossRef]
- Dahl-Halvarsson, M.; Olive, M.; Pokrzywa, M.; Norum, M.; Ejeskär, K.; Tajsharghi, H. Impaired muscle morphology in a Drosophila model of myosin storage myopathy was supressed by overexpression of an E3 ubiquitin ligase. Dis. Models Mech. 2021, 13, dmm047886. [Google Scholar] [CrossRef]
- Plantié, E.; Migocka-Patrzałek, M.; Daczewska, M.; Jagla, K. Model Organisms in the Fight against Muscular Dystrophy: Lessons from Drosophila and Zebrafish. Molecules 2015, 20, 6237–6253. [Google Scholar] [CrossRef]
- Suggs, J.A.; Melkani, G.C.; Glasheen, B.M.; Detor, M.M.; Melkani, A.; Marsan, N.P.; Swank, D.M.; Bernstein, S.I. A Drosophila model of dominant inclusion body myopathy Type 3 shows diminished myosin kinetics that reduce muscle power and yield myofibrillar defects. Dis. Models Mech. 2017, 10, 761–771. [Google Scholar] [CrossRef]
- Taylor, M.V. Comparison of Muscle Development in Drosophila and Vertebrates. In Madame Curie Bioscience Database 2000–2013; Landes Bioscience: Austin, TX, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6226/ (accessed on 31 October 2022).
- Piccirillo, R.; Demontis, F.; Perrimon, N.; Goldberg, A.L. Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev. Dyn. 2014, 243, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Lehmann, R. Isoprenoids Control Germ Cell Migration Downstream of HMGCoA Reductase. Dev. Cell 2004, 6, 283–293. [Google Scholar] [CrossRef]
- Alsehli, A.M.; Liao, S.; Al-Sabri, M.H.; Vasionis, L.; Purohit, A.; Behare, N.; Clemensson, L.E.; Williams, M.J.; Schiöth, H.B. The Statin Target HMG-Coenzyme a Reductase (Hmgcr) Regulates Sleep Homeostasis in Drosophila. Pharmaceuticals 2022, 15, 79. [Google Scholar] [CrossRef]
- Belgacem, Y.H.; Martin, J.-R.R. Hmgcr in the Corpus Allatum Controls Sexual Dimorphism of Locomotor Activity and Body Size via the Insulin Pathway in Drosophila. PLoS ONE 2007, 2, e187. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, Y.H.; Martin, J.-R. Neuroendocrine control of a sexually dimorphic behavior by a few neurons of the pars intercerebralis in Drosophila. Proc. Natl. Acad. Sci. USA 2002, 99, 15154–15158. [Google Scholar] [CrossRef]
- Kucera, W.G. Oxygen Consumption in the Male and Female Fly, Drosophila melanogaster. Physiol. Zool. 1934, 7, 449–458. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; de la Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef]
- The Effects of a Human Food Additive, Titanium Dioxide Nanoparticles E171, on Drosophila Melanogaster—A 20 Generation Dietary Exposure Experiment|Scientific Reports. Available online: https://www.nature.com/articles/s41598-018-36174-w (accessed on 14 September 2022).
- Moulin, T.C.; Ferro, F.; Hoyer, A.; Cheung, P.; Williams, M.J.; Schiöth, H.B. The Drosophila melanogaster Levodopa-Induced Depression Model Exhibits Negative Geotaxis Deficits and Differential Gene Expression in Males and Females. Front. Neurosci. 2021, 15, 560. [Google Scholar] [CrossRef]
- Williams, M.J.; Alsehli, A.M.; Gartner, S.N.; Clemensson, L.E.; Liao, S.; Eriksson, A.; Isgrove, K.; Thelander, L.; Khan, Z.; Itskov, P.M.; et al. The Statin Target Hmgcr Regulates Energy Metabolism and Food Intake through Central Mechanisms. Cells 2022, 11, 970. [Google Scholar] [CrossRef]
- Singh, F.; Zoll, J.; Duthaler, U.; Charles, A.L.; Panajatovic, M.V.; Laverny, G.; McWilliams, T.G.; Metzger, D.; Geny, B.; Krähenbühl, S.; et al. PGC-1β modulates statin-associated myotoxicity in mice. Arch. Toxicol. 2019, 93, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, J.; Wang, Y.; Marsden, A.M.; Orton, T.C.; Westwood, F.R.; Azuma, C.T.; Scott, R.C. Statin-induced myopathy in the rat: Relationship between systemic exposure, muscle exposure and myopathy. Xenobiotica 2009, 39, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Leo, T.K.; Garba, S.; Abubakar, D.; Sazili, A.Q.; Candyrine, S.C.L.; Jahromi, M.F.; Goh, Y.M.; Ronimus, R.; Muetzel, S.; Liang, J.B. Naturally Produced Lovastatin Modifies the Histology and Proteome Profile of Goat Skeletal Muscle. Animals 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.M.; Guapyassu, L.; Gomes, C.; Midlej, V.; Benchimol, M.; Mermelstein, C.; Costa, M.L. Simvastatin and Muscle: Zebrafish and Chicken Show that the Benefits are not Worth the Damage. Front. Cell Dev. Biol. 2022, 10, 488. [Google Scholar] [CrossRef]
- Dubińska-Magiera, M.; Migocka-Patrzałek, M.; Lewandowski, D.; Daczewska, M.; Jagla, K. Zebrafish as a Model for the Study of Lipid-Lowering Drug-Induced Myopathies. Int. J. Mol. Sci. 2021, 22, 5654. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsiao, C.D.; Lin, D.S.; Chow, C.Y.; Chang, C.J.; Liau, I. Imaging of Zebrafish In Vivo with Second-Harmonic Generation Reveals Shortened Sarcomeres Associated with Myopathy Induced by Statin. PLoS ONE 2011, 6, e24764. [Google Scholar] [CrossRef]
- Levine, B.D.; Cagan, R.L. Drosophila Lung Cancer Models Identify Trametinib plus Statin as Candidate Therapeutic. Cell Rep. 2016, 14, 1477–1487. [Google Scholar] [CrossRef]
- Yi, P.; Han, Z.; Li, X.; Olson, E.H. The Mevalonate Pathway Controls Heart Formation in Drosophila by Isoprenylation of Gγ1. Science 2006, 313, 1301–1303. [Google Scholar] [CrossRef]
- Moulin, T.C.; Ferro, F.; Berkins, S.; Hoyer, A.; Williams, M.J.; Schiöth, H.B. Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults. Brain Sci. 2020, 10, 487. [Google Scholar] [CrossRef]
- Chung, H.R.; Vakil, M.; Munroe, M.; Parikh, A.; Meador, B.M.; Wu, P.T.; Jeong, J.H.; Woods, J.A.; Wilund, K.R.; Boppart, M.D. The Impact of Exercise on Statin-Associated Skeletal Muscle Myopathy. PLoS ONE 2016, 11, e0168065. [Google Scholar] [CrossRef]
- Reyes-Lugo, M.; Sánchez, T.; Finol, H.J.; Sánchez, E.E.; Suárez, J.A.; Guerreiro, B.; Rodríguez-Acosta, A. Neurotoxic activity and ultrastructural changes in muscles caused by the brown widow spider Latrodectus geometricus venom. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.D.S.; Rassier, D.E. Sarcomere Length Nonuniformity and Force Regulation in Myofibrils and Sarcomeres. Biophys. J. 2020, 119, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- de Winter, J.M.; Ottenheijm, C.A.C. Sarcomere Dysfunction in Nemaline Myopathy. J. Neuromuscul. Dis. 2017, 4, 99–113. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, N.; Xiao, Y.S.; Schilling, M.A.; Marescal, O.; Liao, K.A.; Schöck, F. Myofibril diameter is set by a finely tuned mechanism of protein oligomerization in Drosophila. eLife 2019, 8, e50496. [Google Scholar] [CrossRef]
- Joureau, B.; de Winter, J.M.; Conijn, S.; Bogaards, S.J.P.; Kovacevic, I.; Kalganov, A.; Persson, M.; Lindqvist, J.; Stienen, G.J.M.; Irving, T.C.; et al. Dysfunctional sarcomere contractility contributes to muscle weakness in ACTA1—Related nemaline myopathy (NEM3). Ann. Neurol. 2018, 83, 269–282. [Google Scholar] [CrossRef]
- Liu, J.; Puolanne, E.; Schwartzkopf, M.; Arner, A. Altered Sarcomeric Structure and Function in Woody Breast Myopathy of Avian Pectoralis Major Muscle. Front. Physiol. 2020, 11, 287. [Google Scholar] [CrossRef]
- Unger, A.; Beckendorf, L.; Böhme, P.; Kley, R.; Von Frieling-Salewsky, M.; Lochmüller, H.; Schröder, R.; Fürst, D.O.; Vorgerd, M.; Linke, W.A. Translocation of molecular chaperones to the titin springs is common in skeletal myopathy patients and affects sarcomere function. Acta Neuropathol. Commun. 2017, 5, 72. [Google Scholar] [CrossRef]
- Dahl-Halvarsson, M.; Olive, M.; Pokrzywa, M.; Ejeskär, K.; Palmer, R.H.; Uv, A.E.; Tajsharghi, H. Drosophila model of myosin myopathy rescued by overexpression of a TRIM-protein family member. Proc. Natl. Acad. Sci. USA 2018, 115, E6566–E6575. [Google Scholar] [CrossRef]
- Baher, W.; Abo Zeid, A.A.; Mohamed Fahmy, B.; Mohamed Hashem, A.; Ashraf Sakr, R.; Ahmed Mohamed, F.; Ali Mohamed, N.; Mounir Halem, M. The effect of low dose statin combined with grapefruit on muscle structure and the possible protective role of mesenchymal stem cells. QJM Int. J. Med. 2018, 111, hcy200-221. [Google Scholar] [CrossRef]
- Voigt, T.; Sebald, H.J.; Schoenauer, R.; Levano, S.; Girard, T.; Hoppeler, H.H.; Babiychuk, E.B.; Draeger, A. Annexin A1 is a biomarker of T-tubular repair in skeletal muscle of nonmyopathic patients undergoing statin therapy. FASEB J. 2013, 27, 2156–2164. [Google Scholar] [CrossRef]
- Campos, L.M.; Rios, E.A.; Midlej, V.; Atella, G.C.; Herculano-Houzel, S.; Benchimol, M.; Mermelstein, C.; Costa, M.L. Structural Analysis of Alterations in Zebrafish Muscle Differentiation Induced by Simvastatin and Their Recovery with Cholesterol. J. Histochem. Cytochem. 2015, 63, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Pierno, S.; Didonna, M.P.; Cippone, V.; De Luca, A.; Pisoni, M.; Frigeri, A.; Nicchia, G.P.; Svelto, M.; Chiesa, G.; Sirtori, C.; et al. Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: A biochemical, histological and electrophysiological study. Br. J. Pharmacol. 2006, 149, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Cantó-Santos, J.; Grau-Junyent, J.M.; Garrabou, G. The Impact of Mitochondrial Deficiencies in Neuromuscular Diseases. Antioxidants 2020, 9, 964. [Google Scholar] [CrossRef]
- Wredenberg, A.; Wibom, R.; Wilhelmsson, H.; Graff, C.; Wiener, H.H.; Burden, S.J.; Oldfors, A.; Westerblad, H.; Larsson, N.G. Increased Mitochondrial Mass in Mitochondrial Myopathy Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 15066–15071. [Google Scholar] [CrossRef] [PubMed]
- Aksu-Menges, E.; Eylem, C.C.; Nemutlu, E.; Gizer, M.; Korkusuz, P.; Topaloglu, H.; Talim, B.; Balci-Hayta, B. Reduced mitochondrial fission and impaired energy metabolism in human primary skeletal muscle cells of Megaconial Congenital Muscular Dystrophy. Sci. Rep. 2021, 11, 18161. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Lin, A.J.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; Ho, T.; Lee, J.L.; Rucker, D.H.; Nguyen, C.Q.; et al. Mitochondrial Dysfunction Is an Early Consequence of Partial or Complete Dystrophin Loss in mdx Mice. Front. Physiol. 2020, 11, 690. [Google Scholar] [CrossRef]
- Houston, B.A.; Judge, D.P.; Brown, E.; Halushka, M.; Barouch, L.A. Giant Ring Mitochondria in a Patient with Heart Failure and Cerebral White Matter Disease Resulting From an MT-TL1 Mitochondrial Gene Mutation. J. Card. Fail. 2017, 23, 652–655. [Google Scholar] [CrossRef]
- Miettinen, T.P.; Björklund, M. Cellular Allometry of Mitochondrial Functionality Establishes the Optimal Cell Size. Dev. Cell 2016, 39, 370–382. [Google Scholar] [CrossRef]
- Corsetti, G.; D’Antona, G.; Ruocco, C.; Stacchiotti, A.; Romano, C.; Tedesco, L.; Dioguardi, F.; Rezzani, R.; Nisoli, E. Dietary supplementation with essential amino acids boosts the beneficial effects of rosuvastatin on mouse kidney. Amino Acids 2014, 46, 2189–2203. [Google Scholar] [CrossRef][Green Version]
- Díaz-Zagoya, J.C.; Marín-Medina, A.; Zetina-Esquivel, A.M.; Blé-Castillo, J.L.; Castell-Rodríguez, A.E.; Juárez-Rojop, I.E.; Miranda-Zamora, R. Effects of high rosuvastatin doses on hepatocyte mitochondria of hypercholesterolemic mice. Sci. Rep. 2021, 11, 15809. [Google Scholar] [CrossRef]
- Christie, C.F.; Fang, D.; Hunt, E.G.; Morris, M.E.; Rovini, A.; Heslop, K.A.; Beeson, G.C.; Beeson, C.C.; Maldonado, E.N. Statin-dependent modulation of mitochondrial metabolism in cancer cells is independent of cholesterol content. FASEB J. 2019, 33, 8186–8201. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, P.; Verma, A.; Maiti, T.K.; Mathew, S.J. Myosin heavy chain mutations that cause Freeman-Sheldon syndrome lead to muscle structural and functional defects in Drosophila. Dev. Biol. 2019, 449, 90–98. [Google Scholar] [CrossRef]
- Bour, B.A.; O’Brien, M.A.; Lockwood, W.L.; Goldstein, E.S.; Bodmer, R.; Taghert, P.H.; Abmayr, S.M.; Nguyen, H.T. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995, 9, 730–741. [Google Scholar] [CrossRef]
- Nongthomba, U.; Cummins, M.; Clark, S.; Vigoreaux, J.O.; Sparrow, J.C. Suppression of Muscle Hypercontraction by Mutations in the Myosin Heavy Chain Gene of Drosophila melanogaster. Genetics 2003, 164, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.I.; Mogami, K.; Donady, J.J.; Emerson, C.P. Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. Nature 1983, 302, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, D.; Reichert, H.; Gunage, R.D.; VijayRaghavan, K. Identification and functional characterization of muscle satellite cells in Drosophila. eLife 2017, 6, e30107. [Google Scholar] [CrossRef]
- Postigo, A.A.; Ward, E.; Skeath, J.B.; Dean, D.C. zfh-1, the Drosophila Homologue of ZEB, Is a Transcriptional Repressor That Regulates Somatic Myogenesis. Mol. Cell. Biol. 1999, 19, 7255–7263. [Google Scholar] [CrossRef]
- Caine, C.; Kasherov, P.; Silber, J.; Lalouette, A. Mef2 Interacts with the Notch Pathway during Adult Muscle Development in Drosophila melanogaster. PLoS ONE 2014, 9, e108149. [Google Scholar] [CrossRef][Green Version]
- Postigo, A.A.; Dean, D.C. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J. 1997, 16, 3935–3943. [Google Scholar] [CrossRef]
- Grunwald, S.A.; Popp, O.; Haafke, S.; Jedraszczak, N.; Grieben, U.; Saar, K.; Patone, G.; Kress, W.; Steinhagen-Thiessen, E.; Dittmar, G.; et al. Statin-induced myopathic changes in primary human muscle cells and reversal by a prostaglandin F2 alpha analogue. Sci. Rep. 2020, 10, 2158. [Google Scholar] [CrossRef]
- Marino, J.S.; Hinds, T.D.; Potter, R.A.; Ondrus, E.; Onion, J.L.; Dowling, A.; McLoughlin, T.J.; Sanchez, E.R.; Hill, J.W. Suppression of protein kinase C theta contributes to enhanced myogenesis In vitro via IRS1 and ERK1/2 phosphorylation. BMC Cell Biol. 2013, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Verity, M.A.; Reue, K. Lipin-1 Regulates Autophagy Clearance and Intersects with Statin Drug Effects in Skeletal Muscle. Cell Metab. 2014, 20, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Michot, C.; Hubert, L.; Romero, N.B.; Gouda, A.; Mamoune, A.; Mathew, S.; Kirk, E.; Viollet, L.; Rahman, S.; Bekri, S.; et al. Study of LPIN1, LPIN2 and LPIN3 in rhabdomyolysis and exercise-induced myalgia. J. Inherit. Metab. Dis. 2012, 35, 1119–1128. [Google Scholar] [CrossRef]
- Bergounioux, J.; Brassier, A.; Rambaud, C.; Bustarret, O.; Michot, C.; Hubert, L.; Arnoux, J.B.; Laquerriere, A.; Bekri, S.; Galene-Gromez, S.; et al. Fatal Rhabdomyolysis in 2 Children with LPIN1 Mutations. J. Pediatr. 2012, 160, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Vigelsø, A.; Dandanell, S.; Prats, C.; Dela, F.; Helge, J.W. Simvastatin-Induced Insulin Resistance May Be Linked to Decreased Lipid Uptake and Lipid Synthesis in Human Skeletal Muscle: The LIFESTAT Study. J. Diabetes Res. 2018, 2018, 9257874. [Google Scholar] [CrossRef]
- Pennisi, E.M.; Garibaldi, M.; Antonini, G. Lipid Myopathies. J. Clin. Med. 2018, 7, 472. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Blaak, E.E.; Van Loon, L.J.C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef]
- Morales, P.E.; Bucarey, J.L.; Espinosa, A. Muscle Lipid Metabolism: Role of Lipid Droplets and Perilipins. J. Diabetes Res. 2017, 2017, 1789395. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Gao, T.-Q.; Wang, Z.; Li, D.-D. Role of insulin/insulin-like growth factor 1 signaling pathway in longevity. World J. Gastroenterol. 2005, 11, 1891–1895. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of Life-Span by Loss of CHICO, a Drosophila Insulin Receptor Substrate Protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef]
- Miron, M.; Lasko, P.; Sonenberg, N. Signaling from Akt to FRAP/TOR Targets both 4E-BP and S6K in Drosophila melanogaster. Mol. Cell. Biol. 2003, 23, 9117–9126. [Google Scholar] [CrossRef] [PubMed]
- Kauwe, G.; Tsurudome, K.; Penney, J.; Mori, M.; Gray, L.; Calderon, M.R.; Elazouzzi, F.; Chicoine, N.; Sonenberg, N.; Haghighi, A.P. Acute Fasting Regulates Retrograde Synaptic Enhancement through a 4E-BP-Dependent Mechanism. Neuron 2016, 92, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Zid, B.M.; Rogers, A.N.; Katewa, S.D.; Vargas, M.A.; Kolipinski, M.C.; Lu, T.A.; Benzer, S.; Kapahi, P. 4E-BP Extends Lifespan upon Dietary Restriction by Enhancing Mitochondrial Activity in Drosophila. Cell 2009, 139, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Warner, K.; Magrane, M. SPIN: Submitting Sequences Determined at Protein Level to UniProt. Curr. Protoc. Bioinform. 2018, 62, e52. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, J.; Goodman, J.L.; Strelets, V.B.; Attrill, H.; Gramates, L.S.; Marygold, S.J.; Matthews, B.B.; Millburn, G.; Antonazzo, G.; Trovisco, V.; et al. FlyBase 2.0: The next generation. Nucleic Acids Res. 2019, 47, D759–D765. [Google Scholar] [CrossRef] [PubMed]
- Palanker, L.; Tennessen, J.M.; Lam, G.; Thummel, C.S. Drosophila HNF4 Regulates Lipid Mobilization and β-Oxidation. Cell Metab. 2009, 9, 228–239. [Google Scholar] [CrossRef]
- Girard, R.; Tremblay, S.; Noll, C.; St.-Jean, S.; Jones, C.; Gélinas, Y.; Maloum-Rami, F.; Perreault, N.; Laplante, M.; Carpentier, A.C.; et al. The transcription factor hepatocyte nuclear factor 4A acts in the intestine to promote white adipose tissue energy storage. Nat. Commun. 2022, 13, 224. [Google Scholar] [CrossRef]
- Baker, S.K. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve 2005, 31, 572–580. [Google Scholar] [CrossRef]
- Weaver, L.N.; Ma, T.; Drummond-Barbosa, D. Analysis of Gal4 Expression Patterns in Adult Drosophila Females. G3 Genes Genomes Genet. 2020, 10, 4147–4158. [Google Scholar] [CrossRef]
- Weaver, L.N.; Drummond-Barbosa, D. The nuclear receptor seven up functions in adipocytes and oenocytes to control distinct steps of Drosophila oogenesis. Dev. Biol. 2019, 456, 179–189. [Google Scholar] [CrossRef]
- Schuster, C.M.; Davis, G.W.; Fetter, R.D.; Goodman, C.S. Genetic Dissection of Structural and Functional Components of Synaptic Plasticity. I. Fasciclin II Controls Synaptic Stabilization and Growth. Neuron 1996, 17, 641–654. [Google Scholar] [CrossRef]
- Viswanathan, M.C.; Blice-Baum, A.C.; Schmidt, W.; Foster, D.B.; Cammarato, A. Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Front. Physiol. 2015, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.; Shane, A.; Zheng, J.; Seroude, L. Characterization of the Drosophila Gene-Switch system in aging studies: A cautionary tale. Aging Cell 2008, 7, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Barwell, T.; DeVeale, B.; Poirier, L.; Zheng, J.; Seroude, F.; Seroude, L. Regulating the UAS/GAL4 system in adult Drosophila with Tet-off GAL80 transgenes. PeerJ 2017, 5, e4167. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, I.; Harvey, I.; Yates, E.R.; Redd, J.R.; Reiter, L.T.; Bridges, D. The role of TORC1 in muscle development in Drosophila. Sci. Rep. 2015, 5, 9676. [Google Scholar] [CrossRef] [PubMed]
- Rose, U.; Derst, C.; Wanischeck, M.; Marinc, C.; Walther, C. Properties and possible function of a hyperpolarisation-activated chloride current in Drosophila. J. Exp. Biol. 2007, 210, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Capella-Gutiérrez, S.; Pryszcz, L.P.; Marcet-Houben, M.; Gabaldón, T. PhylomeDB v4: Zooming into the plurality of evolutionary histories of a genome. Nucleic Acids Res. 2014, 42, D897–D902. [Google Scholar] [CrossRef]
- Flores, C.A.; Niemeyer, M.I.; Sepúlveda, F.V.; Cid, L.P. Two splice variants derived from a Drosophila melanogaster candidate ClC gene generate ClC-2-type Cl− channels. Mol. Membr. Biol. 2006, 23, 149–156. [Google Scholar] [CrossRef]
- Parker, B.A.; Thompson, P.D. Effect of Statins on Skeletal Muscle: Exercise, Myopathy, and Muscle Outcomes. Exerc. Sport Sci. Rev. 2012, 40, 188–194. [Google Scholar] [CrossRef]
- Allard, N.A.E.; Janssen, L.; Aussieker, T.; Stoffels, A.A.F.; Rodenburg, R.J.; Assendelft, W.J.J.; Thompson, P.D.; Snijders, T.; Hopman, M.T.E.; Timmers, S. Moderate Intensity Exercise Training Improves Skeletal Muscle Performance in Symptomatic and Asymptomatic Statin Users. J. Am. Coll. Cardiol. 2021, 78, 2023–2037. [Google Scholar] [CrossRef]
- Altamura, C.; Mangiatordi, G.F.; Nicolotti, O.; Sahbani, D.; Farinato, A.; Leonetti, F.; Carratù, M.R.; Conte, D.; Desaphy, J.F.; Imbrici, P. Mapping ligand binding pockets in chloride ClC-1 channels through an integrated in silico and experimental approach using anthracene-9-carboxylic acid and niflumic acid. Br. J. Pharmacol. 2018, 175, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Bouchè, M.; De Bellis, M.; Cannone, M.; Liantonio, A.; Musaraj, K.; Romano, R.; Smeriglio, P.; Madaro, L.; Giustino, A.; et al. Protein kinase C theta (PKCθ) modulates the ClC-1 chloride channel activity and skeletal muscle phenotype: A biophysical and gene expression study in mouse models lacking the PKCθ. Pflug. Arch. Eur. J. Physiol. 2014, 466, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Zirin, J.; Hu, Y.; Liu, L.; Yang-Zhou, D.; Colbeth, R.; Yan, D.; Ewen-Campen, B.; Tao, R.; Vogt, E.; VanNest, S.; et al. Large-Scale Transgenic Drosophila Resource Collections for Loss- and Gain-of-Function Studies. Genetics 2020, 214, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, P.; Ryan, D.; Johnston, C.A.; Cripps, R.M. A Novel Mechanism for Activation of Myosin Regulatory Light Chain by Protein Kinase C-Delta in Drosophila. Genetics 2020, 216, 177–190. [Google Scholar] [CrossRef]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Resource Review Genevestigator V3: A Reference Expression Database for the Meta-Analysis of Transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef]
- Maier, D.; Nagel, A.C.; Kelp, A.; Preiss, A. Protein Kinase D Is Dispensable for Development and Survival of Drosophila melanogaster. G3 Genes Genomes Genet. 2019, 9, 2477–2487. [Google Scholar] [CrossRef]
- Seroude, L.; Brummel, T.; Kapahi, P.; Benzer, S. Spatio-temporal analysis of gene expression during aging in Drosophila melanogaster. Aging Cell 2002, 1, 47–56. [Google Scholar] [CrossRef]
- Madaro, L.; Pelle, A.; Nicoletti, C.; Crupi, A.; Marrocco, V.; Bossi, G.; Soddu, S.; Bouché, M. PKC Theta Ablation Improves Healing in a Mouse Model of Muscular Dystrophy. PLoS ONE 2012, 7, e31515. [Google Scholar] [CrossRef]
- Marrocco, V.; Fiore, P.; Benedetti, A.; Pisu, S.; Rizzuto, E.; Musarò, A.; Madaro, L.; Lozanoska-Ochser, B.; Bouché, M. Pharmacological Inhibition of PKCθ Counteracts Muscle Disease in a Mouse Model of Duchenne Muscular Dystrophy. eBioMedicine 2017, 16, 150–161. [Google Scholar] [CrossRef]
- Madaro, L.; Marrocco, V.; Carnio, S.; Sandri, M.; Bouché, M. Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J. 2013, 27, 1990–2000. [Google Scholar] [CrossRef]
- Herbert, J.M.; Augereau, J.M.; Gleye, J.; Maffrand, J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990, 172, 993–999. [Google Scholar] [CrossRef]
- Jones, P.H. Statins as the cornerstone of drug therapy for dyslipidemia: Monotherapy and combination therapy options. Am. Heart J. 2004, 148, S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Osaki, Y.; Nakagawa, Y.; Miyahara, S.; Iwasaki, H.; Ishii, A.; Matsuzaka, T.; Kobayashi, K.; Yatoh, S.; Takahashi, A.; Yahagi, N.; et al. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: A model for statin-induced myopathy. Biochem. Biophys. Res. Commun. 2015, 466, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Cassagnol, M. HMG-CoA Reductase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542212/ (accessed on 4 July 2022).
- Hanai, J.; Cao, P.; Tanksale, P.; Imamura, S.; Koshimizu, E.; Zhao, J.; Kishi, S.; Yamashita, M.; Phillips, P.S.; Sukhatme, V.P.; et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J. Clin. Investig. 2007, 117, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Niu, X.; King, M.J.; Noedl, M.T.; Tabin, C.J.; Galloway, J.L. The mevalonate pathway is a critical regulator of tendon cell specification. Development 2020, 147, dev185389. [Google Scholar] [CrossRef]
- Spindler, S.R.; Li, R.; Dhahbi, J.M.; Yamakawa, A.; Mote, P.; Bodmer, R.; Ocorr, K.; Williams, R.T.; Wang, Y.; Ablao, K.P. Statin Treatment Increases Lifespan and Improves Cardiac Health in Drosophila by Decreasing Specific Protein Prenylation. PLoS ONE 2012, 7, e39581. [Google Scholar] [CrossRef]
- Alcázar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1073–1078. [Google Scholar] [CrossRef]
- Shults, C.W.; Haas, R.H.; Passov, D.; Beal, M.F. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann. Neurol. 1997, 42, 261–264. [Google Scholar] [CrossRef]
- Cornelius, N.; Byron, C.; Hargreaves, I.; Guerra, P.F.; Furdek, A.K.; Land, J.; Radford, W.W.; Frerman, F.; Corydon, T.J.; Gregersen, N.; et al. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Hum. Mol. Genet. 2013, 22, 3819–3827. [Google Scholar] [CrossRef]
- Quinzii, C.M.; López, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef]
- Lalani, S.R.; Vladutiu, G.D.; Plunkett, K.; Lotze, T.E.; Adesina, A.M.; Scaglia, F. Isolated Mitochondrial Myopathy Associated with Muscle Coenzyme Q10 Deficiency. Arch. Neurol. 2005, 62, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Woerner, A.C.; Vockley, J. Mitochondrial Disease and Coenzyme Q10 Deficiency: Commentary. J. Pediatr. 2021, 228, 14–15.e1. [Google Scholar] [CrossRef] [PubMed]
- Neergheen, V.; Chalasani, A.; Wainwright, L.; Yubero, D.; Montero, R.; Artuch, R.; Hargreaves, I. Coenzyme Q10 in the Treatment of Mitochondrial Disease. J. Inborn Errors Metab. Screen. 2017, 5, 232640981770777. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Li, Y.; Chen, W.; Heffner, R.R.; Vladutiu, G.D. Histopathologic and Biochemical Evidence for Mitochondrial Disease Among 279 Patients with Severe Statin Myopathy. J. Neuromuscul. Dis. 2017, 4, 77–87. [Google Scholar] [CrossRef]
- Neřoldová, M.; Stránecký, V.; Hodaňová, K.; Hartmannová, H.; Piherová, L.; Přistoupilová, A.; Mrázová, L.; Vrablík, M.; Adámková, V.; Hubáček, J.A.; et al. Rare Variants in Known and Novel Candidate Genes Predisposing to Statin-Associated Myopathy. Pharmacogenomics 2016, 17, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Imbrici, P.; Altamura, C.; Pessia, M.; Mantegazza, R.; Desaphy, J.F.; Camerino, D.C. ClC-1 chloride channels: State-of-the-art research and future challenges. Front. Cell. Neurosci. 2015, 9, 156. [Google Scholar] [CrossRef]
- Adrian, R.H.; Bryant, S.H. On the repetitive discharge in myotonic muscle fibres. J. Physiol. 1974, 240, 505–515. [Google Scholar] [CrossRef]
- Pierno, S.; Liantonio, A.; Camerino, G.M.; De Bellis, M.; Cannone, M.; Dinardo, M.M.; Scaramuzzi, A.; Madaro, L.; Bouchè, M.; Desaphy, J.-F.; et al. Characterization of the Role of PKC-Theta in the Modulation of ClC-1 Chloride Channel Function and Calcium Homeostasis in Fast- and Slow-Twitch Skeletal Muscle by using PKC-Theta Null Mice. Biophys. J. 2012, 102, 332a–333a. [Google Scholar] [CrossRef]
- Steinmeyer, K.; Ortland, C.; Jentsch, T.J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature 1991, 354, 301–304. [Google Scholar] [CrossRef]
- Imbrici, P.; Altamura, C.; Camerino, G.M.; Mangiatordi, G.F.; Conte, E.; Maggi, L.; Brugnoni, R.; Musaraj, K.; Caloiero, R.; Alberga, D.; et al. Multidisciplinary study of a new CIC-1 mutation causing myotonia congenita: A paradigm to understand and treat ion channelopathies. FASEB J. 2016, 30, 3285–3295. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Gramegna, G.; Altamura, C.; Dinardo, M.M.; Imbrici, P.; George, A.L.; Modoni, A.; LoMonaco, M.; Camerino, D.C. Functional characterization of ClC-1 mutations from patients affected by recessive myotonia congenita presenting with different clinical phenotypes. Exp. Neurol. 2013, 248, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Bakker, A.J. The effect of chelerythrine on depolarization-induced force responses in skinned fast skeletal muscle fibres of the rat. Br. J. Pharmacol. 2003, 138, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.V.; Parker, P.J. Protein kinase C—A question of specificity. Trends Biochem. Sci. 1994, 19, 73–77. [Google Scholar] [CrossRef]
- Newton, A.C.; Johnson, J.E. Protein kinase C: A paradigm for regulation of protein function by two membrane-targeting modules. Biochim. Biophys. Acta Rev. Biomembr. 1998, 1376, 155–172. [Google Scholar] [CrossRef]
- Phillips, P.S.; Haas, R.H.; Bannykh, S.; Hathaway, S.; Gray, N.L.; Kimura, B.J.; Vladutiu, G.D.; England, J.D.F.; Scripps Mercy Clinical Research Center. Statin-Associated Myopathy with Normal Creatine Kinase Levels. Ann. Intern. Med. 2002, 137, 581–585. [Google Scholar] [CrossRef]
- Ragheb, R.; Shanab, G.M.L.; Medhat, A.M.; Seoudi, D.M.; Adeli, K.; Fantus, I.G. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: Evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem. Biophys. Res. Commun. 2009, 389, 211–216. [Google Scholar] [CrossRef]
- Abplanalp, W.; Clegg, D.J. Translocation of PKC theta by fatty acids causes hypothalamic insulin resistance. Appetite 2007, 49, 274. [Google Scholar] [CrossRef]
- Boden, G. Obesity and Free Fatty Acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-Induced Insulin Resistance in Human Muscle Is Associated with Changes in Diacylglycerol, Protein Kinase C, and IκB-α. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 2000, 48, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Kapadia, B.; Misra, P.; Saxena, U. Simvastatin may induce insulin resistance through a novel fatty acid mediated cholesterol independent mechanism. Sci. Rep. 2015, 5, 13823. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.; Shan, X.; Dong, Y.; Li, L.; Zhang, S.; Wang, H. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation through NF-ΚB Signaling Pathway Mediated by Nrf2. Front. Pharmacol. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Dvořák, Z.; Ulrichová, J.; Modrianský, M. Conventional protein kinase C isoenzymes undergo dephosphorylation in neutrophil-like HL-60 cells treated by chelerythrine or sanguinarine. Cell Biol. Toxicol. 2008, 24, 39–53. [Google Scholar] [CrossRef]
- Zheng, W.; Qiu, L.; Wang, R.; Feng, X.; Han, Y.; Zhu, Y.; Chen, D.; Liu, Y.; Jin, L.; Li, Y. Selective targeting of PPARγ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency. Sci. Rep. 2015, 5, 12222. [Google Scholar] [CrossRef]
- Niwa, R.; Niwa, Y.S. The Fruit Fly Drosophila melanogaster as a Model System to Study Cholesterol Metabolism and Homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef]
- Bouitbir, J.; Singh, F.; Charles, A.-L.; Schlagowski, A.-I.; Bonifacio, A.; Echaniz-Laguna, A.; Geny, B.; Krähenbühl, S.; Zoll, J. Statins Trigger Mitochondrial Reactive Oxygen Species-Induced Apoptosis in Glycolytic Skeletal Muscle. Antioxid. Redox Signal. 2016, 24, 84–98. [Google Scholar] [CrossRef]
- Bouitbir, J.; Charles, A.L.; Echaniz-Laguna, A.; Kindo, M.; Daussin, F.; Auwerx, J.; Piquard, F.; Geny, B.; Zoll, J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur. Heart J. 2012, 33, 1397–1407. [Google Scholar] [CrossRef]


| Bloomington Stock Numbers | Drosophila Line | Referred to as |
|---|---|---|
| 50652 | y[1] v[1]; P{y[+t7.7]v[+t1.8] = TRiP.HMC03053}attP40 | UAS-Hmgcr RNAi |
| 8176 | w[1118]; P{w[+mW.hs] = GawB}EDTP[DJ694] | EDTP-Gal4 |
| 28355 | y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.JF02991}attP2 | UAS-Pkcdelta RNAi |
| 53337 | P{y[+t7.7] v[+t1.8] = TRiP.HMC03566}attP40/CyO | UAS-ClC-a RNAi |
| 38464 | w[*]; P{w[+mC] = Mhc-RFP.F3-580}2, P{w[+mC] = Mhc-GAL4.F3-580}2/SM6b | Mhc-Gal4 |
| Experimental Group | Solution | Feeding Period | On the Fifth Day |
|---|---|---|---|
| Control | 4.5 mL food and 0.5 mL water | five days | Starved flies for one hour and then transfer to 100 μL of sucrose alone for three hours |
| 0.5 mM Fluvastatin (five days) | 4.5 mL food and 0.5 mL of 5 mM fluvastatin | five days | Starved flies for one hour and then transfer to 100 μL of sucrose alone for three hours |
| 0.5 mM Fluvastatin (three hours) | 4.5 mL food and 0.5 mL of 5 mM fluvastatin | five days | Starved flies for one hour and then transfer to 100 μL of 0.5 mM fluvastatin for three hours |
| Experimental Group | Solution | Feeding Period | On the Fifth Day |
|---|---|---|---|
| Control | 4.5 mL food and 0.5 mL water | five days | Starved flies for one hour and then transfer to 100 μL of sucrose alone for three hours |
| Control with 100 μM CC | 4.5 mL food and 0.5 mL water | five days | Starve flies for one hour and then transfer to 100 μL of 100 μM CC for three hours |
| 0.5 mM Fluvastatin | 4.5 mL food and 0.5 mL 5 mM fluvastatin | five days | Starved flies for one hour and then transfer to 100 μL of 0.5 mM fluvastatin for three hours |
| 0.5 mM Fluvastatin with 100 μM CC | 4.5 mL food and 0.5 mL 5 mM fluvastatin | five days | Starved flies for one hour and then transfer to 100 μL of 100 μM CC for three hours. |
| Group | Stock | Volume Taken | Total Volume with Food |
|---|---|---|---|
| 1.0 mM Fluvastatin | 3 mM fluvastatin | 4 mL | 12 mL |
| 0.5 mM Fluvastatin | 3 mM fluvastatin | 2 mL | 12 mL |
| 0.5 mM Fluvastatin with 100 μM CC | 3 mM fluvastatin 2.9 mM CC | 2 mL fluvastatin 413 μL CC | 12 mL |
| 100 μM CC | 2.9 mM CC | 413 μL CC | 12 mL |
| Control | no | no | 12 mL |
| Group | Stock | Volume Taken | Total Volume with Food |
|---|---|---|---|
| Control | no | 2.4 mL of alcohol | 12 mL |
| 1 mM CPP | 5 mM CPP in alcohol | 2.4 mL of the stock solution | 12 mL |
| 1 mM A9C | 5 mM CPP in alcohol | 2.4 mL of the stock solution | 12 mL |
| Primer Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| Hmgcr | 5′GCTGCACTGCCGTACTGTA3′ | 5′AATGCCCAGCACATATTTGGA3′ |
| CLC-a | 5′TGGGCGAGGATTGGGTATTC3′ | 5′ACTGAACAAAAGGCTGTGACG3′ |
| Pkc98E | 5′TCCATTATGCACGACGATGT3′ | 5′CTCCGGATTTTTGGTGAGAA3′ |
| Pkcdelta | 5′GGCACCAAACACCCGTATCT3′ | 5′CCCATAGAATCTGGCTCGCT3′ |
| RPL32 | 5′AGCATACAGGCCCAAGATCG3′ | 5′TGTTGTCGATACCCTTGGGC3′ |
| AMPK ALPHA | 5′TACCAGGTCATATCGACGCC3′ | 5′ACGCCAGAGATAATCTGCTGAA3′ |
| mdy | 5′CACAAAGTGTGACTCGTGCTG3′ | 5′CCAGTTCACCAGTCCAGAAAAA3′ |
| Dgat2 | 5′CAGATACTGGTCACGGCCTTT3′ | 5′CGGATTGGGTTTTCTTGTGGTG3′ |
| Hmgcr | 5′GCTGCACTGCCGTACTGTA3′ | 5′GCCACAAGCACCAGGATG3′ |
| Mef2 | 5′ATATCACGCATCACCGATGAAC3′ | 5′GGCGTACTGGTACAGCTTGT3′ |
| Mhc | 5′CCAAGACGGTCAAAAACGAT3′ | 5′GATGTTGGCTCCCGAGATAA3′ |
| zfh1 | 5′CCTCCAAGAAGTGCATCAGCA3′ | 5′CGAAGTAGCTCATCGGATGTG3′ |
| Hnf4 | 5′GGCGACGGGCAAACATTATG3′ | 5′CGCAAATCTCGCAAGTGTACTGAT3′ |
| Bmm | 5′GTCTCCTCTGCGATTTGCCAT3′ | 5′CTG AAG GGA CCC AGG GAG TA3′ |
| lip1 | 5′GTG AGCCTGGCCTACTTGC3′ | 5′GGTCGAGGGTGGTGTGATTC3′ |
| Lpin | 5′CACACCGACAACACACTGGA3′ | 5′CTTCTTCTCGCCCTGAAACAG3′ |
| CG1941 | 5′GTCTACGCGAATCACAAGAGAA3′ | 5′CGATAATGCCGAAACAGCCAA3′ |
| CG1946 | 5′CAGACCTGGTACGTCATTC3′ | 5′CGCCGTAGTACAGCAGGATAG3′ |
| Chico | 5′GCGCACTCACCTTATGACCA3′ | 5′GCACACGAATGTCAGGGATTT3′ |
| Thor | 5′CGTCCAGCGGAAAGTTTTCG3′ | 5′GTTTGGTGCCTCCAGGAGTGG3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sabri, M.H.; Behare, N.; Alsehli, A.M.; Berkins, S.; Arora, A.; Antoniou, E.; Moysiadou, E.I.; Anantha-Krishnan, S.; Cosmen, P.D.; Vikner, J.; et al. Statins Induce Locomotion and Muscular Phenotypes in Drosophila melanogaster That Are Reminiscent of Human Myopathy: Evidence for the Role of the Chloride Channel Inhibition in the Muscular Phenotypes. Cells 2022, 11, 3528. https://doi.org/10.3390/cells11223528
Al-Sabri MH, Behare N, Alsehli AM, Berkins S, Arora A, Antoniou E, Moysiadou EI, Anantha-Krishnan S, Cosmen PD, Vikner J, et al. Statins Induce Locomotion and Muscular Phenotypes in Drosophila melanogaster That Are Reminiscent of Human Myopathy: Evidence for the Role of the Chloride Channel Inhibition in the Muscular Phenotypes. Cells. 2022; 11(22):3528. https://doi.org/10.3390/cells11223528
Chicago/Turabian StyleAl-Sabri, Mohamed H., Neha Behare, Ahmed M. Alsehli, Samuel Berkins, Aadeya Arora, Eirini Antoniou, Eleni I. Moysiadou, Sowmya Anantha-Krishnan, Patricia D. Cosmen, Johanna Vikner, and et al. 2022. "Statins Induce Locomotion and Muscular Phenotypes in Drosophila melanogaster That Are Reminiscent of Human Myopathy: Evidence for the Role of the Chloride Channel Inhibition in the Muscular Phenotypes" Cells 11, no. 22: 3528. https://doi.org/10.3390/cells11223528
APA StyleAl-Sabri, M. H., Behare, N., Alsehli, A. M., Berkins, S., Arora, A., Antoniou, E., Moysiadou, E. I., Anantha-Krishnan, S., Cosmen, P. D., Vikner, J., Moulin, T. C., Ammar, N., Boukhatmi, H., Clemensson, L. E., Rask-Andersen, M., Mwinyi, J., Williams, M. J., Fredriksson, R., & Schiöth, H. B. (2022). Statins Induce Locomotion and Muscular Phenotypes in Drosophila melanogaster That Are Reminiscent of Human Myopathy: Evidence for the Role of the Chloride Channel Inhibition in the Muscular Phenotypes. Cells, 11(22), 3528. https://doi.org/10.3390/cells11223528









