The BRCAness Landscape of Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Genome Data across 33 Cancer Types
2.2. Pathogenic Variant Data in BRCAness Genes
2.3. CNV Data in BRCAness Genes
2.4. DNA Methylation Data in BRCAness Genes
2.5. Gene Expression and Functional Enrichment Analysis
2.6. Clinical Relevance of BRCAness Genes
2.7. Identifying Candidate Cancer Types for PARPi Therapy
3. Results
3.1. Overview of the Study
3.2. Pathogenic Variation in BRCAness Genes in Different Cancer Types
3.3. Homozygotic Deletion Patterns between BRCA1/2 and BRCAness Genes in Different Cancer Types
3.4. Methylation and Expression Patterns between BRCA1/2 and BRCAness Genes in Different Cancer Types
3.5. Prognostics of BRCAness Gene Expression across Different Cancer Types
3.6. BRCAness Cancer Types Sharing High Similarity with BRCA and OV Cancer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donovan, P.J.; Livingston, D.M. BRCA1 and BRCA2: Breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis 2010, 31, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Heyer, W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Tutt, A.; Bertwistle, D.; Valentine, J.; Gabriel, A.; Swift, S.; Ross, G.; Griffin, C.; Thacker, J.; Ashworth, A. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 2001, 20, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Taghian, D.G.; DeFrank, J.S.; Zeng, Z.C.; Willers, H.; Iliakis, G.; Powell, S.N. Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc. Natl. Acad. Sci. USA 2001, 98, 8644–8649. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Pierce, A.J.; Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 2001, 7, 263–272. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Rahman, N. The emerging landscape of breast cancer susceptibility. Nat. Genet. 2008, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- John, E.M.; Miron, A.; Gong, G.; Phipps, A.I.; Felberg, A.; Li, F.P.; West, D.W.; Whittemore, A.S. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA 2007, 298, 2869–2876. [Google Scholar] [CrossRef]
- Malone, K.E.; Daling, J.R.; Doody, D.R.; Hsu, L.; Bernstein, L.; Coates, R.J.; Marchbanks, P.A.; Simon, M.S.; McDonald, J.A.; Norman, S.A.; et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006, 66, 8297–8308. [Google Scholar] [CrossRef] [PubMed]
- Schlacher, K.; Christ, N.; Siaud, N.; Egashira, A.; Wu, H.; Jasin, M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 2011, 145, 529–542. [Google Scholar] [CrossRef]
- Turner, N.; Tutt, A.; Ashworth, A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat. Rev. Cancer 2004, 4, 814–819. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef]
- Imyanitov, E.N. Ovarian cancer genome. Methods Mol. Biol. 2013, 1049, 3–7. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Alexandrov, L.B.; Wedge, D.C.; Van Loo, P.; Greenman, C.D.; Raine, K.; Jones, D.; Hinton, J.; Marshall, J.; Stebbings, L.A.; et al. Mutational processes molding the genomes of 21 breast cancers. Cell 2012, 149, 979–993. [Google Scholar] [CrossRef]
- Polak, P.; Kim, J.; Braunstein, L.Z.; Karlic, R.; Haradhavala, N.J.; Tiao, G.; Rosebrock, D.; Livitz, D.; Kubler, K.; Mouw, K.W.; et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat. Genet. 2017, 49, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Lee, E.; McKean-Cowdin, R.; Ma, H.; Spicer, D.V.; Van Den Berg, D.; Bernstein, L.; Ursin, G. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: Results from a population-based study of young women. J. Clin. Oncol. 2011, 29, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Brunet, J.S.; Stefansson, I.M.; Straume, O.; Chappuis, P.O.; Begin, L.R.; Hamel, N.; Goffin, J.R.; Wong, N.; Trudel, M.; et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004, 64, 830–835. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Stefansson, I.M.; Chappuis, P.O.; Begin, L.R.; Goffin, J.R.; Wong, N.; Trudel, M.; Akslen, L.A. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl. Cancer Inst. 2003, 95, 1482–1485. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, W.; Cheng, C.T.; Ren, X.; Somlo, G.; Fong, M.Y.; Chin, A.R.; Li, H.; Yu, Y.; Xu, Y.; et al. TGFbeta induces “BRCAness” and sensitivity to PARP inhibition in breast cancer by regulating DNA-repair genes. Mol. Cancer Res. 2014, 12, 1597–1609. [Google Scholar] [CrossRef]
- Bodily, W.R.; Shirts, B.H.; Walsh, T.; Gulsuner, S.; King, M.C.; Parker, A.; Roosan, M.; Piccolo, S.R. Effects of germline and somatic events in candidate BRCA-like genes on breast-tumor signatures. PLoS ONE 2020, 15, e0239197. [Google Scholar] [CrossRef]
- Wedge, D.C.; Gundem, G.; Mitchell, T.; Woodcock, D.J.; Martincorena, I.; Ghori, M.; Zamora, J.; Butler, A.; Whitaker, H.; Kote-Jarai, Z.; et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat. Genet. 2018, 50, 682–692. [Google Scholar] [CrossRef]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e123. [Google Scholar] [CrossRef]
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Moffitt, R.A.; Yeh, J.J.; Stewart, C.; Robertson, A.G.; Cherniack, A.D.; Gupta, M.; Gad Getz, G.; et al. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.M.; Bailey, P.; Hubschmann, D.; Berger, A.K.; Neoptolemos, J.P.; Jager, D.; Siveke, J.; Springfeld, C. Poly(ADP-ribose) polymerase inhibition in pancreatic cancer. Genes Chromosom. Cancer 2021, 60, 373–384. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Campbell, D.; Patnaik, A.; Shapiro, J.D.; Sautois, B.; Vogelzang, N.J.; Voog, E.G.; Bryce, A.H.; McDermott, R.; Ricci, F.; et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin. Cancer Res. 2020, 26, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Byrum, A.K.; Vindigni, A.; Mosammaparast, N. Defining and Modulating ‘BRCAness’. Trends Cell Biol. 2019, 29, 740–751. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Mermel, C.H.; Schumacher, S.E.; Hill, B.; Meyerson, M.L.; Beroukhim, R.; Getz, G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011, 12, R41. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Chen, X. False discovery rate control for multiple testing based on discrete p-values. Biom. J. 2020, 62, 1060–1079. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, M. Synthetic lethality strategies: Beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020, 111, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254.e236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Demeulemeester, J.; Wedge, D.C.; Vollan, H.K.M.; Pitt, J.J.; Russnes, H.G.; Pandey, B.P.; Nilsen, G.; Nord, S.; Bignell, G.R.; et al. Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors. Nat. Commun. 2017, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Saghafinia, S.; Mina, M.; Riggi, N.; Hanahan, D.; Ciriello, G. Pan-Cancer Landscape of Aberrant DNA Methylation across Human Tumors. Cell Rep. 2018, 25, 1066–1080.e1068. [Google Scholar] [CrossRef]
- Hjortkjaer, M.; Malik Aagaard Jorgensen, M.; Waldstrom, M.; Ornskov, D.; Sogaard-Andersen, E.; Jakobsen, A.; Dahl-Steffensen, K. The clinical importance of BRCAness in a population-based cohort of Danish epithelial ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 166–173. [Google Scholar] [CrossRef]

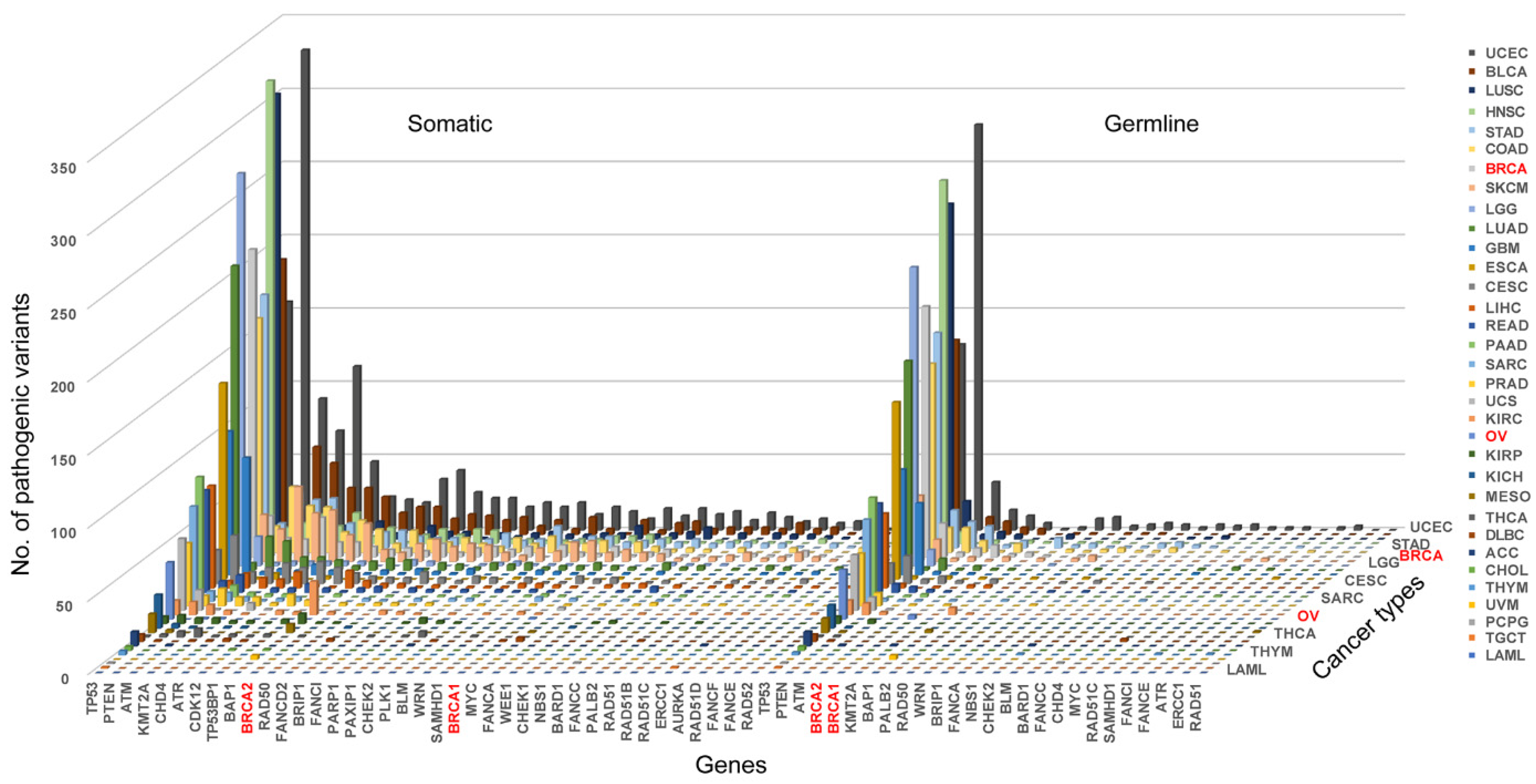
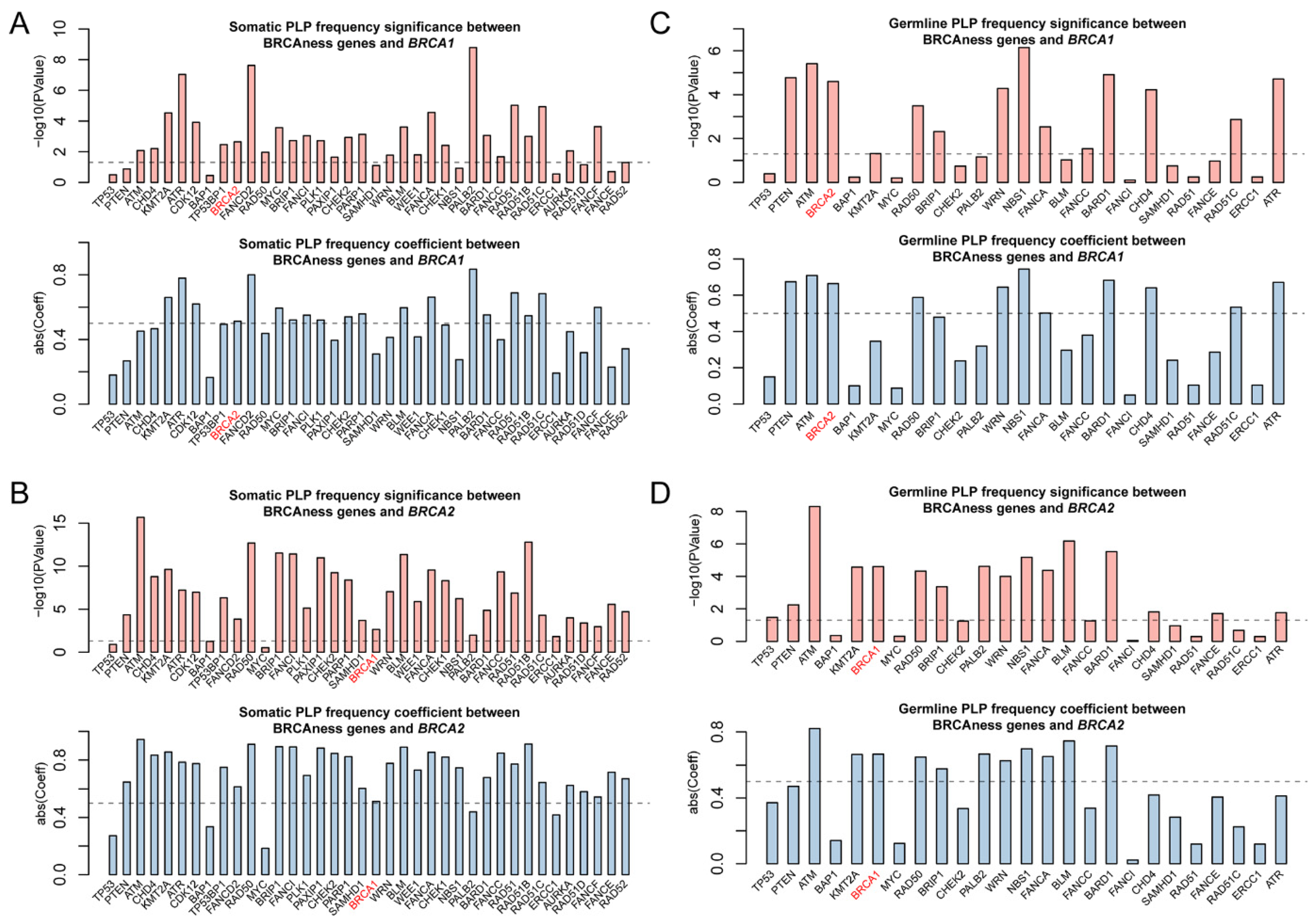

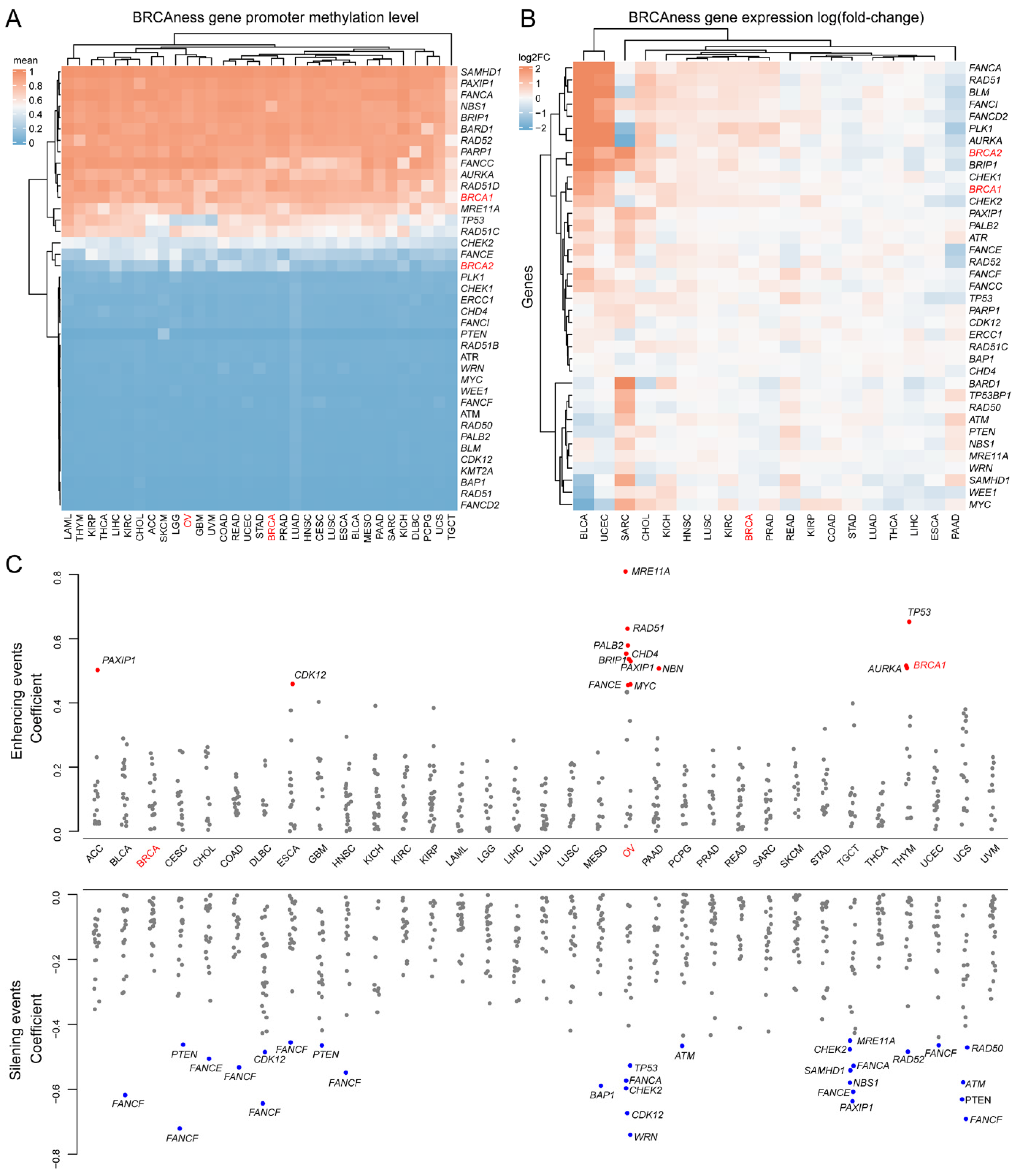

| Cancer | Rank by BRCA Cancer | Rank by OV Cancer | Sum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatic | Germline | Homozygotic | Methylation | Expression | Prognosis | Somatic | Germline | Homozygotic | Methylation | Expression | Prognosis | ||
| UCEC | 5.9 | 4.7 | 0.7 | 1.0 | 7.0 | 3.0 | 11.0 | 17.2 | 0.5 | 1.0 | 7.0 | 3.0 | 62.1 |
| BLCA | 3.8 | 2.0 | 1.3 | 1.0 | 10.5 | 1.0 | 4.8 | 14.6 | 1.0 | 1.0 | 10.5 | 1.0 | 52.6 |
| PAAD | 1.5 | 1.9 | 0.2 | 1.0 | 5.5 | 9.0 | 4.5 | 6.1 | 0.1 | 1.0 | 5.5 | 9.0 | 45.3 |
| LGG | 1.5 | 1.9 | 0.7 | 1.0 | - | 12.0 | 4.6 | 5.7 | 0.5 | 1.1 | - | 12.0 | 41.0 |
| SARC | 1.1 | 1.2 | 1.8 | 1.0 | 8.5 | 4.5 | 2.9 | 4.0 | 1.4 | 1.0 | 8.5 | 4.5 | 40.5 |
| LUAD | 1.8 | 1.5 | 1.0 | 1.0 | 0.0 | 7.0 | 3.5 | 6.9 | 0.8 | 1.0 | 0.0 | 7.0 | 31.6 |
| KICH | 1.2 | 1.2 | 0.1 | 1.0 | 3.0 | 6.5 | 2.9 | 4.2 | 0.1 | 1.0 | 3.0 | 6.5 | 30.5 |
| UCS | 3.2 | 4.0 | 0.7 | 0.9 | - | 0.0 | 9.6 | 10.1 | 0.5 | 0.9 | - | 0.0 | 30.0 |
| ACC | 0.4 | 0.7 | 0.8 | 1.0 | - | 10.0 | 1.6 | 1.4 | 0.6 | 1.0 | - | 10.0 | 27.4 |
| COAD | 2.9 | 2.2 | 0.7 | 1.0 | 0.0 | 1.0 | 5.3 | 11.0 | 0.6 | 1.0 | 0.0 | 1.0 | 26.8 |
| LIHC | 1.1 | 0.8 | 0.7 | 1.0 | 0.5 | 7.0 | 1.8 | 4.4 | 0.6 | 1.0 | 0.5 | 7.0 | 26.4 |
| ESCA | 2.0 | 3.3 | 1.0 | 1.0 | 1.0 | 0.0 | 7.7 | 7.7 | 0.8 | 1.0 | 1.0 | 0.0 | 26.4 |
| HNSC | 2.5 | 2.4 | 0.7 | 1.0 | 0.5 | 1.0 | 5.8 | 9.5 | 0.5 | 1.0 | 0.5 | 1.0 | 26.4 |
| READ | 2.3 | 2.4 | 0.5 | 1.0 | 2.0 | 0.0 | 5.8 | 8.5 | 0.4 | 1.0 | 2.0 | 0.0 | 25.9 |
| STAD | 2.8 | 2.6 | 1.0 | 1.0 | 0.0 | 0.0 | 6.0 | 10.5 | 0.8 | 1.0 | 0.0 | 0.0 | 25.6 |
| SKCM | 2.5 | 0.8 | 1.1 | 1.0 | - | 3.5 | 1.9 | 9.3 | 0.9 | 1.0 | - | 3.5 | 25.5 |
| LUSC | 2.7 | 2.6 | 1.1 | 1.0 | 0.0 | 0.0 | 6.3 | 9.9 | 0.8 | 1.0 | 0.0 | 0.0 | 25.3 |
| MESO | 0.7 | 0.8 | 1.2 | 1.0 | - | 7.0 | 1.9 | 2.4 | 0.9 | 1.0 | - | 7.0 | 23.9 |
| KIRC | 0.5 | 0.3 | 1.0 | 1.0 | 0.5 | 7.0 | 0.8 | 1.8 | 0.8 | 1.0 | 0.5 | 7.0 | 22.3 |
| KIRP | 0.4 | 0.1 | 0.5 | 1.0 | 0.0 | 7.5 | 0.3 | 1.5 | 0.4 | 1.1 | 0.0 | 7.5 | 20.3 |
| PRAD | 0.5 | 0.5 | 3.1 | 1.0 | 1.0 | 3.0 | 1.1 | 2.0 | 2.4 | 1.0 | 1.0 | 3.0 | 19.6 |
| BRCA&OV | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 12.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Wang, S.M. The BRCAness Landscape of Cancer. Cells 2022, 11, 3877. https://doi.org/10.3390/cells11233877
Guo M, Wang SM. The BRCAness Landscape of Cancer. Cells. 2022; 11(23):3877. https://doi.org/10.3390/cells11233877
Chicago/Turabian StyleGuo, Maoni, and San Ming Wang. 2022. "The BRCAness Landscape of Cancer" Cells 11, no. 23: 3877. https://doi.org/10.3390/cells11233877
APA StyleGuo, M., & Wang, S. M. (2022). The BRCAness Landscape of Cancer. Cells, 11(23), 3877. https://doi.org/10.3390/cells11233877






