New Insights into Mechanisms of Ferroptosis Associated with Immune Infiltration in Neonatal Hypoxic-Ischemic Brain Damage
Abstract
:1. Introduction
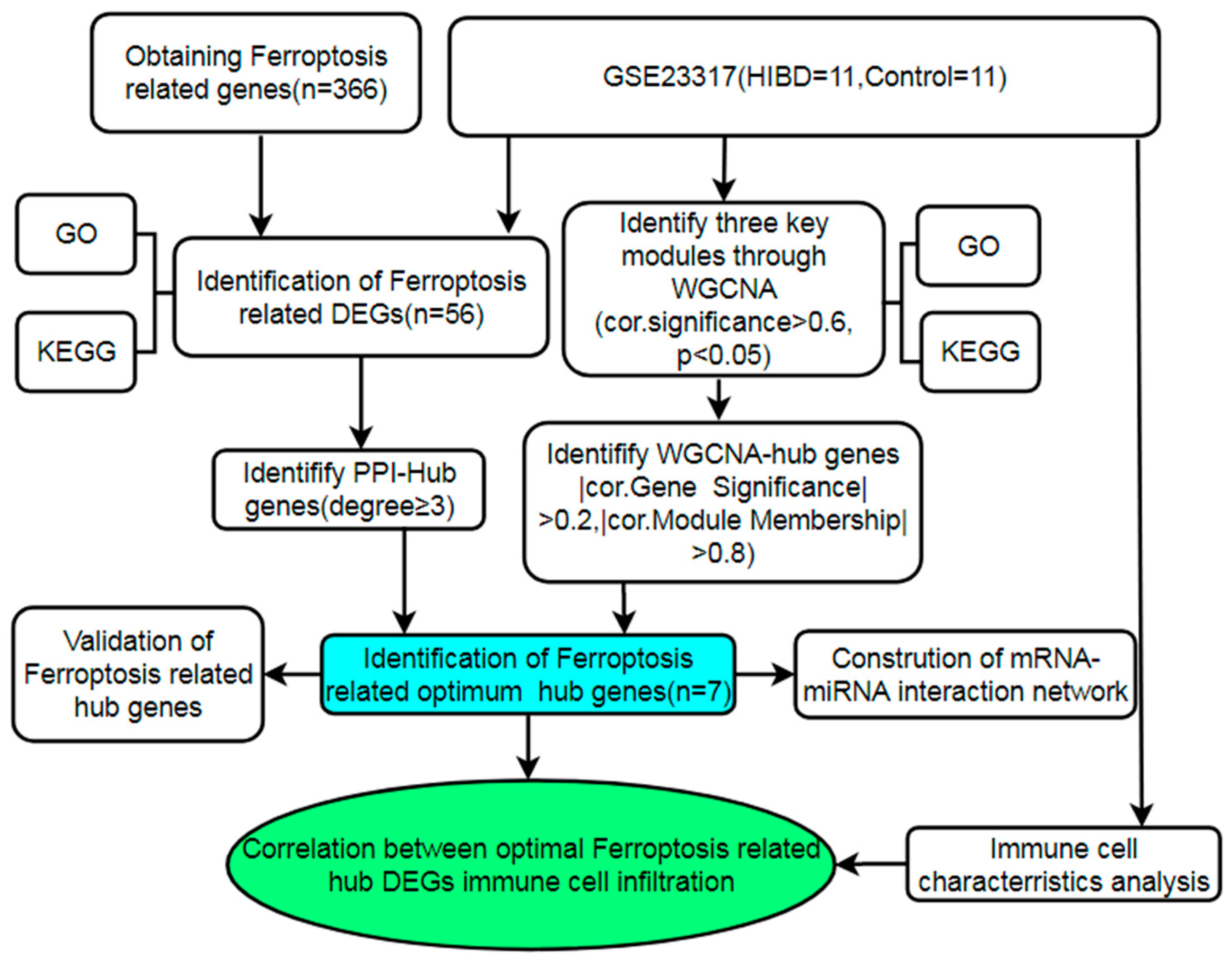
2. Materials and Methods
2.1. Data Acquisition and Processing
2.2. Variation Analysis of mRNA and miRNA
2.3. Ferroptosis-Related Gene Acquisition
2.4. Functional Enrichment and Signaling Pathway Analysis
2.5. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.6. Construction of Protein-Protein Interaction (PPI) Network
2.7. Identification and Validation of the Optimal Hub FRDEGs
2.8. Construction of Ferroptosis-Related mRNA-miRNA Regulatory Network
2.9. Immune Cell Infiltration Analysis
3. Result
3.1. Determination of Ferroptosis-Related DEGs
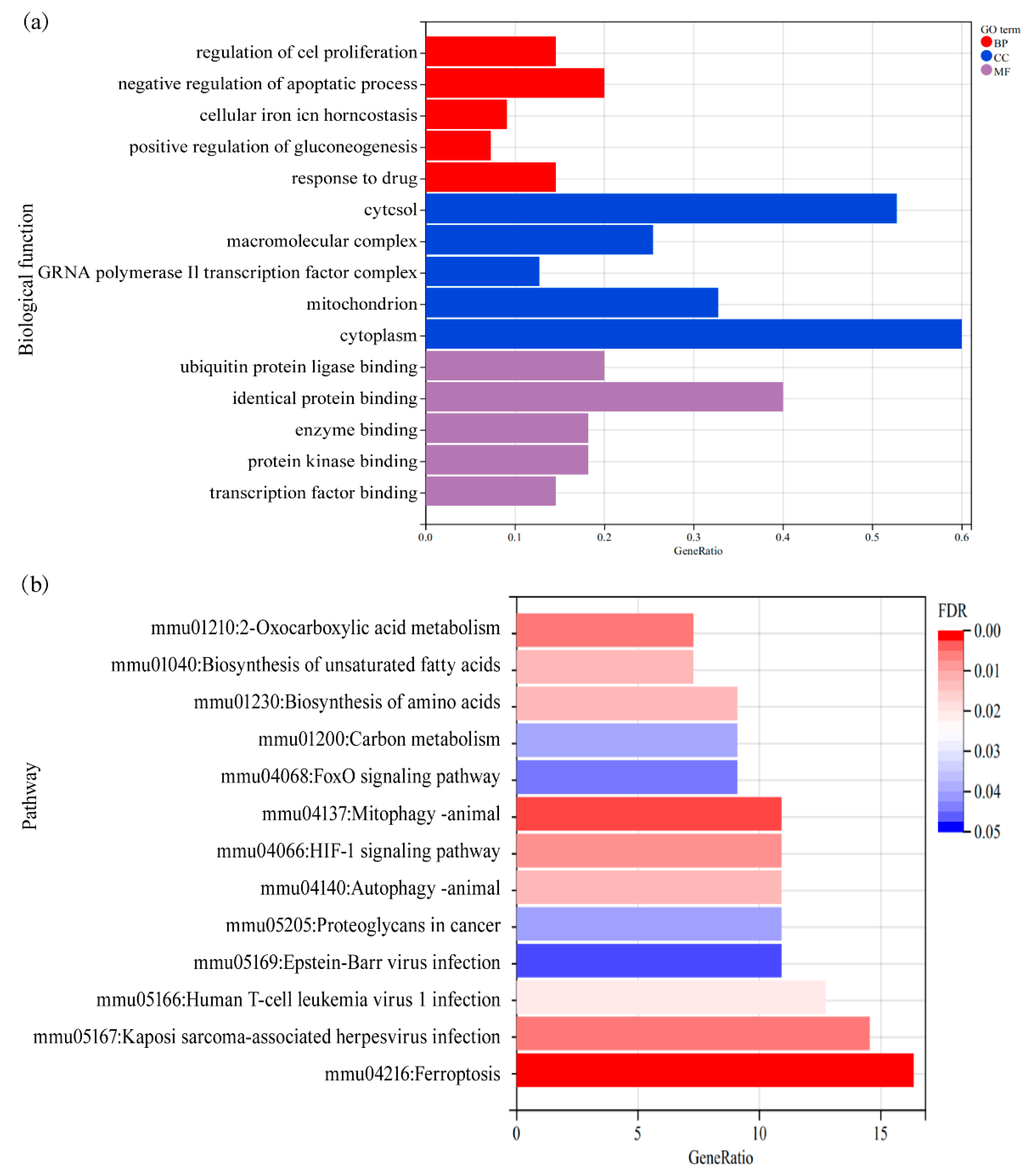
3.2. Enrichment Analysis of Ferroptosis-Related DEGs
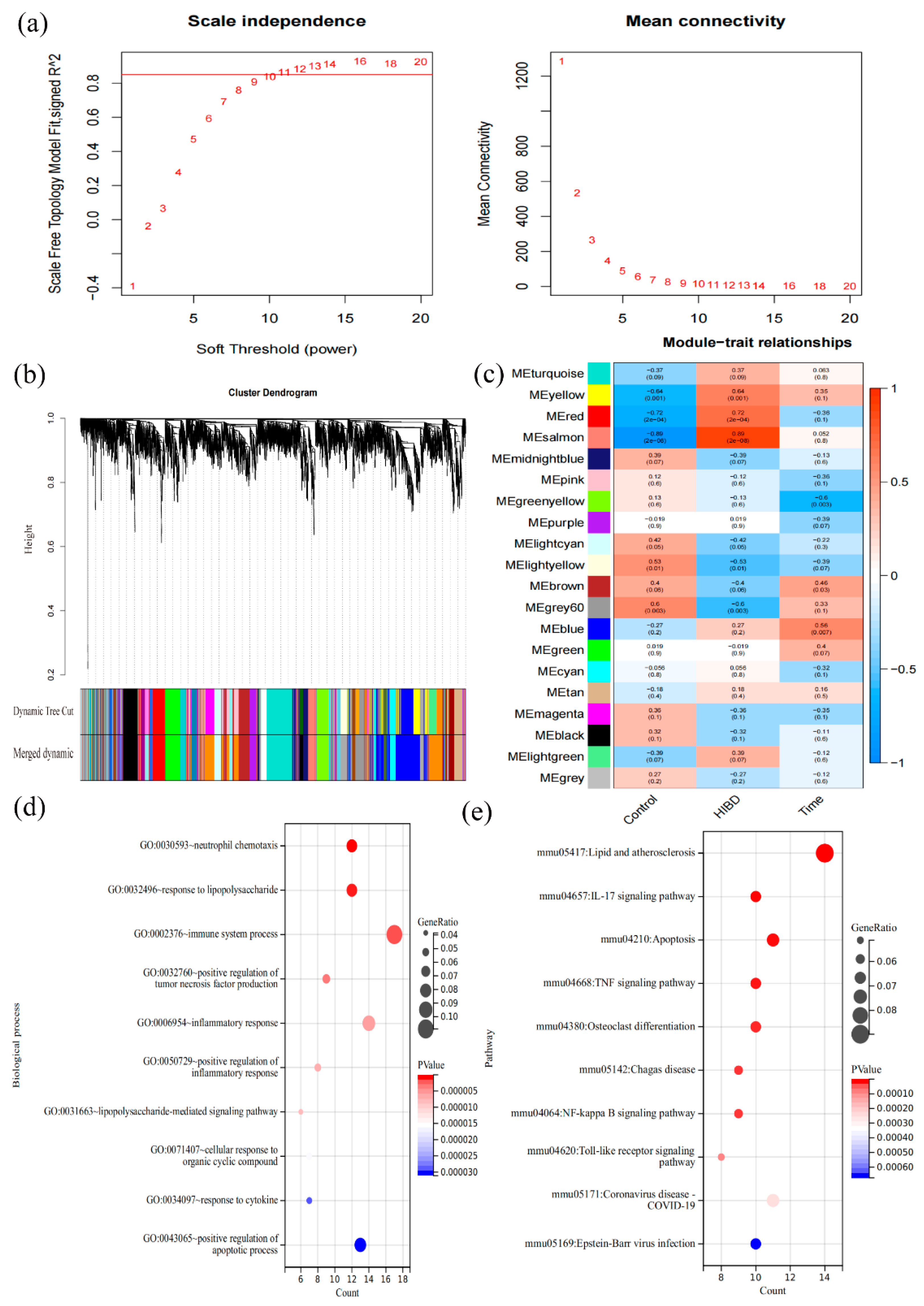
3.3. Construction of Co-Expression Modules and Determination of Key Modules
3.4. Determination and Validation of the Optimal Hub FRDEGs
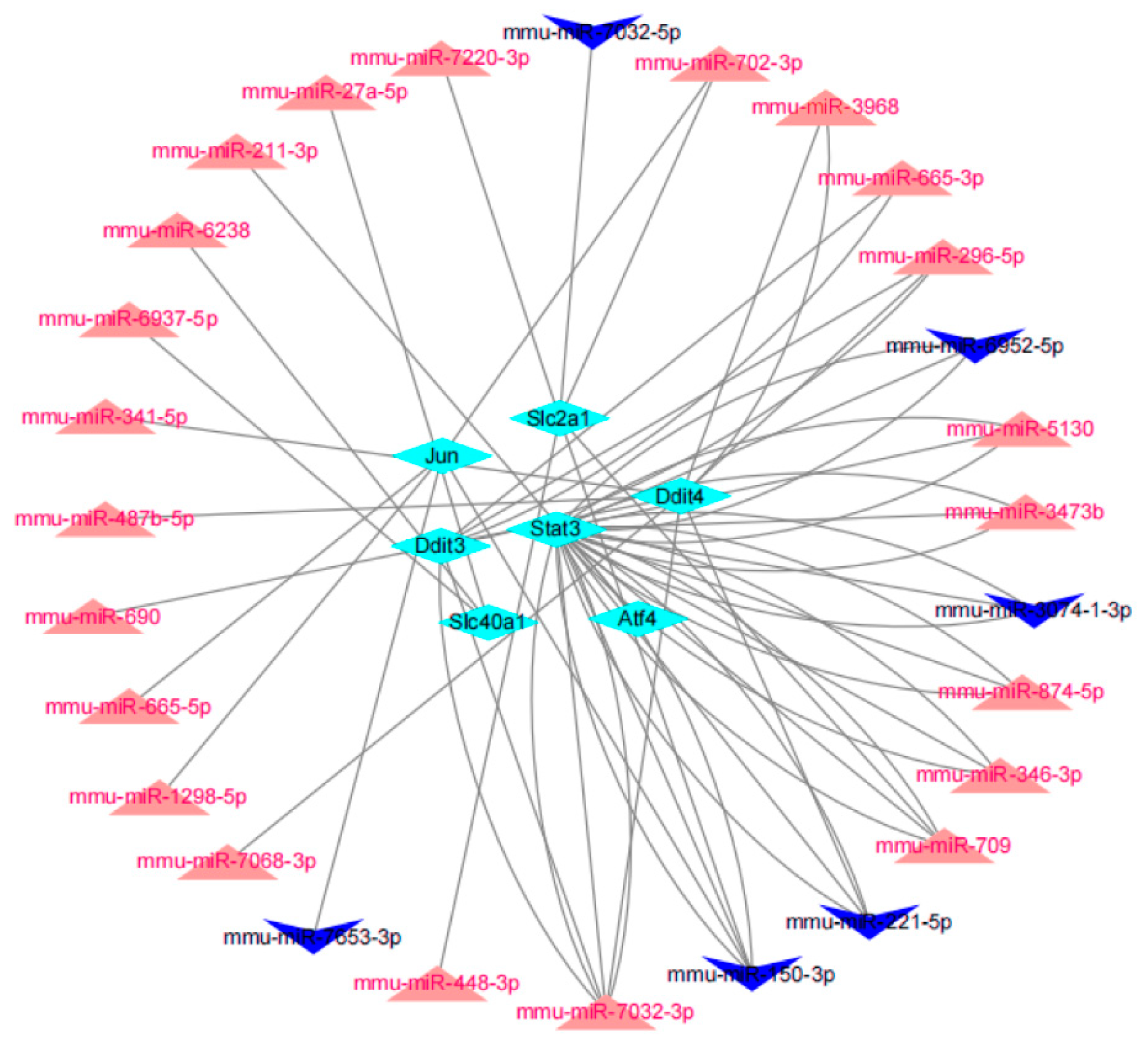
3.5. miRNA Prediction
3.6. Immune Infiltration Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wachtel, E.V.; Hendricks-Muñoz, K.D. Current Management of the Infant Who Presents with Neonatal Encephalopathy. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Glass, H.C. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalopathy. Clin. Exp. Pediatr. 2021, 64, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Gonzalez, F.F. Erythropoietin: A novel therapy for hypoxic-ischaemic encephalopathy? Dev. Med. Child Neurol. 2015, 57 (Suppl. 3), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Packer, C.H.; Hersh, A.R.; Sargent, J.A.; Caughey, A.B. Therapeutic hypothermia in severe hypoxic-ischemic encephalopathy: A cost-effectiveness analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Leaw, B.; Mallard, C.; Nair, S.; Jinnai, M.; Hagberg, H. Cell Death in the Developing Brain after Hypoxia-Ischemia. Front. Cell. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Dasgupta, C.; Shen, G.; Li, Y.; Zhang, L. MicroRNA-210 downregulates TET2 and contributes to inflammatory response in neonatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2021, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Hu, D.W.; Zhang, G.; Lin, L.; Yu, X.J.; Wang, F.; Lin, Q. Dynamic Changes in Brain Iron Metabolism in Neonatal Rats after Hypoxia-Ischemia. J. Stroke Cerebrovasc. Dis. 2022, 31, 106352. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, T.; Zheng, J.; Zhou, Y.; Lin, Z.; Fu, X. Ferroptosis is Involved in Hypoxic-ischemic Brain Damage in Neonatal Rats. Neuroscience 2022, 487, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhu, X.; Sun, S.; Yang, W.; Liu, S.; Tang, Z.; Zhang, R.; Li, J.; Shen, T.; Hei, M. Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp. Neurol. 2021, 345, 113828. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Su, X.; Hu, X.; Zhou, Y.; Huang, L.; Fan, Y.; Li, J.; Lu, L. Melatonin improves hypoxic-ischemic brain damage through the Akt/Nrf2/Gpx4 signaling pathway. Brain Res. Bull. 2020, 163, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Bendix, I.; Felderhoff-Müser, U. Peripheral immune cells and perinatal brain injury: A double-edged sword? Pediatr. Res. 2022, 91, 392–403. [Google Scholar] [CrossRef]
- Min, Y.-J.; Ling, E.-A.; Li, F. Immunomodulatory Mechanism and Potential Therapies for Perinatal Hypoxic-Ischemic Brain Damage. Front. Pharmacol. 2020, 11, 580428. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, Y.-Q. Ferroptosis: A Critical Moderator in the Life Cycle of Immune Cells. Front. Immunol. 2022, 13, 877634. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.; Yip, P.K. The role of microRNAs in newborn brain development and hypoxic ischaemic encephalopathy. Neuropharmacology 2019, 149, 55–65. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Hu, H.; Duan, Q.; Chen, J.; Li, L. MiR-363-3p attenuates neonatal hypoxic-ischemia encephalopathy by targeting DUSP5. Neurosci. Res. 2021, 171, 103–113. [Google Scholar] [CrossRef]
- Wang, W.; Jia, L. Regulatory Mechanism of MicroRNA-30b on Neonatal Hypoxic-Ischemic Encephalopathy (HIE). J. Stroke Cerebrovasc. Dis. 2021, 30, 105553. [Google Scholar] [CrossRef]
- Zhang, G.; Ye, M.; Li, M. Deregulated miR-384 serves as a biomarker in neonatal hypoxic-ischemic encephalopathy and alleviates microglia-mediated neuroinflammation. Mol. Biol. Rep. 2020, 47, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, J.; Zhang, L.; Hou, Y.; Pu, S.; Chu, A.; Bai, M.; Zhang, Z. Weighted Gene Co-Expression Network Analysis Identifies Critical Genes in the Development of Heart Failure After Acute Myocardial Infarction. Front Genet. 2019, 10, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupré, N.; Derambure, C.; Le Dieu-Lugon, B.; Hauchecorne, M.; Detroussel, Y.; Gonzalez, B.J.; Marret, S.; Leroux, P. Hypoxia-Ischemia Induced Age-Dependent Gene Transcription Effects at Two Development Stages in the Neonate Mouse Brain. Front. Mol. Neurosci. 2020, 13, 587815. [Google Scholar] [CrossRef]
- Pașca, A.M.; Park, J.-Y.; Shin, H.-W.; Qi, Q.; Revah, O.; Krasnoff, R.; O’Hara, R.; Willsey, A.J.; Palmer, T.; Pașca, S.P. Human 3D cellular model of hypoxic brain injury of prematurity. Nat. Med. 2019, 25, 784–791. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Zhou, C.; Wang, H.; Yu, L.; Xu, Y.; Xu, L.; Wang, Q. Transcriptome-Wide Gene Expression in a Murine Model of Ventilator-Induced Lung Injury. Dis. Markers 2021, 2021, 5535890. [Google Scholar] [CrossRef] [PubMed]
- Shouman, B.O.; Mesbah, A.; Aly, H. Iron metabolism and lipid peroxidation products in infants with hypoxic ischemic encephalopathy. J. Perinatol. 2008, 28, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Song, J.; Wang, Y.; Wang, X.; Culmsee, C.; Zhu, C. The Potential Role of Ferroptosis in Neonatal Brain Injury. Front. Neurosci. 2019, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bana, S.M.; Maher, S.E.; Gaber, A.F.; Aly, S.S. Serum and Urinary Malondialdehyde (MDA), Uric acid, and Protein as markers of perinatal asphyxia. Electron. Physician 2016, 8, 2614–2619. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Li, X.; Yang, X.; Yan, J.; Shi, P.; Ba, L.; Cao, Y.; Wang, P. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 2019, 235, 116795. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef] [Green Version]
- Walsh, B.H.; Broadhurst, D.I.; Mandal, R.; Wishart, D.S.; Boylan, G.B.; Kenny, L.C.; Murray, D.M. The Metabolomic Profile of Umbilical Cord Blood in Neonatal Hypoxic Ischaemic Encephalopathy. PLoS ONE 2012, 7, e50520. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.-K. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Chen, L.; Gao, X.; Shen, S.; Sheng, W.; Min, J.; Wang, F. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci. Bull. 2021, 66, 1806–1816. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Liu, Y.; Wu, Q.; Zhang, X.; Bian, Z.; Zhang, Y.; Pan, Q.; Sun, F. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell. Signal. 2019, 63, 109384. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [PubMed] [Green Version]
- Wu, X.; Luo, J.; Liu, H.; Cui, W.; Guo, W.; Zhao, L.; Guo, H.; Bai, H.; Guo, K.; Feng, D.; et al. Recombinant adiponectin peptide promotes neuronal survival after intracerebral haemorrhage by suppressing mitochondrial and ATF4-CHOP apoptosis pathways in diabetic mice via Smad3 signalling inhibition. Cell Prolif. 2020, 53, e12759. [Google Scholar] [CrossRef]
- Gao, H.; Bai, Y.; Jia, Y.; Zhao, Y.; Kang, R.; Tang, D.; Dai, E. Ferroptosis is a lysosomal cell death process. Biochem. Biophys. Res. Commun. 2018, 503, 1550–1556. [Google Scholar] [CrossRef]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T.; et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Mi, J.; Song, L.; Guo, Y.; Li, Y.; Yin, Y.; Zhang, C. SLC40A1 Mediates Ferroptosis and Cognitive Dysfunction in Type 1 Diabetes. Neuroscience 2021, 463, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ye, G.; Liu, Y.; Huang, D.; Luo, Q.; Chen, W.; Qi, Z. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci. Lett. 2022, 779, 136635. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wang, Y.-P.; Zhang, X.; Li, G.-X.; Zheng, K.; Duan, C.-Z. lncRNA Mtss1 promotes inflammatory responses and secondary brain injury after intracerebral hemorrhage by targeting miR-709 in mice. Brain Res. Bull. 2020, 162, 20–29. [Google Scholar] [CrossRef]
- O’Hare, F.M.; Watson, R.W.; O’Neill, A.; Blanco, A.; Donoghue, V.; Molloy, E.J. Persistent systemic monocyte and neutrophil ac-tivation in neonatal encephalopathy. J. Matern. Fetal Neonatal Med. 2016, 29, 309–316. [Google Scholar] [CrossRef]
- Melo, A.M.; Taher, N.A.; Doherty, D.G.; Molloy, E.J. The role of lymphocytes in neonatal encephalopathy. Brain Behav. Immun.-Health 2021, 18, 100380. [Google Scholar] [CrossRef]
- Herz, J.; Köster, C.; Crasmöller, M.; Abberger, H.; Hansen, W.; Felderhoff-Müser, U.; Bendix, I. Peripheral T Cell Depletion by FTY720 Exacerbates Hypoxic-Ischemic Brain Injury in Neonatal Mice. Front. Immunol. 2018, 9, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-C.; Lin, S.-J. Neonatal Natural Killer Cell Function: Relevance to Antiviral Immune Defense. Clin. Dev. Immunol. 2013, 2013, 427696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathali, N.; Ostrowski, R.P.; Hasegawa, Y.; Lekic, T.; Tang, J.; Zhang, J.H. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl. Stroke Res. 2013, 4, 208–219. [Google Scholar] [CrossRef]
- Ceran, B.; Dizdar, E.A.; Beser, E.; Karaçağlar, N.B.; Sari, F.N. Diagnostic role of systemic inflammatory indices in infants with moderate to severe hypoxic ischemic encephalopathy. Am. J. Perinatol. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]



| Dataset | Platform | Species | No. of Samples (Control/HIBD) | Type | Tissue | Experimental Model |
|---|---|---|---|---|---|---|
| GSE23317 | GPL6885 | Mus musculus | P8: 3 h: 4/3 8 h: 3/4 24 h: 4/4 | mRNA | Cerebral cortex | Unilateral carotid artery ligation+hypoxia (8% oxygen/92% N2, 1 h) |
| GSE144456 | GPL10333 | Mus musculus | P5, P10: 3 h: 3/3 6 h: 3/3 12 h: 3/3 24 h: 3/3 | mRNA | Forebrain | Unilateral carotid artery ligation+hypoxia (8% oxygen, 40 min) |
| GSE112137 | GPL20301 | Homo sapiens (extreme prematurity) | 24 h: 8/8 48 h: 8/8 | mRNA | Cortical progenitors | At day 74–78 of in vitro differentiation, hCS are exposed for 24 h and 48 h at <1% O2 in a gas-controlled culture chamber. Control hCS are maintained at 21% O2 throughout. |
| GSE184939 | GPL21626 | Mus musculus | P9: 4/4 | microRNA | Cerebral cortex | Unilateral carotid artery ligation+hypoxia (8% oxygen, 30 min) |
| Type | DEGs |
|---|---|
| Driver | Ireb2, Cs, Vdac2, Scp2, Tfrc, Go1t, Ncoa4, Phkg2, G6pdx, Becn1, Map1lc3a, Wipi2, Sat1, Hif1a, Taz, Sirt1, Fbxw7, Dnajb6, Bach1, Elovl5, Usp7, Aat4, Apat3, Pex2, Lyrm1, Slc25a28,Mdm2 |
| Suppressor | Slc7a11, Gpx4, Hspb1, Slc40a1, Atf4, Fads2, Stat3, Cdkn1a, Vdac2, Cbs, Hif1a, Jun, Lamp2, Zfp36, Cav1, Fzd7, Acot1, Cp, Idh2, Rela |
| Marker | Dusp1, Slc7a11, Ddit4, Ddit3, Txnip, Gpt2, Cbs, Atf4, Klhl24, Rela, Agpat3, Tfrc Mafg, Slc40a1, Gpx4, Hspb1, Slc2a1, Slc2a3, Slc2a6, Ireb2, Nnmt, Capg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wan, L.; Sun, J.; Yan, W.; Wang, J.; Gao, X.; Ren, C.; Hao, L. New Insights into Mechanisms of Ferroptosis Associated with Immune Infiltration in Neonatal Hypoxic-Ischemic Brain Damage. Cells 2022, 11, 3778. https://doi.org/10.3390/cells11233778
Li S, Wan L, Sun J, Yan W, Wang J, Gao X, Ren C, Hao L. New Insights into Mechanisms of Ferroptosis Associated with Immune Infiltration in Neonatal Hypoxic-Ischemic Brain Damage. Cells. 2022; 11(23):3778. https://doi.org/10.3390/cells11233778
Chicago/Turabian StyleLi, Shangbin, Li Wan, Jingfei Sun, Weichen Yan, Jie Wang, Xiong Gao, Changjun Ren, and Ling Hao. 2022. "New Insights into Mechanisms of Ferroptosis Associated with Immune Infiltration in Neonatal Hypoxic-Ischemic Brain Damage" Cells 11, no. 23: 3778. https://doi.org/10.3390/cells11233778





