Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Construct
2.2. Growth Conditions
2.3. Western Blot Analysis
2.4. Confocal Laser Scanning Microscopy and Image Analysis
2.5. VA-TIRF Microscopy and Single-Particle Fluorescence Image
2.6. Fluorescence-Correlation Spectroscopy Analysis
2.7. Heterologous Expression of AMT1;3-EGFP in Yeast
3. Results
3.1. Ubiquitination Is Correlated with the Degradation of AMT1;3 under High Ammonium Stress
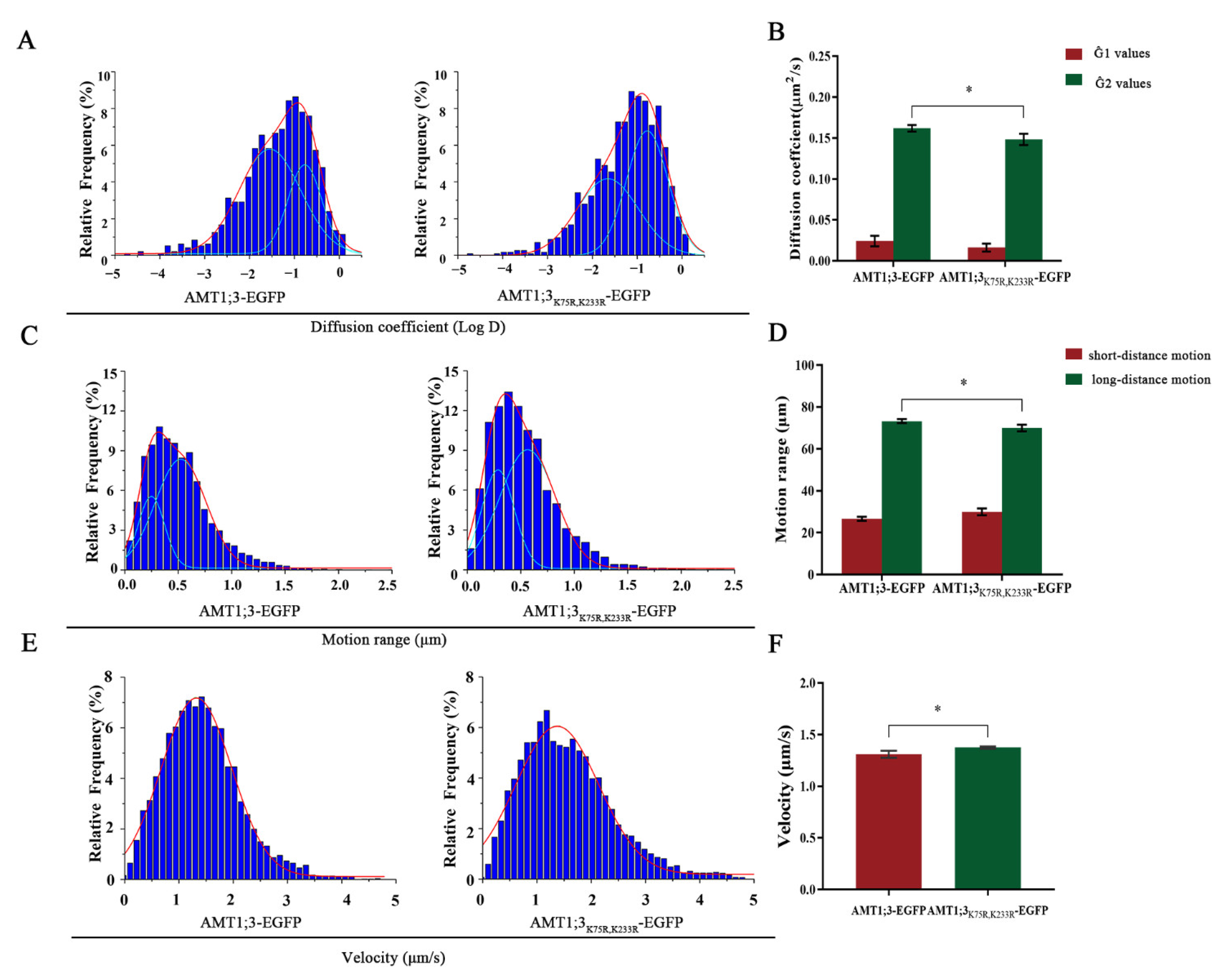
3.2. Ubiquitination Affects the Dynamics of AMT1;3 under High Ammonium Stress
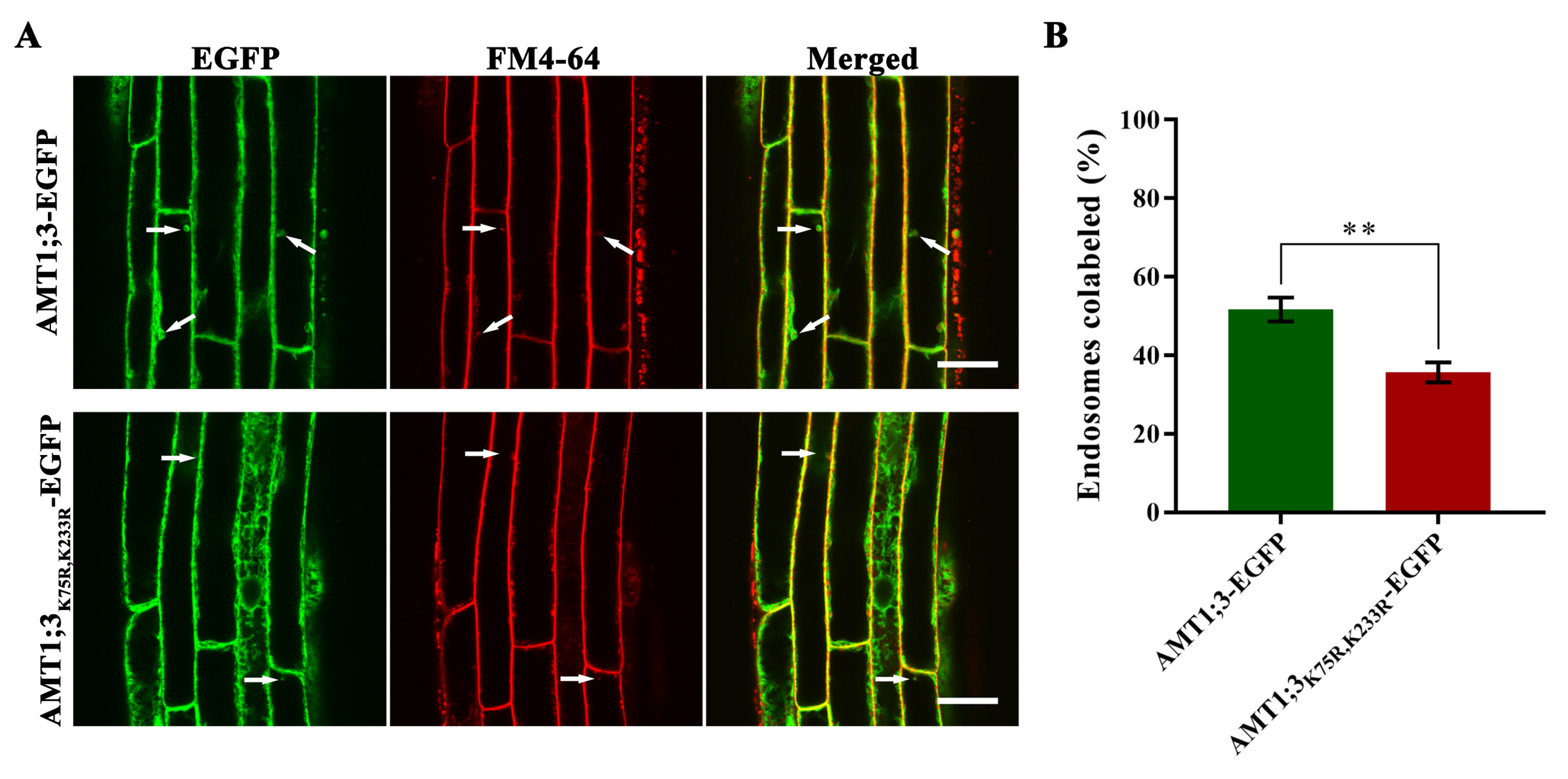
3.3. Loss of AMT1;3 Ubiquitination Impairs Endocytosis under High Ammonium Stress
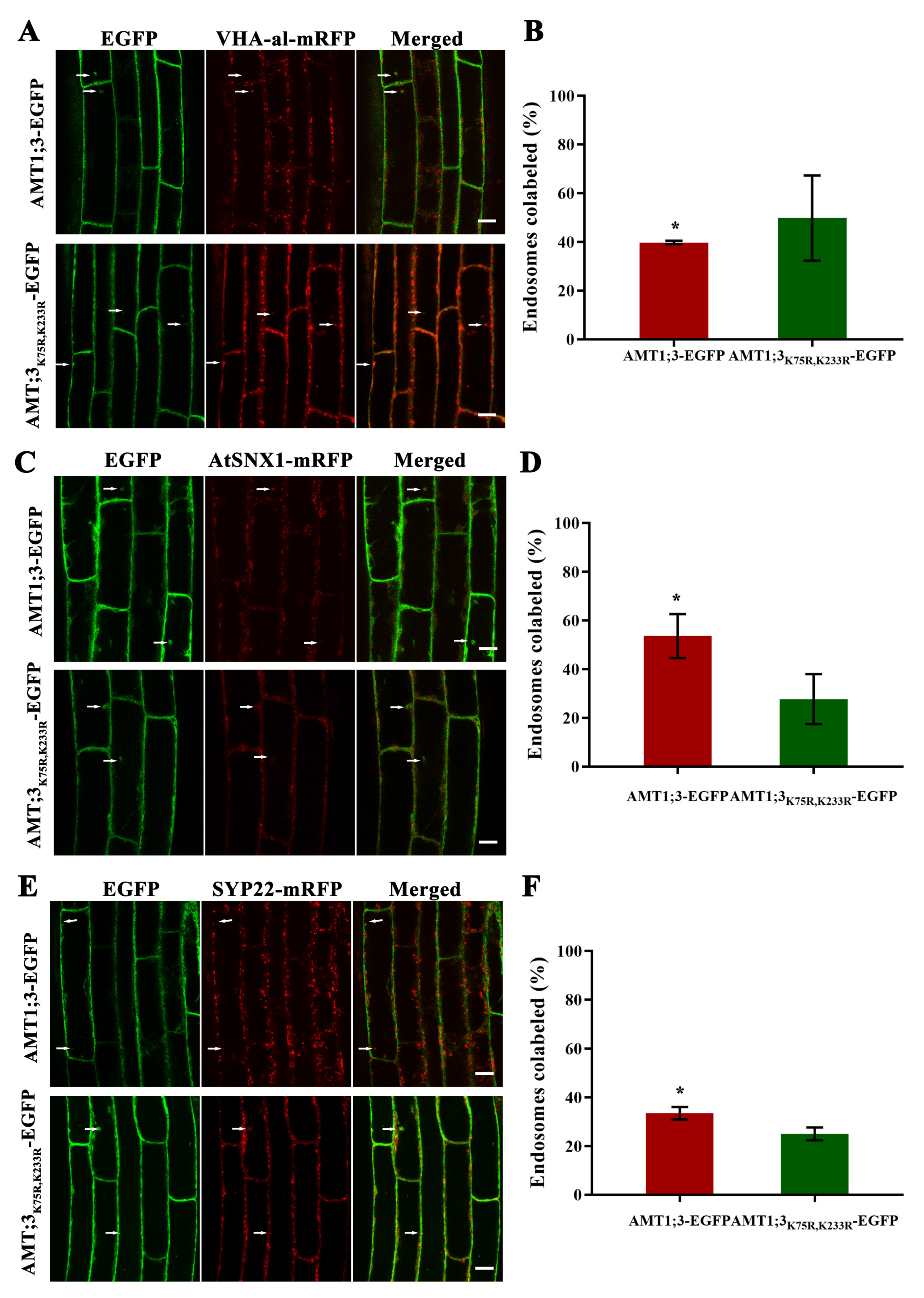
3.4. Ubiquitination Affects Vesicular Transport of the AMT1;3 Protein under High Ammonium Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miflin, B.J.; Lea, P.J. The pathway of nitrogen assimilation in plants. Phytochemistry 1976, 15, 873–885. [Google Scholar] [CrossRef]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–916. [Google Scholar] [CrossRef]
- Crawford, N.M.; Forde, B.G. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Martin, M.H.; Marschner, H. Mineral nutrition of higher plants. J. Ecol. 1988, 76, 1250. [Google Scholar] [CrossRef] [Green Version]
- Von Wirén, N.; Merrick, M. Regulation and function of ammonium carriers in bacteria, fungi, and plants. Top. Curr. Genet. 2004, 9, 95–120. [Google Scholar]
- Sonia, G.; Laurence, L.; Alain, G.; Olaf, N.; Wolf, B.F.; Nicolaus, V.W. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937–947. [Google Scholar]
- Yuan, L.; Loque, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wirén, N. The organization of High-Affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-Type transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef] [Green Version]
- Loqué, D.; Yuan, L.; Kojima, S.; Gojon, A.; Wirth, J.; Gazzarrini, S.; Ishiyama, K.; Takahashi, H.; Von Wirén, N. Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 2010, 48, 522–534. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhao, Y.Y.; Luo, W.X.; Li, R.L.; He, Q.H.; Fang, X.H.; Michele, R.D.; Ast, C.; Wirén, N.V.; Lin, J.X. Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc. Natl. Acad. Sci. USA 2013, 110, 13204–13209. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Gu, R.; Xuan, Y.; Smith-Valle, E.; Loqué, D.; Frommer, W.B.; von Wirén, N. Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo. Plant Cell 2013, 25, 974–984. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Huarancca Reyes, T.; Uemura, T.; Baral, A.; Fujimaki, A.; Luo, Y.; Morita, Y.; Saeki, Y.; Maekawa, S.; Yasuda, S.; et al. The TGN/EE SNARE protein SYP61 and the ubiquitin ligase ATL31 cooperatively regulate plant responses to carbon/nitrogen conditions in Arabidopsis. Plant Cell 2022, 29, 34. [Google Scholar] [CrossRef]
- Eugene, O.; David, A.; Michael, R. Principles of Ubiquitin-Dependent Signaling. Annu. Rev. Cell Dev. Biol. 2018, 34, 137–162. [Google Scholar]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, E450–E458. [Google Scholar] [CrossRef] [Green Version]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 2018, 69, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Kasai, K.; Takano, J.; Miwa, K.; Toyoda, A.; Fujiwara, T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J. Biol. Chem. 2011, 286, 6175–6183. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, A.; Hosokawa, T.; Beier, M.P.; Oshima, K.; Ogino, Y.; Hori, C.; Takasuka, T.E.; Fukao, Y.; Fujiwara, T.; Takano, J. Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation. Plant Cell 2021, 33, 420–438. [Google Scholar] [CrossRef]
- Korbei, B.; Moulinier-Anzola, J.; De-Araujo, L.; Lucyshyn, D.; Retzer, K.; Khan, M.A.; Luschnig, C. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 2013, 23, 2500–2505. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.Y.; He, Q.Z.H.; Qi, Z.X.; Zhang, Y.; Lu, L.; Xue, J.Y.; Li, R.L.; Lin, J.X. Dynamics and endocytosis of flot1 in Arabidopsis require CPI1 function. Int. J. Mol. Sci. 2020, 21, 1552. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Liu, H.J.; Dong, Z.Y.; Xiao, J.W.; Su, B.D.; Fan, L.S.; Komis, G.; Samaj, J.; Lin, J.X.; Li, R.L. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J. Physiol. 2017, 215, 73–84. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.J.; Yu, M.; Li, R.L.; Fan, L.S.; Zhu, Y.F.; Lin, J.X. Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development 2018, 145, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.R.; Vega-Sánchez, M.E.; Zhu, T.; Wang, G.L. Ubiquitination-mediated protein degradation and modification: An emerging theme in plant-microbe interactions. Cell Res. 2006, 16, 413–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suenaga, A.; Moriya, K.; Sonoda, Y.; Ikeda, A.; Von Wirén, N.; Hayakawa, T.; Yamaguchi, J.; Yamaya, T. Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol. 2003, 44, 206–211. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, X.; Zhang, N.; Feng, X.; Huang, Y.; Zeng, Q.; Wu, J.; Zhang, J.; Qi, Y. Genome-wide identification and transcriptional analysis of ammonium transporters in Saccharum. Genomics 2021, 113, 1671–1680. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Zhang, B.; Hao, Y.; Ma, F. Genome-wide identification and expression analysis of AMT gene family in Apple (Malus domestica Borkh.). Horticulturae 2022, 8, 457. [Google Scholar] [CrossRef]
- Wu, X.; Liu, T.; Zhang, Y.; Duan, F.; Neuhäuser, B.; Ludewig, U.; Schulze, W.X.; Yuan, L. Ammonium and nitrate regulate NH4+ uptake activity of Arabidopsis ammonium transporter AtAMT1;3 via phosphorylation at multiple C-terminal sites. J. Exp. Bot. 2019, 70, 4919–4930. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, D.; Riezman, H. Proteasome-Independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Isono, E.; Kalinowska, K. ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants. Curr. Opin. Plant Biol. 2017, 40, 49–55. [Google Scholar] [CrossRef]
- Lindy, A.; René, B.; Nenad, M.; Tomasz, P.; Justyna, W.; Jeanette, C.M.; Tobias, S.; Jirí, F.; Christian, L. Intracellular trafficking and proteolysis of the Arabidopsis auxin efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar]
- Vera, G.; Thomas, S.; Heidrun, H.; Sophia, M.; Tobias, M.; Thomas, B.; Marta, D.T.; John, W.M.; Silke, R. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008, 18, 1824–1832. [Google Scholar]
- Hyun, K.L.; Seok, K.C.; Ora, S.; Xu, Z.Y.; Inhwan, H.; Woo, T.K. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 2009, 21, 622–641. [Google Scholar]
- Jentsch, T.J. Discovery of CLC transport proteins: Cloning, structure, function and pathophysiology. J. Physiol. 2015, 593, 4091–4109. [Google Scholar] [CrossRef]
- Haglund, K.; Sigismund, S.; Polo, S.; Szymkiewicz, I.; Di Fiore, P.P.; Dikic, I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003, 5, 461–466. [Google Scholar] [CrossRef]
- Guo, X.; Shen, S.; Song, S.; He, S.; Cui, Y.; Xing, G.; Wang, J.; Yin, Y.; Fan, L.; He, F.; et al. The E3 ligase Smurf1 regulates Wolfram syndrome protein stability at the endoplasmic reticulum. J. Biol. Chem. 2011, 286, 18037–18047. [Google Scholar] [CrossRef] [Green Version]
- Nakatsu, F.; Sakuma, M.; Matsuo, Y.; Arase, H.; Yamasaki, S.; Nakamura, N.; Saito, T.; Ohno, H. A Di-leucine signal in the ubiquitin moiety. Possible involvement in ubiquitination mediated endocytosis. J. Biol. Chem. 2000, 275, 26213–26219. [Google Scholar] [CrossRef] [Green Version]
- Terrell, J.; Shih, S.; Dunn, R.; Hicke, L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1998, 1, 193–202. [Google Scholar] [CrossRef]
- Ma, X.; Claus, L.A.; Leslie, M.E.; Tao, K.; Wu, Z.; Liu, J.; Yu, X.; Li, B.; Zhou, J.; Savatin, D.V.; et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 2020, 581, 199–203. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Dohmann, E.; Cayrel, A.; Johnson, A.; Fischer, W.; Pojer, F.; Satiat-Jeunemaître, B.; Jaillais, Y.; Chory, J.; Geldner, N.; et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun. 2015, 6, 6151. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Cao, Y.; Ge, Y.; Xu, J.; Li, R.; Yang, M.; Chen, Y.; Wu, D.; Xiao, J.; Li, R. Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress. Cells 2022, 11, 3651. https://doi.org/10.3390/cells11223651
Zhao R, Cao Y, Ge Y, Xu J, Li R, Yang M, Chen Y, Wu D, Xiao J, Li R. Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress. Cells. 2022; 11(22):3651. https://doi.org/10.3390/cells11223651
Chicago/Turabian StyleZhao, Ran, Yangyang Cao, Yanrui Ge, Jing Xu, Ruofan Li, Mei Yang, Yingying Chen, Dingjie Wu, Jianwei Xiao, and Ruili Li. 2022. "Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress" Cells 11, no. 22: 3651. https://doi.org/10.3390/cells11223651




