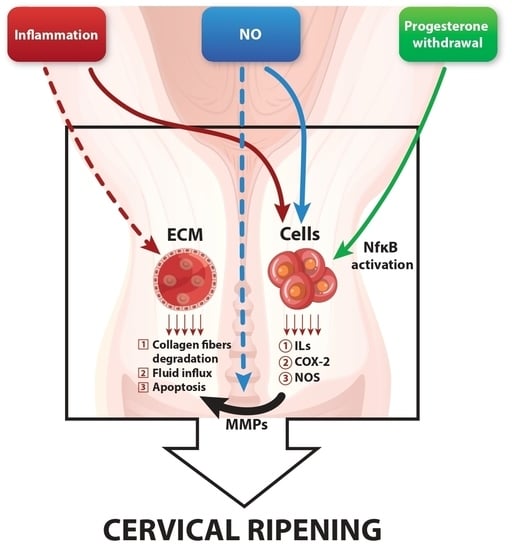
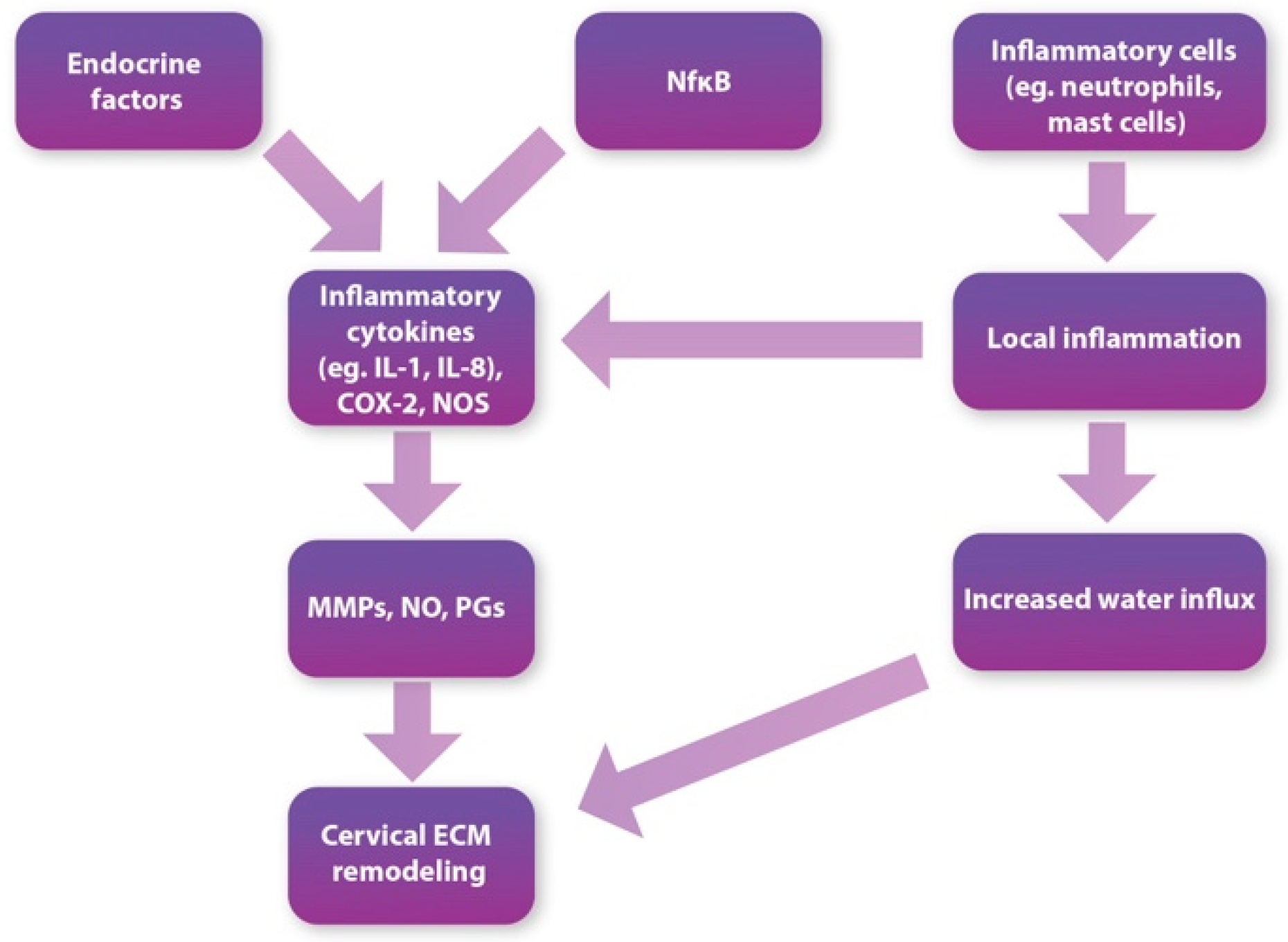
Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery
Abstract
:1. Introduction
2. Anatomy and Physiology of Cervix
3. Cervical Ripening
3.1. Glycosaminoglycans
3.2. Matrix Metalloproteinases
3.3. Inflammatory Process and Immune Cells
4. Regulation of Cervical Ripening
4.1. Inflammatory Cytokines
4.1.1. Interleukins
4.1.2. Tumor Necrosis Factor
4.2. Apoptosis
4.3. Hyaluronic Acid
4.4. Erythropoietin
4.5. Mechanical Factors
4.6. Endocrine Regulation
4.6.1. Relaxin
4.6.2. Corticotropin-Releasing Protein Hormone
4.6.3. Glucocorticoids and NF-κB
4.6.4. Dehydroepiandrosterone 3-Sulfate and Interleukin-8
4.6.5. Estrogen and Insulin Growth Factor-1
4.6.6. Progesterone
4.6.7. Oxytocin
4.7. Nitric Oxide
4.8. Prostaglandins
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, J.M.; Kellar, L.C.; Henning, G.F.; Waheed, A.; Colon-Gonzalez, M.; Ural, S. The Association between the Regular Use of Preventive Labour Induction and Improved Term Birth Outcomes: Findings of a Systematic Review and Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Petitjean, P.; Carbonne, B.; Deneux-Tharaux, C.; Salomé, M.; Goffinet, F.; Le Ray, C. Comparison of Effectiveness and Safety of Cervical Ripening Methods for Induction of Labour: A Population-Based Study Using Coarsened Exact Matching. Paediatr. Perinat. Epidemiol. 2019, 33, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative Developmental Biology of the Mammalian Uterus. Curr. Top. Dev. Biol. 2005, 68, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Jordan, J.A. The Functional Anatomy of the Cervix, the Cervical Epithelium and the Stroma. Cervix Second Ed. 2009, 13–37. [Google Scholar] [CrossRef]
- Leppert, P.C. Anatomy and Physiology of Cervical Ripening. Clin. Obstet. Gynecol. 1995, 38, 267–279. [Google Scholar] [CrossRef]
- Martyn, F.; McAuliffe, F.M.; Wingfield, M. The Role of the Cervix in Fertility: Is It Time for a Reappraisal? Hum. Reprod. Oxf. Engl. 2014, 29, 2092–2098. [Google Scholar] [CrossRef]
- Mahendroo, M.; Nallasamy, S. Cervix. Encycl. Reprod. 2018, 2, 339–346. [Google Scholar] [CrossRef]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A New Paradigm for the Role of Smooth Muscle Cells in the Human Cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1–478.e11. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, A. The Physiology of Cervical Ripening and the Induction of Labour: A Potential Role for the Nitric Oxide Donor Isosorbide Mononitrate; University of Glasgow: Glasgow, UK, 2001. [Google Scholar]
- Schlembach, D.; Mackay, L.; Shi, L.; Maner, W.L.; Garfield, R.E.; Maul, H. Cervical Ripening and Insufficiency: From Biochemical and Molecular Studies to in Vivo Clinical Examination. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S70–S76. [Google Scholar] [CrossRef]
- Iwahashi, M.; Muragaki, Y.; Ooshima, A.; Umesaki, N. Decreased Type I Collagen Expression in Human Uterine Cervix during Pregnancy. J. Clin. Endocrinol. Metab. 2003, 88, 2231–2235. [Google Scholar] [CrossRef]
- Uldbjerg, N.; Ekman, G.; Malmström, A.; Olsson, K.; Ulmsten, U. Ripening of the Human Uterine Cervix Related to Changes in Collagen, Glycosaminoglycans, and Collagenolytic Activity. Am. J. Obstet. Gynecol. 1983, 147, 662–666. [Google Scholar] [CrossRef]
- Akgul, Y.; Holt, R.; Mummert, M.; Word, A.; Mahendroo, M. Dynamic Changes in Cervical Glycosaminoglycan Composition during Normal Pregnancy and Preterm Birth. Endocrinology 2012, 153, 3493–3503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppert, P.C.; Keller, S.; Cerreta, J.; Hosannah, Y.; Mandl, I. The Content of Elastin in the Uterine Cervix. Arch. Biochem. Biophys. 1983, 222, 53–58. [Google Scholar] [CrossRef]
- Yoshida, K.; Jayyosi, C.; Lee, N.; Mahendroo, M.; Myers, K.M. Mechanics of Cervical Remodelling: Insights from Rodent Models of Pregnancy. Interface Focus 2019, 9, 20190026. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Ruscheinsky, M.; De la Motte, C.; Mahendroo, M. Hyaluronan and Its Binding Proteins during Cervical Ripening and Parturition: Dynamic Changes in Size, Distribution and Temporal Sequence. Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Maradny, E.E.; Kanayama, N.; Kobayashi, H.; Hossain, B.; Khatun, S.; Liping, S.; Kobayashi, T.; Terao, T. The Role of Hyaluronic Acid as a Mediator and Regulator of Cervical Ripening. Hum. Reprod. Oxf. Engl. 1997, 12, 1080–1088. [Google Scholar] [CrossRef]
- Almond, A. Hyaluronan. Cell Mol. Life Sci. CMLS 2007, 64, 1591–1596. [Google Scholar] [CrossRef]
- Norman, M.; Ekman, G.; Malmström, A. Changed Proteoglycan Metabolism in Human Cervix Immediately after Spontaneous Vaginal Delivery. Obstet. Gynecol. 1993, 81, 217–223. [Google Scholar]
- Gahlot, S.C.; Kumaresan, A.; Kumar, S.; Yadav, S.; Saraf, K.; Karan, P.; Verma, K. Incomplete Cervical Dilatation in Animals—An Update. Int. J. Sci. Environ. 2017, 6, 1036–1048. [Google Scholar]
- Osmers, R.; Rath, W.; Adelmann-Grill, B.C.; Fittkow, C.; Kuloczik, M.; Szeverényi, M.; Tschesche, H.; Kuhn, W. Origin of Cervical Collagenase during Parturition. Am. J. Obstet. Gynecol. 1992, 166, 1455–1460. [Google Scholar] [CrossRef]
- Nagaset, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, M.A.; Denison, F.C.; Riley, S.C.; Norman, J.E. Matrix Metalloproteinases-2 and -9 and Their Inhibitors Are Produced by the Human Uterine Cervix but Their Secretion Is Not Regulated by Nitric Oxide Donors. Hum. Reprod. Oxf. Engl. 1999, 14, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Uldbjerg, N.; Ulmsten, U.; Ekman, G. The Ripening of the Human Uterine Cervix in Terms of Connective Tissue Biochemistry. Clin. Obstet. Gynecol. 1983, 26, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennström, M.B.; Brauner, A.; Byström, B.; Malmström, A.; Ekman, G. Matrix Metalloproteinase-8 Correlates with the Cervical Ripening Process in Humans. Acta Obstet. Gynecol. Scand. 2003, 82, 904–911. [Google Scholar] [CrossRef]
- Bollopragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term Labor Is Associated with a Core Inflammatory Response in Human Fetal Membranes, Myometrium, and Cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e11. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Guilbert, L.J.; Olson, D.M. Invasion of the Leukocytes into the Fetal-Maternal Interface during Pregnancy. J. Leukoc. Biol. 2010, 88, 625–633. [Google Scholar] [CrossRef]
- Helmig, B.R.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Bujold, E.; Gomez, R.; Ohlsson, K.; Uldbjerg, N. Neutrophil Elastase and Secretory Leukocyte Protease Inhibitor in Prelabor Rupture of Membranes, Parturition and Intra-Amniotic Infection. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2002, 12, 237–246. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Zugaib, M.; Montes, G.S.; Toledo, O.M.; Krisztán, R.M.; Shigihara, K.M. Morphologic and Histochemical Evidence for the Occurrence of Collagenolysis and for the Role of Neutrophilic Polymorphonuclear Leukocytes during Cervical Dilation. Am. J. Obstet. Gynecol. 1980, 138, 273–281. [Google Scholar] [CrossRef]
- Pavlov, O.; Pavlova, O.; Ailamazyan, E.; Selkov, S. Characterization of Cytokine Production by Human Term Placenta Macrophages in Vitro. Am. J. Reprod. Immunol. 2008, 60, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Shepherd, M.C.; Nibbs, R.J.; Nelson, S.M. The Role of Mast Cells and Their Mediators in Reproduction, Pregnancy and Labour. Hum. Reprod. Update 2011, 17, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune Cells in Term and Preterm Labor. Cell. Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juremalm, M.; Nilsson, G. Chemokine Receptor Expression by Mast Cells. Chem. Immunol. Allergy 2005, 87, 130–144. [Google Scholar] [CrossRef]
- Rudolph, M.I.; Reinicke, K.; Cruz, M.A.; Gallardo, V.; Gonzalez, C.; Bardisa, L. Distribution of Mast Cells and the Effect of Their Mediators on Contractility in Human Myometrium. Br. J. Obstet. Gynaecol. 1993, 100, 1125–1130. [Google Scholar] [CrossRef]
- Willets, J.M.; Taylor, A.H.; Shaw, H.; Konje, J.C.; Challiss, R.A.J. Selective Regulation of H1 Histamine Receptor Signaling by G Protein-Coupled Receptor Kinase 2 in Uterine Smooth Muscle Cells. Mol. Endocrinol. Baltim. Md 2008, 22, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Bytautiene, E.; Romero, R.; Vedernikov, Y.P.; El-Zeky, F.; Saade, G.R.; Garfield, R.E. Induction of Premature Labor and Delivery by Allergic Reaction and Prevention by Histamine H 1 Receptor Antagonist. Am. J. Obstet. Gynecol. 2004, 191, 1356–1361. [Google Scholar] [CrossRef]
- Van Engelen, E.; De Groot, M.W.; Breeveld-Dwarkasing, V.N.A.; Everts, M.E.; Van Der Weyden, G.C.; Taverne, M.A.M.; Rutten, V.P.M.G. Cervical Ripening and Parturition in Cows Are Driven by a Cascade of Pro-Inflammatory Cytokines. Reprod. Domest. Anim. Zuchthyg. 2009, 44, 834–841. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Chapter 2—Inflammation. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 23–51. ISBN 978-0-12-813792-5. [Google Scholar]
- Peplow, P.V. Actions of Cytokines in Relation to Arachidonic Acid Metabolism and Eicosanoid Production. Prostaglandins Leukot. Essent. Fatty Acids 1996, 54, 303–317. [Google Scholar] [CrossRef]
- Sennström, M.B.; Ekman, G.; Westergren-Thorsson, G.; Malmström, A.; Byström, B.; Endrésen, U.; Mlambo, N.; Norman, M.; Ståbi, B.; Brauner, A. Human Cervical Ripening, an Inflammatory Process Mediated by Cytokines. Mol. Hum. Reprod. 2000, 6, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Bowen, J.M.; Chamley, L.; Keelan, J.A.; Mitchell, M.D. Cytokines of the Placenta and Extra-Placental Membranes: Roles and Regulation during Human Pregnancy and Parturition. Placenta 2002, 23, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Edwin, S.S.; Lundin-Schiller, S.; Silver, R.M.; Smotkin, D.; Trautman, M.S. Mechanism of Interleukin-1 Beta Stimulation of Human Amnion Prostaglandin Biosynthesis: Mediation via a Novel Inducible Cyclooxygenase. Placenta 1993, 14, 615–625. [Google Scholar] [CrossRef]
- Kniss, D.A.; Zimmerman, P.D.; Garver, C.L.; Fertel, R.H. Interleukin-1 Receptor Antagonist Blocks Interleukin-1-Induced Expression of Cyclooxygenase-2 in Endometrium. Am. J. Obstet. Gynecol. 1997, 177, 559–567. [Google Scholar] [CrossRef]
- Hageman, J.R.; Caplan, M.S. An Introduction to the Structure and Function of Inflammatory Mediators for Clinicians. Clin. Perinatol. 1995, 22, 251–261. [Google Scholar] [CrossRef]
- Watari, M.; Watari, H.; DiSanto, M.E.; Chacko, S.; Shi, G.P.; Strauss, J.F. Pro-Inflammatory Cytokines Induce Expression of Matrix-Metabolizing Enzymes in Human Cervical Smooth Muscle Cells. Am. J. Pathol. 1999, 154, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Hirano, H.; Tsubaki, H.; Kodama, H.; Tanaka, T. The Role of Cytokines in Cervical Ripening: Correlations between the Concentrations of Cytokines and Hyaluronic Acid in Cervical Mucus and the Induction of Hyaluronic Acid Production by Inflammatory Cytokines by Human Cervical Fibroblasts. Am. J. Obstet. Gynecol. 1998, 179, 105–110. [Google Scholar] [CrossRef]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. The Effect of Interleukin-1 in Rabbit Cervical Ripening. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 60, 75–80. [Google Scholar] [CrossRef]
- Brennan, K.; Zheng, J. Interleukin 8. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–4. ISBN 978-0-08-055232-3. [Google Scholar]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory Processes in Preterm and Term Parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef]
- Osmers, R.G.W.; Blaser, J.; Kuhn, W.; Tschesche, H. Interleukin-8 Synthesis and the Onset of Labor. Obstet. Gynecol. 1995, 86, 223–229. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.C.; Ruck, P.; Marx, T.; Kaiserlîng, E.; Oberpichler, A.; Tschesche, H.; Rath, W. Parturition at Term: Parallel Increases in Interleukin-8 and Proteinase Concentrations and Neutrophil Count in the Lower Uterine Segment. Hum. Reprod. Oxf. Engl. 1999, 14, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.W.; Illingworth, P.; Baldie, G.; Leask, R.; Brouwer, S.; Calder, A.A. Progesterone Control of Interleukin-8 Production in Endometrium and Chorio-Decidual Cells Underlines the Role of the Neutrophil in Menstruation and Parturition. Hum. Reprod. Oxf. Engl. 1994, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Fischer, D.C.; Hlubek, M.; Van De Leur, E.; Haubeck, H.D.; Rath, W. Interleukin-1beta and Interleukin-8 Concentrations in the Lower Uterine Segment during Parturition at Term. Obstet. Gynecol. 1998, 91, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Kanayama, N.; Belayet, H.M.; Yonezawa, M.; Kobayashi, T.; Terao, T. Interleukin-8 Potentiates the Effect of Interleukin-1-Induced Uterine Contractions. Hum. Reprod. Oxf. Engl. 1999, 14, 560–565. [Google Scholar] [CrossRef] [Green Version]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. Interleukin-8 Induces Cervical Ripening in Rabbits. Am. J. Obstet. Gynecol. 1994, 171, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Idriss, H.T.; Naismith, J.H. TNF Alpha and the TNF Receptor Superfamily: Structure-Function Relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, C.; Gieling, R.G.; Kida, Y.; Garner, W.; Li, W.; Han, Y.P. Tumor Necrosis Factor-Alpha Induced Expression of Matrix Metalloproteinase-9 through P21-Activated Kinase-1. BMC Immunol. 2009, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Leppert, P.C.; Yu, S.Y. Apoptosis in the Cervix of Pregnant Rats in Association with Cervical Softening. Gynecol. Obstet. Investig. 1994, 37, 150–154. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Vink, J.; Medina, P.M.B.; Menon, R. Oxidative Stress Promotes Cellular Damages in the Cervix: Implications for Normal and Pathologic Cervical Function in Human Pregnancy†. Biol. Reprod. 2021, 105, 204–216. [Google Scholar] [CrossRef]
- Straach, K.J.; Shelton, J.M.; Richardson, J.A.; Hascall, V.C.; Mahendroo, M.S. Regulation of Hyaluronan Expression during Cervical Ripening. Glycobiology 2005, 15, 55–65. [Google Scholar] [CrossRef]
- Downing, S.J.; Sherwood, O.D. The Physiological Role of Relaxin in the Pregnant Rat. IV. The Influence of Relaxin on Cervical Collagen and Glycosaminoglycans. Endocrinology 1986, 118, 471–479. [Google Scholar] [CrossRef]
- Rath, W.; Osmers, R.; Adelmann-Grill, B.C.; Stuhlsatz, H.W.; Szvereny, M.; Kuhn, W. Biochemical Changes in Human Cervical Connective Tissue after Intracervical Application of Prostaglandin E2. Prostaglandins 1993, 45, 375–384. [Google Scholar] [CrossRef]
- Cabrol, D.; Carbonne, B.; Bienkiewicz, A.; Dallot, E.; Alj, A.E.; Cedard, L. Induction of Labor and Cervical Maturation Using Mifepristone (RU 486) in the Late Pregnant Rat. Influence of a Cyclooxygenase Inhibitor (Diclofenac). Prostaglandins 1991, 42, 71–79. [Google Scholar] [CrossRef]
- Hakansson, L.; Hallgren, R.; Venge, P. Regulation of Granulocyte Function by Hyaluronic Acid. In Vitro and in Vivo Effects on Phagocytosis, Locomotion, and Metabolism. J. Clin. Investig. 1980, 66, 298–305. [Google Scholar] [CrossRef] [Green Version]
- McKee, C.M.; Lowenstein, C.J.; Horton, M.R.; Wu, J.; Bao, C.; Chin, B.Y.; Choi, A.M.K.; Noble, P.W. Hyaluronan Fragments Induce Nitric-Oxide Synthase in Murine Macrophages through a Nuclear Factor KappaB-Dependent Mechanism. J. Biol. Chem. 1997, 272, 8013–8018. [Google Scholar] [CrossRef] [Green Version]
- Obara, M.; Hirano, H.; Ogawa, M.; Tsubaki, H.; Hosoya, N.; Yoshida, Y.; Miyauchi, S.; Tanaka, T. Changes in Molecular Weight of Hyaluronan and Hyaluronidase Activity in Uterine Cervical Mucus in Cervical Ripening. Acta Obstet. Gynecol. Scand. 2001, 80, 492–496. [Google Scholar] [PubMed]
- Sampson, P.M.; Rochester, C.L.; Freundlich, B.; Elias, J.A. Cytokine Regulation of Human Lung Fibroblast Hyaluronan (Hyaluronic Acid) Production. Evidence for Cytokine-Regulated Hyaluronan (Hyaluronic Acid) Degradation and Human Lung Fibroblast-Derived Hyaluronidase. J. Clin. Investig. 1992, 90, 1492–1503. [Google Scholar] [CrossRef] [Green Version]
- Byers, B.D.; Bytautiene, E.; Costantine, M.M.; Buhimschi, C.S.; Buhimschi, I.; Saade, G.R.; Goharkhay, N. Hyaluronidase Modifies the Biomechanical Properties of the Rat Cervix and Shortens the Duration of Labor Independent of Myometrial Contractility. Am. J. Obstet. Gynecol. 2010, 203, 596.e1–596.e5. [Google Scholar] [CrossRef]
- Kavanagh, J.; Kelly, A.J.; Thomas, J. Hyaluronidase for Cervical Ripening and Induction of Labour. Cochrane Database Syst. Rev. 2006, 2009, CD003097. [Google Scholar] [CrossRef] [PubMed]
- Spallicci, M.D.B.; Chiea, M.A.; Singer, J.M.; Albuquerque, P.B.; Bittar, R.E.; Zugaib, M. Use of Hyaluronidase for Cervical Ripening: A Randomized Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 46–50. [Google Scholar] [CrossRef]
- Goharkhay, N.; Byers, B.; Betancourt, A.; Orise, P.; Saade, G.; Bytautiene, E. 321: Cervical Ripening with Hyaluronidase: Effect on Myometrial Contractility in Vivo and in Vitro. Am. J. Obstet. Gynecol. 2009, 201, S128. [Google Scholar] [CrossRef]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayle, D.A.; Beloosesky, R.; Desai, M.; Amidi, F.; Nuñez, S.E.; Ross, M.G. Maternal LPS Induces Cytokines in the Amniotic Fluid and Corticotropin Releasing Hormone in the Fetal Rat Brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1024–R1029. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, X.; Huang, C.; Pei, Z.; Xiao, H.; Luo, X.; Huang, S.; Chang, Y. Erythropoietin Prevents LPS-Induced Preterm Birth and Increases Offspring Survival. Am. J. Reprod. Immunol. 2020, 84, e13283. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, N.; Fukamizu, H. Mechanical Stretching Increases Prostaglandin E2 in Cultured Human Amnion Cells. Gynecol. Obstet. Investig. 1989, 28, 123–126. [Google Scholar] [CrossRef] [PubMed]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Terao, T. Stretching of Fetal Membranes Increases the Concentration of Interleukin-8 and Collagenase Activity. Am. J. Obstet. Gynecol. 1996, 174, 843–849. [Google Scholar] [CrossRef]
- Jorge, S.; Chang, S.; Barzilai, J.J.; Leppert, P.; Segars, J.H. Mechanical Signaling in Reproductive Tissues: Mechanisms and Importance. Reprod. Sci. Thousand Oaks Calif 2014, 21, 1093–1107. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.J.; Macinga, D.; Rorke, E.A. Relaxin Modulates Human Cervical Stromal Cell Activity. J. Clin. Endocrinol. Metab. 1996, 81, 3379–3384. [Google Scholar] [CrossRef]
- MacLennan, A.H. Relaxin–a Review. Aust. N. Z. J. Obstet. Gynaecol. 1981, 21, 195–202. [Google Scholar] [CrossRef]
- Weiss, G. Relaxin Used to Produce the Cervical Ripening of Labor. Clin. Obstet. Gynecol. 1995, 38, 293–300. [Google Scholar] [CrossRef]
- Teichman, S.L.; Unemori, E.; Teerlink, J.R.; Cotter, G.; Metra, M. Relaxin: Review of Biology and Potential Role in Treating Heart Failure. Curr. Heart Fail. Rep. 2010, 7, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Brennand, J.E.; Calder, A.A.; Leitch, C.R.; Greer, I.A.; Chou, M.M.; MacKenzie, I.Z. Recombinant Human Relaxin as a Cervical Ripening Agent. Br. J. Obstet. Gynaecol. 1997, 104, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.J.; Kavanagh, J.; Thomas, J. Relaxin for Cervical Ripening and Induction of Labour. Cochrane Database Syst. Rev. 2001, 2001, CD003103. [Google Scholar] [CrossRef] [PubMed]
- Luque, E.H.; Muñoz De Toro, M.M.; Ramos, J.G.; Rodriguez, H.A.; Sherwood, O.D. Role of Relaxin and Estrogen in the Control of Eosinophilic Invasion and Collagen Remodeling in Rat Cervical Tissue at Term. Biol. Reprod. 1998, 59, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, S.H.; Ng, K.H.; Johgalingam, V.T.; Ragavan, M. The Effects of Oestradiol and Relaxin on Extensibility and Collagen Organisation of the Pregnant Rat Cervix. J. Endocrinol. 1995, 146, 331–337. [Google Scholar] [CrossRef]
- Szlachter, N.; O’Byrne, E.; Goldsmith, L.; Steinetz, B.G.; Weiss, G. Myometrial Inhibiting Activity of Relaxin-Containing Extracts of Human Corpora Lutea of Pregnancy. Am. J. Obstet. Gynecol. 1980, 136, 584–586. [Google Scholar] [CrossRef]
- Heng, K.; Ivell, R.; Wagaarachchi, P.; Anand-Ivell, R. Relaxin Signalling in Primary Cultures of Human Myometrial Cells. Mol. Hum. Reprod. 2008, 14, 603–611. [Google Scholar] [CrossRef]
- Downing, S.J.; Hollingsworth, M. Action of Relaxin on Uterine Contractions–a Review. J. Reprod. Fertil. 1993, 99, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Goland, R.S.; Wardlaw, S.L.; Blum, M.; Tropper, P.J.; Stark, R.I. Biologically Active Corticotropin-Releasing Hormone in Maternal and Fetal Plasma during Pregnancy. Am. J. Obstet. Gynecol. 1988, 159, 884–890. [Google Scholar] [CrossRef]
- Perkins, A.V.; Eben, F.; Wolfe, C.D.A.; Schulte, H.M.; Linton, E.A. Plasma Measurements of Corticotrophin-Releasing Hormone-Binding Protein in Normal and Abnormal Human Pregnancy. J. Endocrinol. 1993, 138, 149–157. [Google Scholar] [CrossRef]
- Majzoub, J.A.; Karalis, K.P. Placental Corticotropin-Releasing Hormone: Function and Regulation. Am. J. Obstet. Gynecol. 1999, 180, S242–S246. [Google Scholar] [CrossRef]
- Dudley, D.J. Immunoendocrinology of Preterm Labor: The Link between Corticotropin-Releasing Hormone and Inflammation. Am. J. Obstet. Gynecol. 1999, 180, S251–S256. [Google Scholar] [CrossRef]
- Klimaviciute, A.; Calciolari, J.; Bertucci, E.; Abelin-Tornblöm, S.; Stjernholm-Vladic, Y.; Byström, B.; Petraglia, F.; Ekman-Ordeberg, G. Corticotropin-Releasing Hormone, Its Binding Protein and Receptors in Human Cervical Tissue at Preterm and Term Labor in Comparison to Non-Pregnant State. Reprod. Biol. Endocrinol. RBE 2006, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Challis, J.R.G. Corticotropin-Releasing Hormone and Urocortin Induce Secretion of Matrix Metalloproteinase-9 (MMP-9) without Change in Tissue Inhibitors of MMP-1 by Cultured Cells from Human Placenta and Fetal Membranes. J. Clin. Endocrinol. Metab. 2005, 90, 6569–6574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggelidou, E.; Hillhouse, E.W.; Grammatopoulos, D.K. Up-Regulation of Nitric Oxide Synthase and Modulation of the Guanylate Cyclase Activity by Corticotropin-Releasing Hormone but Not Urocortin II or Urocortin III in Cultured Human Pregnant Myometrial Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3300–3305. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Liu, J.; Xu, C.; Liu, W.; Zhu, X.; Li, Y.; Sun, Q.; Gu, H.; Ni, X. Corticotropin-Releasing Hormone (CRH) Promotes Inflammation in Human Pregnant Myometrium: The Evidence of CRH Initiating Parturition? J. Clin. Endocrinol. Metab. 2014, 99, E199–E208. [Google Scholar] [CrossRef] [Green Version]
- Gerlo, S.; Kooijman, R.; Beck, I.M.; Kolmus, K.; Spooren, A.; Haegeman, G. Cyclic AMP: A Selective Modulator of NF-ΚB Action. Cell. Mol. Life Sci. CMLS 2011, 68, 3823–3841. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Nicholson, R.C.; King, B.; Chan, E.-C.; Fitter, J.T.; Smith, R. Glucocorticoid Stimulation of Corticotropin-Releasing Hormone Gene Expression Requires a Cyclic Adenosine 3′,5′-Monophosphate Regulatory Element in Human Primary Placental Cytotrophoblast Cells. J. Clin. Endocrinol. Metab. 2000, 85, 1937–1945. [Google Scholar] [CrossRef] [Green Version]
- Strähle, U.; Boshart, M.; Klock, G.; Stewart, F.; Schütz, G. Glucocorticoid- and Progesterone-Specific Effects Are Determined by Differential Expression of the Respective Hormone Receptors. Nature 1989, 339, 629–632. [Google Scholar] [CrossRef] [Green Version]
- McKay, L.I.; Cidlowski, J.A. Molecular Control of Immune/Inflammatory Responses: Interactions between Nuclear Factor-Kappa B and Steroid Receptor-Signaling Pathways. Endocr. Rev. 1999, 20, 435–459. [Google Scholar] [CrossRef] [Green Version]
- Stjernholm-Vladic, Y.; Stygar, D.; Mansson, C.; Masironi, B.; Akerberg, S.; Wang, H.; Ekman-Ordeberg, G.; Sahlin, L. Factors Involved in the Inflammatory Events of Cervical Ripening in Humans. Reprod. Biol. Endocrinol. RBE 2004, 2, 74. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, D.A.; Sykes, L.; Teoh, T.G.; Bennett, P.R. Prevention of Preterm Labour via the Modulation of Inflammatory Pathways. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2012, 25 (Suppl. S1), 17–20. [Google Scholar] [CrossRef]
- Zuidema, L.J.; Khan-Dawood, F.; Dawood, M.Y.; Work, B.A. Hormones and Cervical Ripening: Dehydroepiandrosterone Sulfate, Estradiol, Estriol, and Progesterone. Am. J. Obstet. Gynecol. 1986, 155, 1252–1254. [Google Scholar] [CrossRef]
- Maradny, E.; Kanayama, N.; Maehara, K.; Kobayashi, T.; Terao, T. Dehydroepiandrosterone Sulfate Potentiates the Effect of Interleukin-8 on the Cervix. Gynecol. Obstet. Investig. 1996, 42, 191–195. [Google Scholar] [CrossRef]
- Kanayama, N.; El Maradny, E.; Goto, J.; Terao, T. Effect of Dehydroepiandrosterone Sulfate on Interleukin-8 Receptor during Cervical Ripening. Eur. J. Endocrinol. 1998, 138, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Nakamura, T.; Takagaki, K.; Funahashi, M.; Saito, Y.; Endo, M. Regulation of Hyaluronate Metabolism by Progesterone in Cultured Fibroblasts from the Human Uterine Cervix. FEBS Lett. 1997, 402, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Belayet, H.M.; Kanayama, N.; Khatun, S.; Tokunaga, N.; Sugimura, M.; Yamashita, M.; Kobayashi, T.; Terao, T. Dehydroepiandrosterone Sulphate Promotes Hyaluronic Acid-Induced Cervical Ripening in Rabbits. Hum. Reprod. Oxf. Engl. 1999, 14, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challis, J.R. Endocrinology of Parturition. Mead Johnson Symp. Perinat. Dev. Med. 1980, 8–15, 50–59. [Google Scholar] [CrossRef]
- Stjernholm, Y.; Sahlin, L.; Akerberg, S.; Elinder, A.; Eriksson, H.A.; Malmstrom, A.; Ekman, G. Cervical Ripening in Humans: Potential Roles of Estrogen, Progesterone, and Insulin-like Growth Factor-I. Am. J. Obstet. Gynecol. 1996, 174, 1065–1071. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Anderson, L.L. Stimulation of Collagen Secretion by Relaxin and Effect of Oestrogen on Relaxin Binding in Uterine Cervical Cells of Pigs. J. Reprod. Fertil. 1993, 98, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Stites, D.P.; Siiteri, P.K. Steroids as Immunosuppressants in Pregnancy. Immunol. Rev. 1983, 75, 117–138. [Google Scholar] [CrossRef]
- Rajabi, M.R.; Dodge, G.R.; Solomon, S.; Poole, A.R. Immunochemical and Immunohistochemical Evidence of Estrogen-Mediated Collagenolysis as a Mechanism of Cervical Dilatation in the Guinea Pig at Parturition. Endocrinology 1991, 128, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the Estrogen Receptor through Phosphorylation by Mitogen-Activated Protein Kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Rádestad, A.; Christensen, N.J.; Strömberg, L. Induced Cervical Ripening with Mifepristone in First Trimester Abortion. A Double-Blind Randomized Biomechanical Study. Contraception 1988, 38, 301–312. [Google Scholar] [CrossRef]
- Bokström, H.; Norström, A. Effects of Mifepristone and Progesterone on Collagen Synthesis in the Human Uterine Cervix. Contraception 1995, 51, 249–254. [Google Scholar] [CrossRef]
- Hapangama, D.; Neilson, J.P. Mifepristone for Induction of Labour. Cochrane Database Syst. Rev. 2009, 2009, CD002865. [Google Scholar] [CrossRef]
- Imada, K.; Ito, A.; Sato, T.; Namiki, M.; Nagase, H.; Mori, Y. Hormonal Regulation of Matrix Metalloproteinase 9/Gelatinase B Gene Expression in Rabbit Uterine Cervical Fibroblasts. Biol. Reprod. 1997, 56, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.W.; Leask, R.; Calder, A.A. Choriodecidual Production of Interleukin-8 and Mechanism of Parturition. Lancet Lond. Engl. 1992, 339, 776–777. [Google Scholar] [CrossRef]
- Kelly, R.W. Pregnancy Maintenance and Parturition: The Role of Prostaglandin in Manipulating the Immune and Inflammatory Response. Endocr. Rev. 1994, 15, 684–706. [Google Scholar] [CrossRef]
- Stjernholm, Y.; Sahlin, L.; Malmström, A.; Barchan, K.; Eriksson, H.A.; Ekman, G. Potential Roles for Gonadal Steroids and Insulin-like Growth Factor I during Final Cervical Ripening. Obstet. Gynecol. 1997, 90, 375–380. [Google Scholar] [CrossRef]
- Walsh, S.W.; Stanczyk, F.Z.; Novy, M.J. Daily Hormonal Changes in the Maternal, Fetal, and Amniotic Fluid Compartments before Parturition in a Primate Species. J. Clin. Endocrinol. Metab. 1984, 58, 629–639. [Google Scholar] [CrossRef]
- Tulchinsky, D.; Hobel, C.J.; Yeager, E.; Marshall, J.R. Plasma Estrone, Estradiol, Estriol, Progesterone, and 17-Hydroxyprogesterone in Human Pregnancy. I. Normal Pregnancy. Am. J. Obstet. Gynecol. 1972, 112, 1095–1100. [Google Scholar] [CrossRef]
- Leonhardt, S.A.; Boonyaratanakornkit, V.; Edwards, D.P. Progesterone Receptor Transcription and Non-Transcription Signaling Mechanisms. Steroids 2003, 68, 761–770. [Google Scholar] [CrossRef]
- Merlino, A.A.; Welsh, T.N.; Tan, H.; Li, J.Y.; Cannon, V.; Mercer, B.M.; Mesiano, S. Nuclear Progesterone Receptors in the Human Pregnancy Myometrium: Evidence That Parturition Involves Functional Progesterone Withdrawal Mediated by Increased Expression of Progesterone Receptor-A. J. Clin. Endocrinol. Metab. 2007, 92, 1927–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangrande, P.H.; Kimbrel, E.A.; Edwards, D.P.; McDonnell, D.P. The Opposing Transcriptional Activities of the Two Isoforms of the Human Progesterone Receptor Are Due to Differential Cofactor Binding. Mol. Cell. Biol. 2000, 20, 3102–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegeto, E.; Shahbaz, M.M.; Wen, D.X.; Goldman, M.E.; O’Malley, B.W.; McDonnell, D.P. Human Progesterone Receptor A Form Is a Cell- and Promoter-Specific Repressor of Human Progesterone Receptor B Function. Mol. Endocrinol. Baltim. Md 1993, 7, 1244–1255. [Google Scholar] [CrossRef]
- Mesiano, S.; Wang, Y.; Norwitz, E.R. Progesterone Receptors in the Human Pregnancy Uterus: Do They Hold the Key to Birth Timing? Reprod. Sci. Thousand Oaks Calif 2011, 18, 6–19. [Google Scholar] [CrossRef]
- Mesiano, S.; Chan, E.C.; Fitter, J.T.; Kwek, K.; Yeo, G.; Smith, R. Progesterone Withdrawal and Estrogen Activation in Human Parturition Are Coordinated by Progesterone Receptor A Expression in the Myometrium. J. Clin. Endocrinol. Metab. 2002, 87, 2924–2930. [Google Scholar] [CrossRef]
- Allport, V.C.; Pieber, D.; Slater, D.M.; Newton, R.; White, J.O.; Bennett, P.R. Human Labour Is Associated with Nuclear Factor-KappaB Activity Which Mediates Cyclo-Oxygenase-2 Expression and Is Involved with the “Functional Progesterone Withdrawal”. Mol. Hum. Reprod. 2001, 7, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Kalkhoven, E.; Wissink, S.; Van Der Saag, P.T.; Van Der Burg, B. Negative Interaction between the RelA(P65) Subunit of NF-KappaB and the Progesterone Receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef] [Green Version]
- Bakker, R.; Pierce, S.; Myers, D. The Role of Prostaglandins E1 and E2, Dinoprostone, and Misoprostol in Cervical Ripening and the Induction of Labor: A Mechanistic Approach. Arch. Gynecol. Obstet. 2017, 296, 167–179. [Google Scholar] [CrossRef]
- Tripathy, S.; Nallasamy, S.; Mahendroo, M. Progesterone and Its Receptor Signaling in Cervical Remodeling: Mechanisms of Physiological Actions and Therapeutic Implications. J. Steroid Biochem. Mol. Biol. 2022, 223, 106137. [Google Scholar] [CrossRef] [PubMed]
- Shyken, J.M.; Petrie, R.H. The Use of Oxytocin. Clin. Perinatol. 1995, 22, 907–931. [Google Scholar] [CrossRef]
- Plant, T.M.; Zeleznik, A.J.; Albertini, D.F.; Goodman, R.L.; Herbison, A.E.; McCarthy, M.M.; Muglia, L.J.; Richards, J.A.S. Knobil and Neill’s Physiology of Reproduction: Two-Volume Set. Knobil Neills Physiol. Reprod. Two-Vol. Set 2014, 1–2, 1987–1995. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Husslein, P.; Fuchs, F. Oxytocin and the Initiation of Human Parturition. II. Stimulation of Prostaglandin Production in Human Decidua by Oxytocin. Am. J. Obstet. Gynecol. 1981, 141, 694–697. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S. Oxytocin Receptors in the Human Uterus during Pregnancy and Parturition. Am. J. Obstet. Gynecol. 1984, 150, 734–741. [Google Scholar] [CrossRef]
- Knowles, R.G.; Moncada, S. Nitric Oxide Synthases in Mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef]
- Murad, F. Discovery of Some of the Biological Effects of Nitric Oxide and Its Role in Cell Signaling. Biosci. Rep. 2004, 24, 452–474. [Google Scholar] [CrossRef]
- Tschugguel, W.; Schneeberger, C.; Lass, H.; Stonek, F.; Zaghlula, M.B.; Czerwenka, K.; Schatten, C.; Kaider, A.; Husslein, P.; Huber, J.C. Human Cervical Ripening Is Associated with an Increase in Cervical Inducible Nitric Oxide Synthase Expression. Biol. Reprod. 1999, 60, 1367–1372. [Google Scholar] [CrossRef] [Green Version]
- Laskin, D.L.; Pendino, K.J. Macrophages and Inflammatory Mediators in Tissue Injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef]
- Buhimschi, I.; Ali, M.; Jain, V.; Chwalisz, K.; Garfield, R.E. Differential Regulation of Nitric Oxide in the Rat Uterus and Cervix during Pregnancy and Labour. Hum. Reprod. Oxf. Engl. 1996, 11, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Nakanishi, T.; Kimura, Y.; Hattori, T.; Sasaki, K.; Norimatsu, H.; Takahashi, K.; Takigawa, M. Nitric Oxide Mediates Interleukin-1-Induced Matrix Degradation and Basic Fibroblast Growth Factor Release in Cultured Rabbit Articular Chondrocytes: A Possible Mechanism of Pathological Neovascularization in Arthritis. Endocrinology 1996, 137, 3729–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Väisänen-Tommiska, M.R.H. Nitric Oxide in the Human Uterine Cervix: Endogenous Ripening Factor. Ann. Med. 2008, 40, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Masferrer, J.L. Interactions of Nitric Oxide with Cyclooxygenase: In Vitro, Ex Vivo, and in Vivo Studies. Methods Enzymol. 1996, 269, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Ekerhovd, E.; Weijdegård, B.; Brännström, M.; Mattsby-Baltzer, I.; Norström, A. Nitric Oxide Induced Cervical Ripening in the Human: Involvement of Cyclic Guanosine Monophosphate, Prostaglandin F(2 Alpha), and Prostaglandin E(2). Am. J. Obstet. Gynecol. 2002, 186, 745–750. [Google Scholar] [CrossRef]
- Ledingham, M.A.; Denison, F.C.; Kelly, R.W.; Young, A.; Norman, J.E. Nitric Oxide Donors Stimulate Prostaglandin F(2alpha) and Inhibit Thromboxane B(2) Production in the Human Cervix during the First Trimester of Pregnancy. Mol. Hum. Reprod. 1999, 5, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Väisänen-Tommiska, M.; Mikkola, T.S.; Ylikorkala, O. Misoprostol Induces Cervical Nitric Oxide Release in Pregnant, but Not in Nonpregnant, Women. Am. J. Obstet. Gynecol. 2005, 193, 790–796. [Google Scholar] [CrossRef]
- Corriveau, C.C.; Madara, P.J.; Van Dervort, A.L.; Tropea, M.M.; Wesley, R.A.; Danner, R.L. Effects of Nitric Oxide on Chemotaxis and Endotoxin-Induced Interleukin-8 Production in Human Neutrophils. J. Infect. Dis. 1998, 177, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.K.M.; Zamora, R.; Petrosko, P.; Billiar, T.R. The Regulatory Role of Nitric Oxide in Apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Ekerhovd, E.; Brännström, M.; Weijdegård, B.; Norström, A. Nitric Oxide Synthases in the Human Cervix at Term Pregnancy and Effects of Nitric Oxide on Cervical Smooth Muscle Contractility. Am. J. Obstet. Gynecol. 2000, 183, 610–616. [Google Scholar] [CrossRef]
- Groeneveld, P.H.P.; Kwappenberg, K.M.C.; Langermans, J.A.M.; Nibbering, P.H.; Curtis, L. Relation between Pro- and Anti-Inflammatory Cytokines and the Production of Nitric Oxide (NO) in Severe Sepsis. Cytokine 1997, 9, 138–142. [Google Scholar] [CrossRef]
- Stadler, J.; Stefanovic-Racic, M.; Billiar, T.R.; Curran, R.D.; McIntyre, L.A.; Georgescu, H.I.; Simmons, R.L.; Evans, C.H. Articular Chondrocytes Synthesize Nitric Oxide in Response to Cytokines and Lipopolysaccharide. J. Immunol. Baltim. Md 1950 1991, 147, 3915–3920. [Google Scholar]
- Garfield, R.E.; Saade, G.; Buhimschi, C.; Buhimschi, I.; Shi, L.; Shi, S.Q.; Chwalisz, K. Control and Assessment of the Uterus and Cervix during Pregnancy and Labour. Hum. Reprod. Update 1998, 4, 673–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chwalisz, K.; Garfield, R.E. Role of Nitric Oxide in the Uterus and Cervix: Implications for the Management of Labor. J. Perinat. Med. 1998, 26, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Shao-Qing, S.; Garfield, R.E.; Beier, H.M. Cervical Ripening in Guinea-Pigs after a Local Application of Nitric Oxide. Hum. Reprod. Oxf. Engl. 1997, 12, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sladek, S.M.; Regenstein, A.C.; Lykins, D.; Roberts, J.M. Nitric Oxide Synthase Activity in Pregnant Rabbit Uterus Decreases on the Last Day of Pregnancy. Am. J. Obstet. Gynecol. 1993, 169, 1285–1291. [Google Scholar] [CrossRef]
- Kublickiene, K.R.; Cockell, A.P.; Nisell, H.; Poston, L. Role of Nitric Oxide in the Regulation of Vascular Tone in Pressurized and Perfused Resistance Myometrial Arteries from Term Pregnant Women. Am. J. Obstet. Gynecol. 1997, 177, 1263–1269. [Google Scholar] [CrossRef]
- Learmont, J.G.; Poston, L. Nitric Oxide Is Involved in Flow-Induced Dilation of Isolated Human Small Fetoplacental Arteries. Am. J. Obstet. Gynecol. 1996, 174, 583–588. [Google Scholar] [CrossRef]
- Ghosh, A.; Lattey, K.R.; Kelly, A.J. Nitric Oxide Donors for Cervical Ripening and Induction of Labour. Cochrane Database Syst. Rev. 2016, 12, CD006901. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Giampietro, F. Inhibition of Nitric Oxide Synthesis Causes Preterm Delivery in the Mouse. Hum. Reprod. Oxf. Engl. 2000, 15, 1838–1842. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, A.E.; Mackenzie, F.; Greer, I.A.; Norman, J.E. Vaginal Application of the Nitric Oxide Donor Isosorbide Mononitrate for Preinduction Cervical Ripening: A Randomized Controlled Trial to Determine Effects on Maternal and Fetal Hemodynamics. Am. J. Obstet. Gynecol. 2001, 184, 958–964. [Google Scholar] [CrossRef]
- Bullarbo, M.; Orrskog, M.E.; Andersch, B.; Granström, L.; Norström, A.; Ekerhovd, E. Outpatient Vaginal Administration of the Nitric Oxide Donor Isosorbide Mononitrate for Cervical Ripening and Labor Induction Postterm: A Randomized Controlled Study. Am. J. Obstet. Gynecol. 2007, 196, 50.e1–50.e5. [Google Scholar] [CrossRef] [PubMed]
- Chatsis, V.; Frey, N. Misoprostol for Cervical Ripening and Induction of Labour: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538944/ (accessed on 21 September 2022).
- Papanikolaou, E.G.; Plachouras, N.; Drougia, A.; Andronikou, S.; Vlachou, C.; Stefos, T.; Paraskevaidis, E.; Zikopoulos, K. Comparison of Misoprostol and Dinoprostone for Elective Induction of Labour in Nulliparous Women at Full Term: A Randomized Prospective Study. Reprod. Biol. Endocrinol. RBE 2004, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Denguezli, W.; Trimech, A.; Haddad, A.; Hajjaji, A.; Saidani, Z.; Faleh, R.; Sakouhi, M. Efficacy and Safety of Six Hourly Vaginal Misoprostol versus Intracervical Dinoprostone: A Randomized Controlled Trial. Arch. Gynecol. Obstet. 2007, 276, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z.; Aflaifel, N.; Weeks, A. Oral Misoprostol for Induction of Labour. Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Granstrom, E. Prostaglandin Biochemistry, Pharmacy and Physiological Function. The Prostaglandins, Thromboxanes and Leukotrienes. Acta Obstet. Gynecol. Scand. Suppl. 1983, 113, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Terzidou, V. Preterm Labour. Biochemical and Endocrinological Preparation for Parturition. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 729–756. [Google Scholar] [CrossRef]
- Garavito, R.M.; Dewitt, D.L. The Cyclooxygenase Isoforms: Structural Insights into the Conversion of Arachidonic Acid to Prostaglandins. Biochim. Biophys. Acta 1999, 1441, 278–287. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in Biology and Disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E Receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [Green Version]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Astle, S.; Thornton, S.; Slater, D.M. Identification and Localization of Prostaglandin E2 Receptors in Upper and Lower Segment Human Myometrium during Pregnancy. Mol. Hum. Reprod. 2005, 11, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Chien, E.K.; MacGregor, C. Expression and Regulation of the Rat Prostaglandin E2 Receptor Type 4 (EP4) in Pregnant Cervical Tissue. Am. J. Obstet. Gynecol. 2003, 189, 1501–1510. [Google Scholar] [CrossRef]
- El Maradny, E.; Kanayama, N.; Halim, A.; Maehara, K.; Sumimoto, K.; Terao, T. Biochemical Changes in the Cervical Tissue of Rabbit Induced by Interleukin-8, Interleukin-1beta, Dehydroepiandrosterone Sulphate and Prostaglandin E2: A Comparative Study. Hum. Reprod. Oxf. Engl. 1996, 11, 1099–1104. [Google Scholar] [CrossRef]
- Carbonne, B.; Jannet, D.; Dallot, E.; Pannier, E.; Ferré, F.; Cabrol, D. Synthesis of Glycosaminoglycans by Human Cervical Fibroblasts in Culture: Effects of Prostaglandin E 2 and Cyclic AMP. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 70, 101–105. [Google Scholar] [CrossRef]
- Lindsey, J.D.; Kashiwagi, K.; Boyle, D.; Kashiwagi, F.; Firestein, G.S.; Weinreb, R.N. Prostaglandins Increase ProMMP-1 and ProMMP-3 Secretion by Human Ciliary Smooth Muscle Cells. Curr. Eye Res. 1996, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Denison, F.C.; Calder, A.A.; Kelly, R.W. The Action of Prostaglandin E2 on the Human Cervix: Stimulation of Interleukin 8 and Inhibition of Secretory Leukocyte Protease Inhibitor. Am. J. Obstet. Gynecol. 1999, 180, 614–620. [Google Scholar] [CrossRef]
- Winkler, M.; Kemp, B.; Hauptmann, S.; Rath, W. Parturition: Steroids, Prostaglandin E2, and Expression of Adhesion Molecules by Endothelial Cells. Obstet. Gynecol. 1997, 89, 398–402. [Google Scholar] [CrossRef]
- Erkinheimo, T.-L.; Saukkonen, K.; Narko, K.; Jalkanen, J.; Ylikorkala, O.; Ristimäki, A. Expression of Cyclooxygenase-2 and Prostanoid Receptors by Human Myometrium. J. Clin. Endocrinol. Metab. 2000, 85, 3468–3475. [Google Scholar] [CrossRef]
- Madsen, G.; Zakar, T.; Ku, C.Y.; Sanborn, B.M.; Smith, R.; Mesiano, S. Prostaglandins Differentially Modulate Progesterone Receptor-A and -B Expression in Human Myometrial Cells: Evidence for Prostaglandin-Induced Functional Progesterone Withdrawal. J. Clin. Endocrinol. Metab. 2004, 89, 1010–1013. [Google Scholar] [CrossRef] [Green Version]
- Pierce, S.; Bakker, R.; Myers, D.A.; Edwards, R.K. Clinical Insights for Cervical Ripening and Labor Induction Using Prostaglandins. AJP Rep. 2018, 8, e307–e314. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, M.W.; Flis, W.; Pietrus, M.; Wartęga, M.; Stankiewicz, M. Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery. Cells 2022, 11, 3690. https://doi.org/10.3390/cells11223690
Socha MW, Flis W, Pietrus M, Wartęga M, Stankiewicz M. Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery. Cells. 2022; 11(22):3690. https://doi.org/10.3390/cells11223690
Chicago/Turabian StyleSocha, Maciej W., Wojciech Flis, Miłosz Pietrus, Mateusz Wartęga, and Martyna Stankiewicz. 2022. "Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery" Cells 11, no. 22: 3690. https://doi.org/10.3390/cells11223690
APA StyleSocha, M. W., Flis, W., Pietrus, M., Wartęga, M., & Stankiewicz, M. (2022). Signaling Pathways Regulating Human Cervical Ripening in Preterm and Term Delivery. Cells, 11(22), 3690. https://doi.org/10.3390/cells11223690