CRISPR/Cas9-Mediated Mutagenesis of Sex-Specific Doublesex Splicing Variants Leads to Sterility in Spodoptera frugiperda, a Global Invasive Pest
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sexing
2.2. Molecular Cloning of Sfdsx
2.3. Synthesis of Sfdsx sgRNAs In Vitro
2.4. Embryo Microinjection
2.5. Genomic DNA Extraction and Mutagenesis Analysis
2.6. Phenotypic Impacts of Mutagenesis
2.7. Real Time Quantitative PCR (RT-qPCR)
3. Results
3.1. Identification and Cloning of Sfdsx
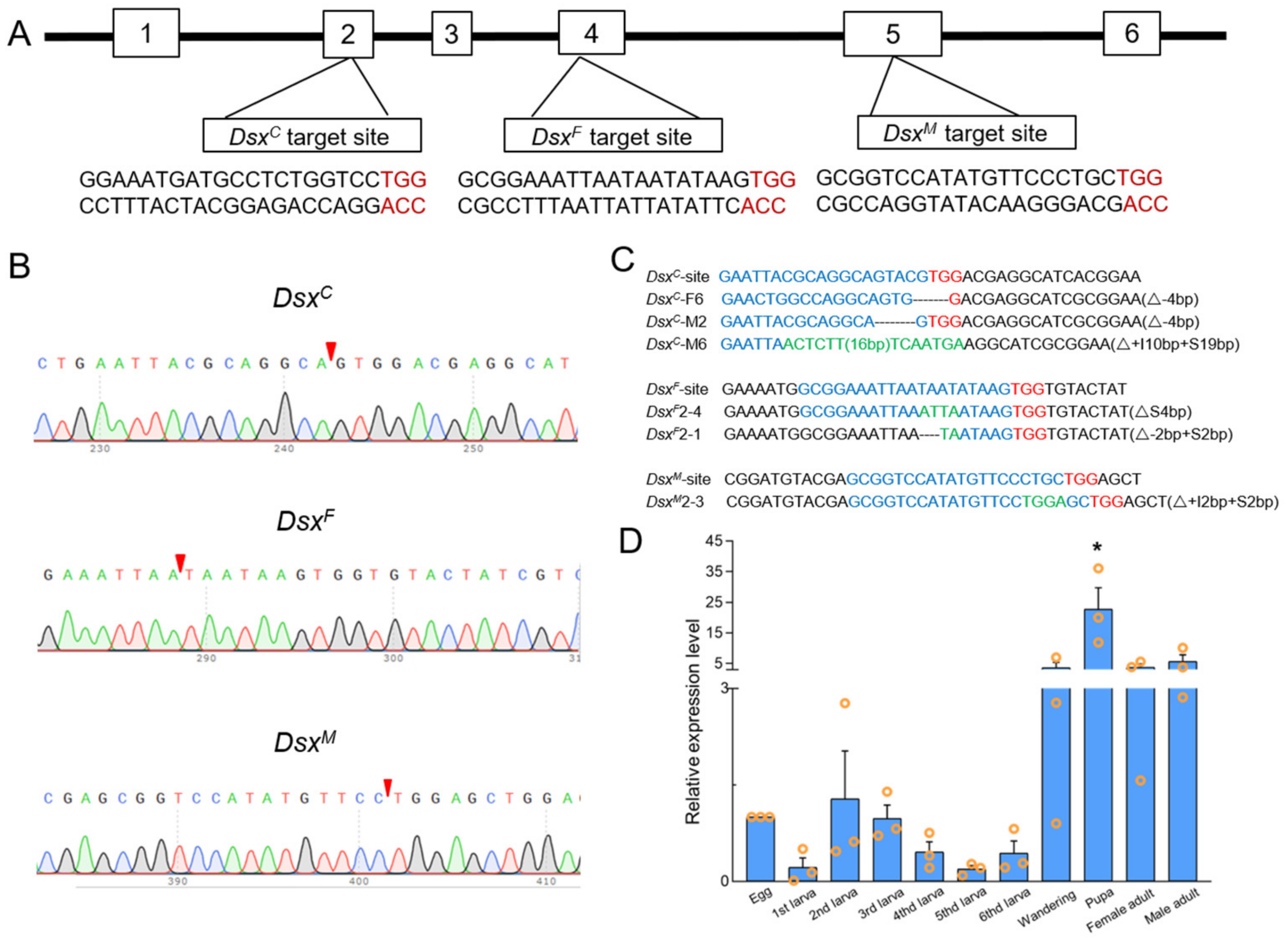
3.2. CRISPR/Cas9-Mediated Sfdsx Mutagenesis
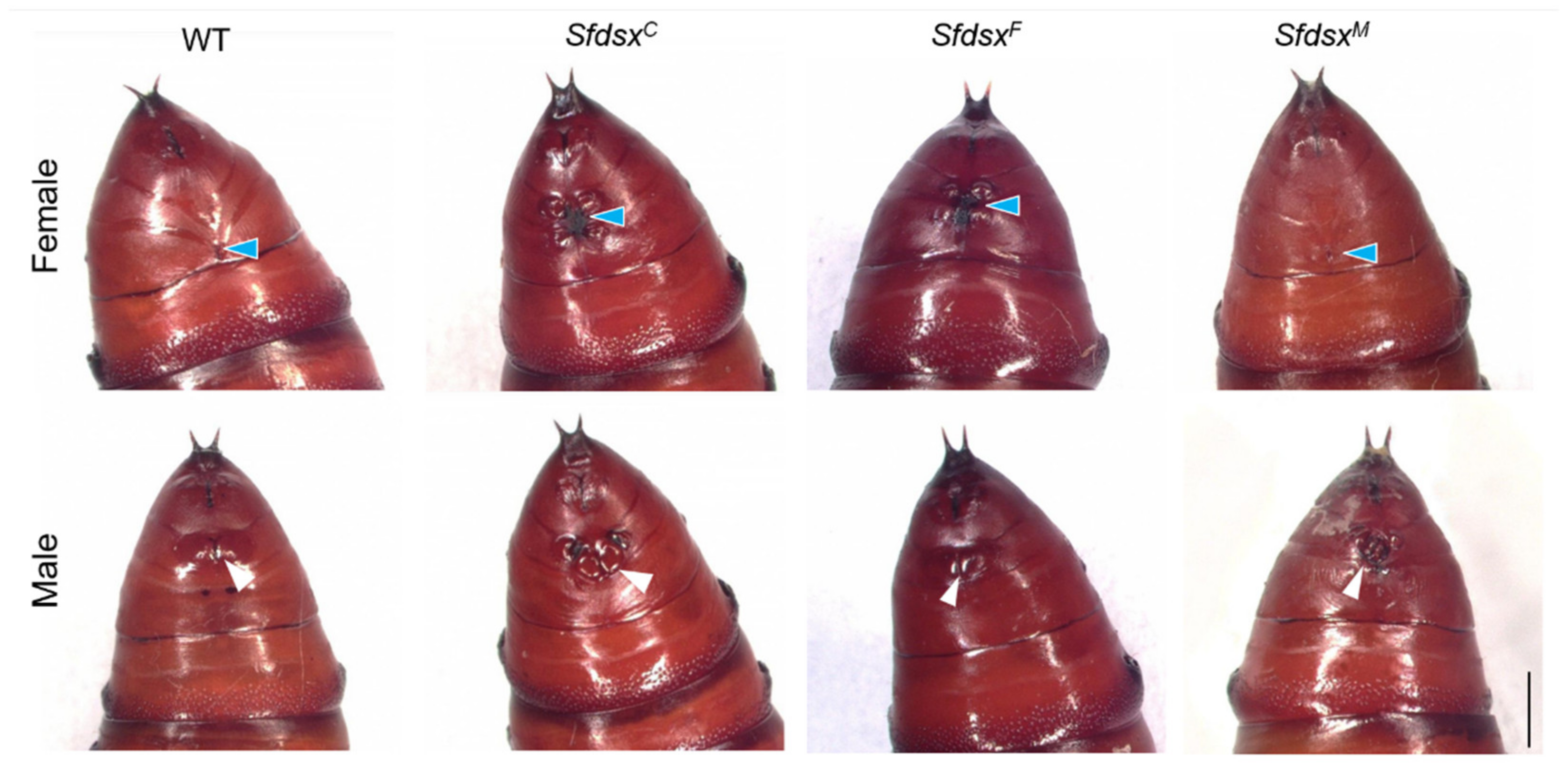
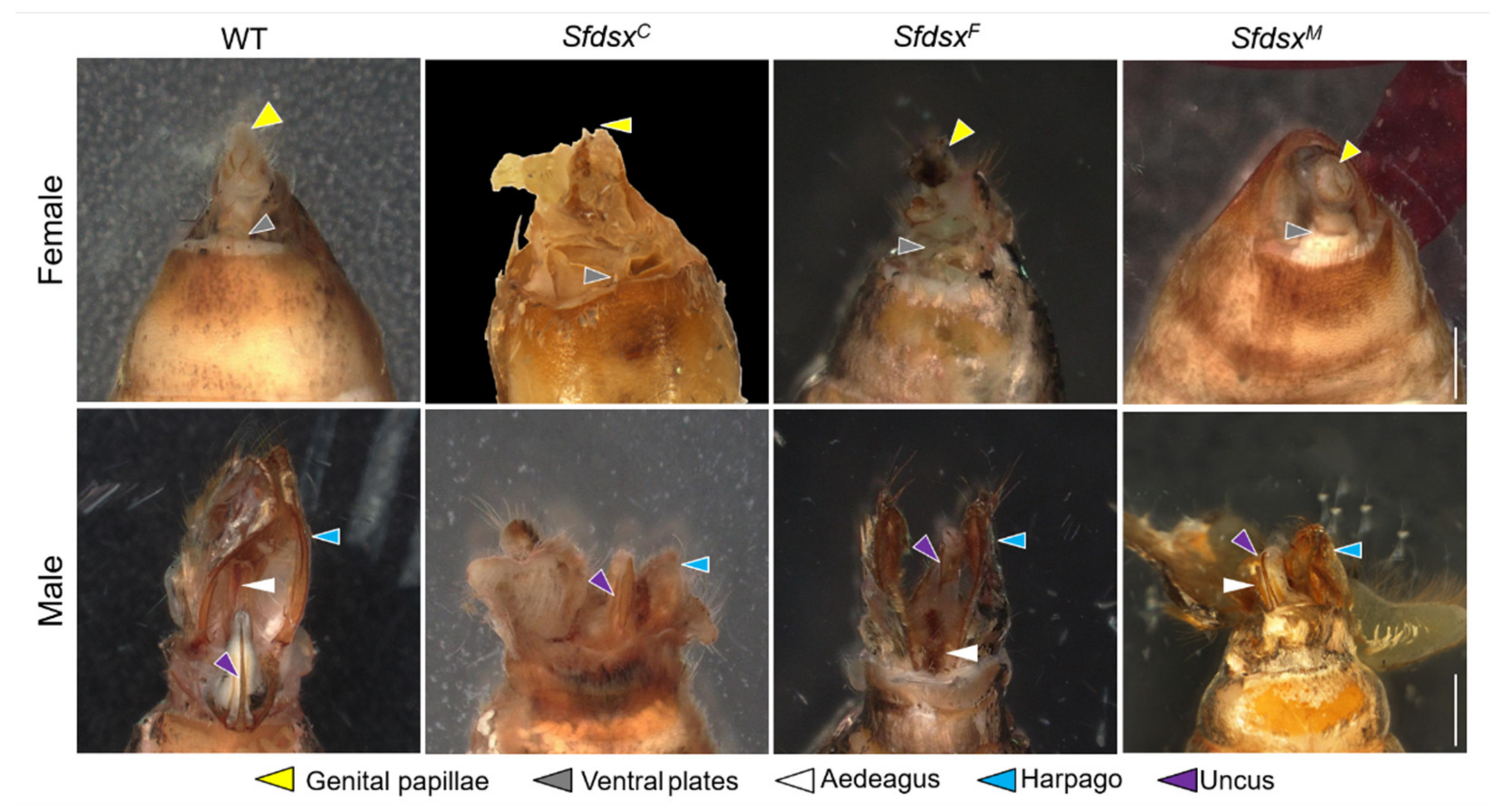
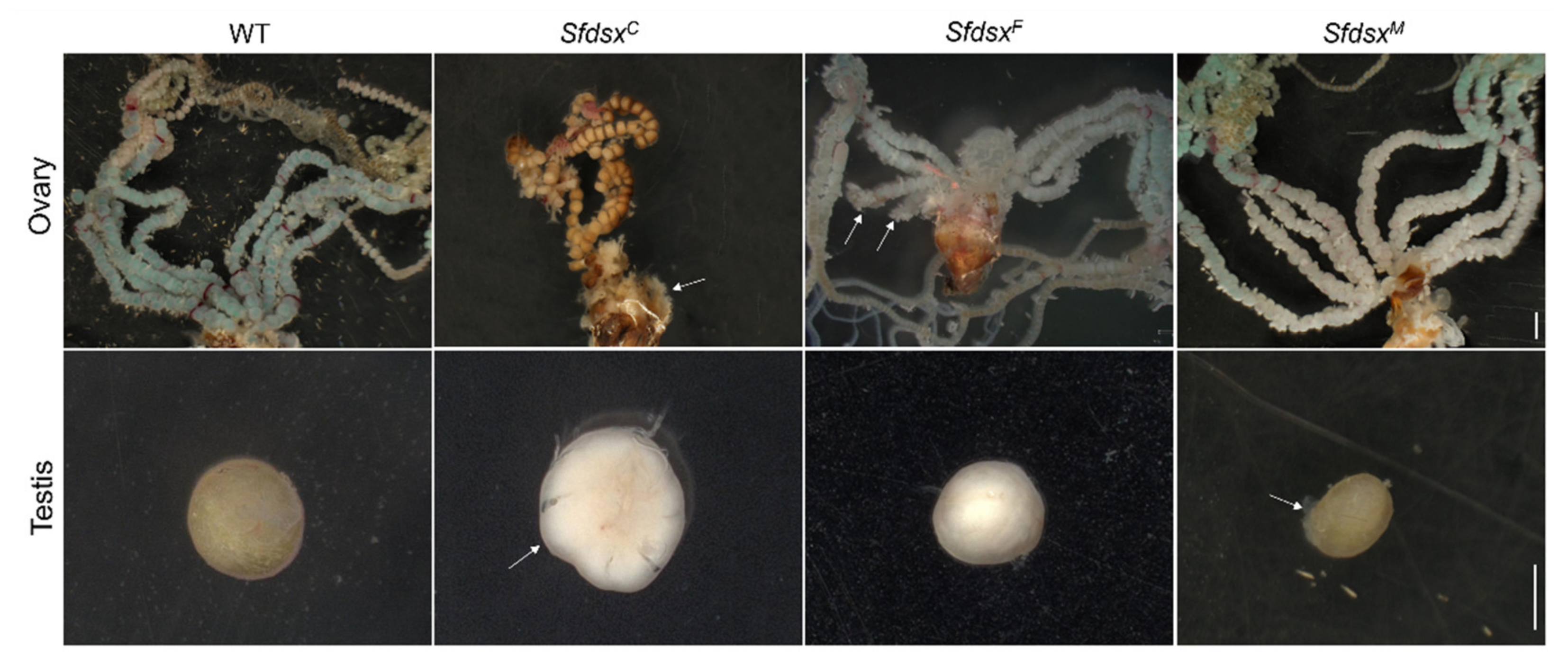
3.3. Phenotypic Impacts of Sfdsx Mutagenesis
3.3.1. External Genitalia
3.3.2. Internal Genitalia
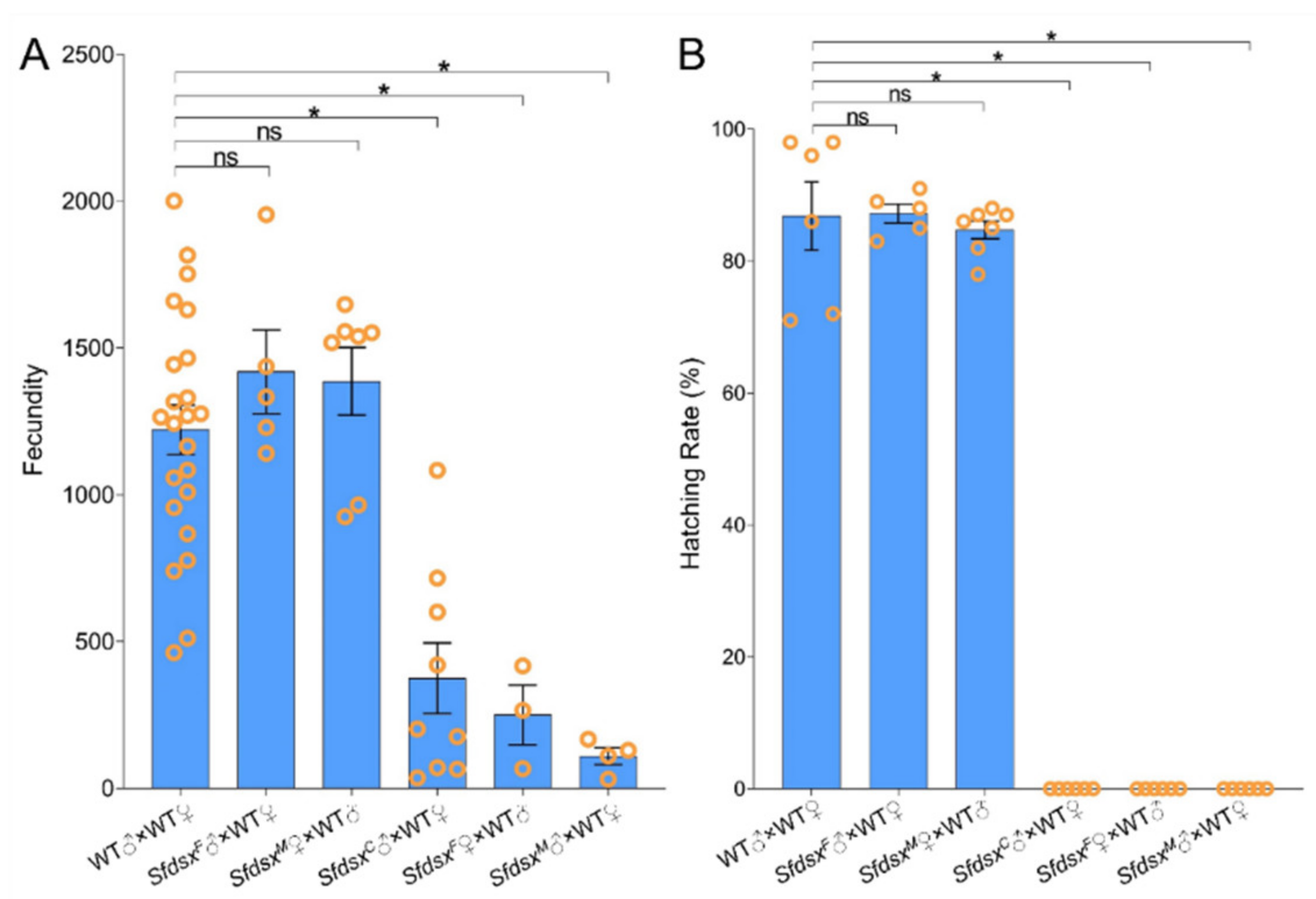
3.4. Physiological Impacts
3.5. Pleiotropic Impacts
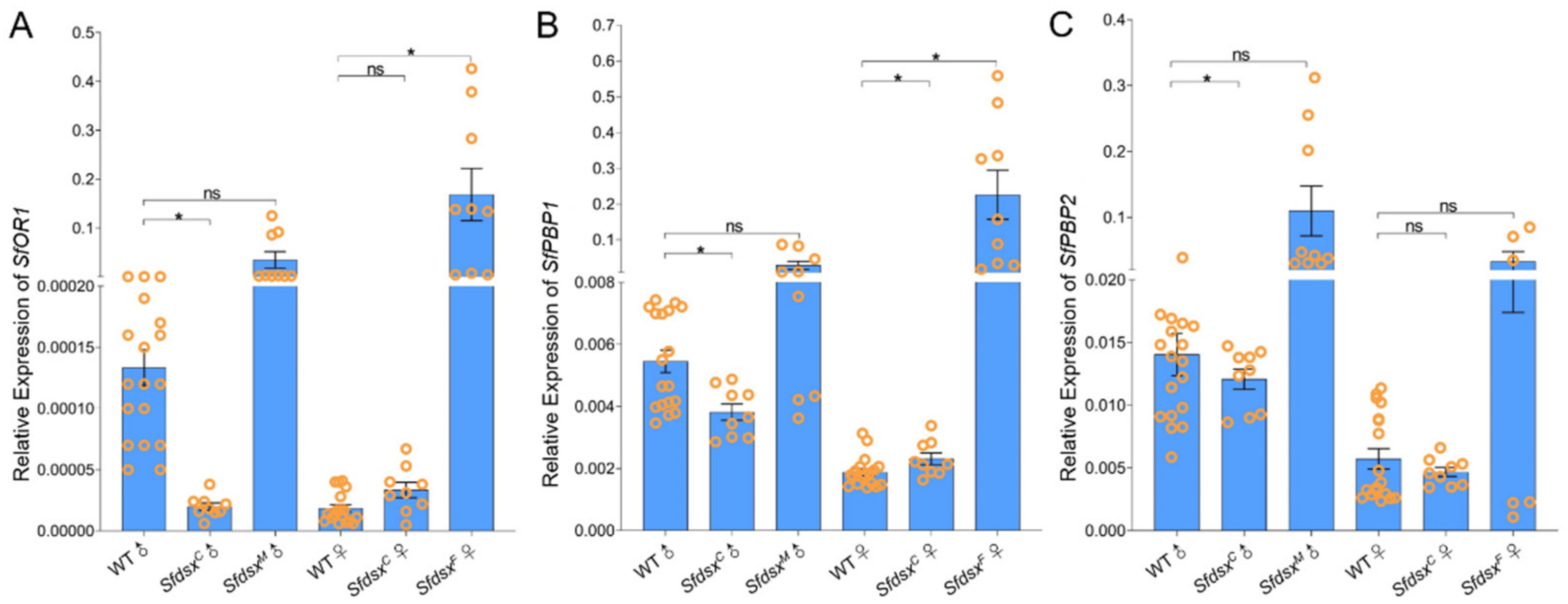
3.6. Sex-Specific Expression of Sfdsx
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagoshi, R.N.; Nagoshi, B.Y.; Canarte, E.; Navarrete, B.; Solorzano, R.; Garces-Carrera, S. Genetic characterization of fall armyworm (Spodoptera frugiperda) in Ecuador and comparisons with regional populations identify likely migratory relationships. PLoS ONE 2019, 14, e0222332. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Pashley, D.P.J.E. Quantitative genetics, development, and physiological adaptation in host strains of fall armyworm. Evolution 1988, 42, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; He, L.M.; Shen, X.J.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Estimation of the Potential Infestation Area of Newly-invaded Fall Armyworm Spodoptera frugiperda in the Yangtze River Valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Montezano, D.G.; Specht, A.; Sosa-Gomez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Xiao, H.; Ye, X.; Xu, H.; Mei, Y.; Yang, Y.; Chen, X.; Yang, Y.; Liu, T.; Yu, Y.; Yang, W.; et al. The genetic adaptations of fall armyworm Spodoptera frugiperda facilitated its rapid global dispersal and invasion. Mol. Ecol. Res. 2020, 20, 1050–1068. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.; Padovez, F.E.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.C.; Bolzan, A.; Nascimento, A.R.; Amaral, F.S.; Kanno, R.H.; Kaiser, I.S.; Omoto, C. Resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to spinetoram: Inheritance and cross-resistance to spinosad. Pest Manag. Sci. 2020, 76, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.; Hou, R.; Zhao, K.; Wang, Y.; Huang, S.; Cheng, D.; Zhang, Z. Examination of acephate absorption, transport, and accumulation in maize after root irrigation for Spodoptera frugiperda control. Environ. Sci. Pollut. Res. Int. 2021, 28, 57361–57371. [Google Scholar] [CrossRef]
- Feng, B.; Zhi, H.; Chen, H.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Cui, H.; Zeng, Z. Development of Chlorantraniliprole and Lambda Cyhalothrin Double-Loaded Nano-Microcapsules for Synergistical Pest Control. Nanomaterials 2021, 11, 2730. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, J.; Prasanna, B.M.; Midega, C.A.O.; Ronald, P.C.; Carriere, Y.; Tabashnik, B.E. Managing Fall Armyworm in Africa: Can Bt Maize Sustainably Improve Control? J. Econ. Entomol. 2021, 114, 1934–1949. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, C.J.; Li, N.; Zhao, Y.; Chen, Z.M.; Yi, S.J.; Li, Z.Q.; Adang, M.J.; Huang, G.H. Novel strategies for the biocontrol of noctuid pests (Lepidoptera) based on improving ascovirus infectivity using Bacillus thuringiensis. Insect Sci. 2021, 28, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, B.; Zheng, W.; Liu, C.; Zhang, D.; Zhao, S.; Li, Z.; Xu, P.; Wilson, K.; Withers, A.; et al. Genetic structure and insecticide resistance characteristics of fall armyworm populations invading China. Mol. Ecol. Res. 2020, 20, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F delta-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef]
- Burtis, K.C.; Baker, B.S.J.C. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 1989, 56, 997–1010. [Google Scholar] [CrossRef]
- Wang, Y.; Rensink, A.H.; Fricke, U.; Riddle, M.C.; Trent, C.; van de Zande, L.; Verhulst, E.C. Doublesex regulates male-specific differentiation during distinct developmental time windows in a parasitoid wasp. Insect Biochem. Mol. Biol. 2022, 142, 103724. [Google Scholar] [CrossRef]
- Cho, S.; Huang, Z.Y.; Zhang, J. Sex-specific splicing of the honeybee doublesex gene reveals 300 million years of evolution at the bottom of the insect sex-determination pathway. Genetics 2007, 177, 1733–1741. [Google Scholar] [CrossRef]
- Scali, C.; Catteruccia, F.; Li, Q.; Crisanti, A. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. J. Exp. Biol. 2005, 208, 3701–3709. [Google Scholar] [CrossRef]
- Salvemini, M.; Mauro, U.; Lombardo, F.; Milano, A.; Zazzaro, V.; Arca, B.; Polito, L.C.; Saccone, G. Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC Evol. Biol. 2011, 11, 41. [Google Scholar] [CrossRef]
- Price, D.C.; Egizi, A.; Fonseca, D.M. Characterization of the doublesex gene within the Culex pipiens complex suggests regulatory plasticity at the base of the mosquito sex determination cascade. BMC Evol. Biol. 2015, 15, 108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Concha, C.; Li, F.; Scott, M.J. Conservation and sex-specific splicing of the doublesex gene in the economically important pest species Lucilia cuprina. J. Genet. 2010, 89, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.; Burghardt, G.; Siegenthaler, C.; Buser, N.; Hilfiker-Kleiner, D.; Dubendorfer, A.; Bopp, D. Sex determination in Drosophila melanogaster and Musca domestica converges at the level of the terminal regulator doublesex. Dev. Genes Evol. 2004, 214, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.N.; Palli, S.R. Doublesex target genes in the red flour beetle, Tribolium castaneum. Sci. Rep. 2012, 2, 948. [Google Scholar] [CrossRef]
- Gotoh, H.; Miyakawa, H.; Ishikawa, A.; Ishikawa, Y.; Sugime, Y.; Emlen, D.J.; Lavine, L.C.; Miura, T. Developmental Link between Sex and Nutrition; doublesex Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles. PLoS Genet. 2014, 10, e1004098. [Google Scholar] [CrossRef]
- Ito, Y.; Harigai, A.; Nakata, M.; Hosoya, T.; Araya, K.; Oba, Y.; Ito, A.; Ohde, T.; Yaginuma, T.; Niimi, T. The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep. 2013, 14, 561–567. [Google Scholar] [CrossRef]
- Kunte, K.; Zhang, W.; Tenger-Trolander, A.; Palmer, D.H.; Martin, A.; Reed, R.D.; Mullen, S.P.; Kronforst, M.R. doublesex is a mimicry supergene. Nature 2014, 507, 229–232. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Li, Z.; Ling, L.; Zeng, B.; James, A.A.; Tan, A.; Huang, Y. Transcription activator-like effector nuclease (TALEN)-mediated female-specific sterility in the silkworm, Bombyx mori. Insect Mol. Biol. 2014, 23, 800–807. [Google Scholar] [CrossRef]
- Prakash, A.; Monteiro, A. Molecular mechanisms of secondary sexual trait development in insects. Curr. Opin. Insect Sci. 2016, 17, 40–48. [Google Scholar] [CrossRef]
- Morrow, J.L.; Riegler, M.; Frommer, M.; Shearman, D.C. Expression patterns of sex-determination genes in single male and female embryos of two Bactrocera fruit fly species during early development. Insect Mol. Biol. 2014, 23, 754–767. [Google Scholar] [CrossRef]
- Robinett, C.C.; Vaughan, A.G.; Knapp, J.M.; Baker, B.S. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010, 8, e1000365. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, K.; Wang, Y.; Yang, D.; Huang, Y. The Sex Determination Cascade in the Silkworm. Genes 2021, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Sun, L.; Tomchaney, M.; Sullivan, G.; Adams, H.; Piscoya, A.S.; Severson, D.W.; Syed, Z.; Duman-Scheel, M. siRNA-Mediated Silencing of doublesex during Female Development of the Dengue Vector Mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e0004213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Liu, Z.; Xu, J.; Li, X.; Bi, H.; Andongma, A.A.; Niu, C.; Huang, Y. Mutation of doublesex induces sex-specific sterility of the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 112, 103180. [Google Scholar] [CrossRef]
- Du, Q.; Wen, L.; Zheng, S.C.; Bi, H.L.; Huang, Y.P.; Feng, Q.L.; Liu, L. Identification and functional characterization of doublesex gene in the testis of Spodoptera litura. Insect Sci. 2019, 26, 1000–1010. [Google Scholar] [CrossRef]
- Bi, H.; Li, X.; Xu, X.; Wang, Y.; Zhou, S.; Huang, Y. Masculinizer and Doublesex as Key Factors Regulate Sexual Dimorphism in Ostrinia furnacalis. Cells 2022, 11, 2161. [Google Scholar] [CrossRef]
- Roth, A.; Vleurinck, C.; Netschitailo, O.; Bauer, V.; Otte, M.; Kaftanoglu, O.; Page, R.E.; Beye, M. A genetic switch for worker nutrition-mediated traits in honeybees. PLoS Biol. 2019, 17, e3000171. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef]
- Shukla, J.; Nagaraju, J.J.J.O.G. Doublesex: A conserved downstream gene controlled by diverse upstream regulators. J. Genet. 2010, 89, 341–356. [Google Scholar] [CrossRef]
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636. [Google Scholar] [CrossRef]
- Katsuma, S.; Sugano, Y.; Kiuchi, T.; Shimada, T. Two Conserved Cysteine Residues Are Required for the Masculinizing Activity of the Silkworm Masc Protein. J. Biol. Chem. 2015, 290, 26114–26124. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sumitani, M.; Chikami, Y.; Yahata, K.; Uchino, K.; Kiuchi, T.; Katsuma, S.; Aoki, F.; Sezutsu, H.; Suzuki, M.G. Transgenic Expression of the piRNA-Resistant Masculinizer Gene Induces Female-Specific Lethality and Partial Female-to-Male Sex Reversal in the Silkworm, Bombyx mori. PLoS Genet. 2016, 12, e1006203. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ozaki, K.; Ishikawa, Y.; Matsuo, T. Identification of candidate odorant receptors in Asian corn borer Ostrinia furnacalis. PLoS ONE 2015, 10, e0121261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Liu, H.; Bi, H.; Wang, Y.; Chen, X.; Wu, N.; Xu, J.; Zhang, Z.; Huang, Y.; et al. Mutation of doublesex in Hyphantria cunea results in sex-specific sterility. Pest Manag. Sci. 2020, 76, 1673–1682. [Google Scholar] [CrossRef]
- Xu, J.; Zhan, S.; Chen, S.; Zeng, B.; Li, Z.; James, A.A.; Tan, A.; Huang, Y. Sexually dimorphic traits in the silkworm, Bombyx mori, are regulated by doublesex. Insect Biochem. Mol. Biol. 2017, 80, 42–51. [Google Scholar] [CrossRef]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Gribble, M.; Morselli, G.; Burt, A.; et al. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat. Biotechnol. 2020, 38, 1054–1060. [Google Scholar] [CrossRef]
- Krzywinska, E.; Ferretti, L.; Li, J.; Li, J.C.; Chen, C.H.; Krzywinski, J. femaleless Controls Sex Determination and Dosage Compensation Pathways in Females of Anopheles Mosquitoes. Curr. Biol. 2021, 31, 1084–1091.e4. [Google Scholar] [CrossRef]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef]
- Rodriguez-de la Noval, C.; Rodriguez-Cabrera, L.; Izquierdo, L.; Espinosa, L.A.; Hernandez, D.; Ponce, M.; Moran-Bertot, I.; Tellez-Rodriguez, P.; Borras-Hidalgo, O.; Huang, S.; et al. Functional expression of a peritrophin A-like SfPER protein is required for larval development in Spodoptera frugiperda (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 2630. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Williams, T.M.; Selegue, J.E.; Werner, T.; Gompel, N.; Kopp, A.; Carroll, S.B. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 2008, 134, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Carroll, S.B. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat. Rev. Genet. 2009, 10, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Monteiro, A. Doublesex Mediates the Development of Sex-Specific Pheromone Organs in Bicyclus Butterflies via Multiple Mechanisms. Mol. Biol. Evol. 2020, 37, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Zhan, S.; Tan, A.; Palli, S.R.; Huang, Y. Disruption of sex-specific doublesex exons results in male- and female-specific defects in the black cutworm, Agrotis ipsilon. Pest Manag. Sci. 2019, 75, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.; Pettis, J.S.; Tarpy, D.R.; Foster, L.J. Feminizer and doublesex knock-outs cause honey bees to switch sexes. PLoS Biol. 2019, 17, e3000256. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Niu, B.L.; Ji, D.F.; Li, M.W.; Li, K.; James, A.A.; Tan, A.J.; Huang, Y.P. Silkworm genetic sexing through W chromosome-linked, targeted gene integration. Proc. Natl. Acad. Sci. USA 2018, 115, 8752–8756. [Google Scholar] [CrossRef]
- Rideout, E.J.; Dornan, A.J.; Neville, M.C.; Eadie, S.; Goodwin, S.F. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 2010, 13, 458–466. [Google Scholar] [CrossRef]
- Matsushima, D.; Kasahara, R.; Matsuno, K.; Aoki, F.; Suzuki, M.G. Involvement of Ecdysone Signaling in the Expression of the doublesex Gene during Embryonic Development in the Silkworm, Bombyx mori. Sex. Dev. 2019, 13, 151–163. [Google Scholar] [CrossRef]
- Shirangi, T.R.; Wong, A.M.; Truman, J.W.; Stern, D.L. Doublesex Regulates the Connectivity of a Neural Circuit Controlling Drosophila Male Courtship Song. Dev. Cell 2016, 37, 533–544. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Uppendahl, L.D.; Chowdhury, M.A.; Ip, P.L.; Siegal, M.L. The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development 2011, 138, 1099–1109. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Abd-Alla, A.M.M.; Bourtzis, K.; Bouyer, J.; Caceres, C.; de Beer, C.; Oliveira Carvalho, D.; Maiga, H.; Mamai, W.; Nikolouli, K.; et al. The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten Years (2010–2020) of Research and Development, Achievements and Challenges in Support of the Sterile Insect Technique. Insects 2021, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and Its Applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef] [PubMed]
| sgRNA | Injected 1 | Hatched 2 | Pupate 3 | P Mutant 4 | Adult (F/M) 5 | A Mutant (F/M) 6 |
|---|---|---|---|---|---|---|
| dsxC | 815 | 585 (71.8%) | 130 (22.2%) | 50 (38.5%) | 117 (39/78) | 48 (1/47) |
| dsxF | 702 | 449 (64.0%) | 79 (17.6%) | 28 (35.4%) | 73 (31/42) | 22 (22/0) |
| dsxM | 1325 | 461 (34.8%) | 37 (6.9%) | 13 (35.1%) | 36 (17/19) | 12 (0/12) |
| WT | 776 | 665 (85.7%) | 469 (70.5%) | - | 366 (177/189) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Wang, J.; Bi, H.; Li, X.; Merchant, A.; Zhang, P.; Zhang, Q.; Zhou, X. CRISPR/Cas9-Mediated Mutagenesis of Sex-Specific Doublesex Splicing Variants Leads to Sterility in Spodoptera frugiperda, a Global Invasive Pest. Cells 2022, 11, 3557. https://doi.org/10.3390/cells11223557
Gu J, Wang J, Bi H, Li X, Merchant A, Zhang P, Zhang Q, Zhou X. CRISPR/Cas9-Mediated Mutagenesis of Sex-Specific Doublesex Splicing Variants Leads to Sterility in Spodoptera frugiperda, a Global Invasive Pest. Cells. 2022; 11(22):3557. https://doi.org/10.3390/cells11223557
Chicago/Turabian StyleGu, Junwen, Jingyi Wang, Honglun Bi, Xuehai Li, Austin Merchant, Porui Zhang, Qi Zhang, and Xuguo Zhou. 2022. "CRISPR/Cas9-Mediated Mutagenesis of Sex-Specific Doublesex Splicing Variants Leads to Sterility in Spodoptera frugiperda, a Global Invasive Pest" Cells 11, no. 22: 3557. https://doi.org/10.3390/cells11223557
APA StyleGu, J., Wang, J., Bi, H., Li, X., Merchant, A., Zhang, P., Zhang, Q., & Zhou, X. (2022). CRISPR/Cas9-Mediated Mutagenesis of Sex-Specific Doublesex Splicing Variants Leads to Sterility in Spodoptera frugiperda, a Global Invasive Pest. Cells, 11(22), 3557. https://doi.org/10.3390/cells11223557








