Modeling of Red Blood Cells in Capillary Flow Using Fluid–Structure Interaction and Gas Diffusion
Abstract
1. Introduction
2. Immersed Finite Element Method Review
2.1. Formulations of IFEM
2.2. Computational Algorithm of IFEM
- (1)
- Given the structure configuration xs,n, and the fluid velocity vn at time step n,
- (2)
- Evaluate the nodal interaction forces fFSI,s,n for solid material points, using Equation (19),
- (3)
- Distribute the material nodal force onto the fluid mesh, from fFSI,s,n to fFSI,n using the delta function as in Equation (20),
- (4)
- Solve for the fluid velocities vn+1 and the pressure pn+1 implicitly using Equations (21) and (22),
- (5)
- Interpolate the velocities in the fluid domain onto the material points, i.e., from vn+1 to vs,n+1 as in Equation (23), and
- (6)
- Update the positions of the structure using us,n+1 = vs,n+1∆t and go back to step 1.
3. The Model of RBC and CO Diffusion
3.1. Discrete RBC Model
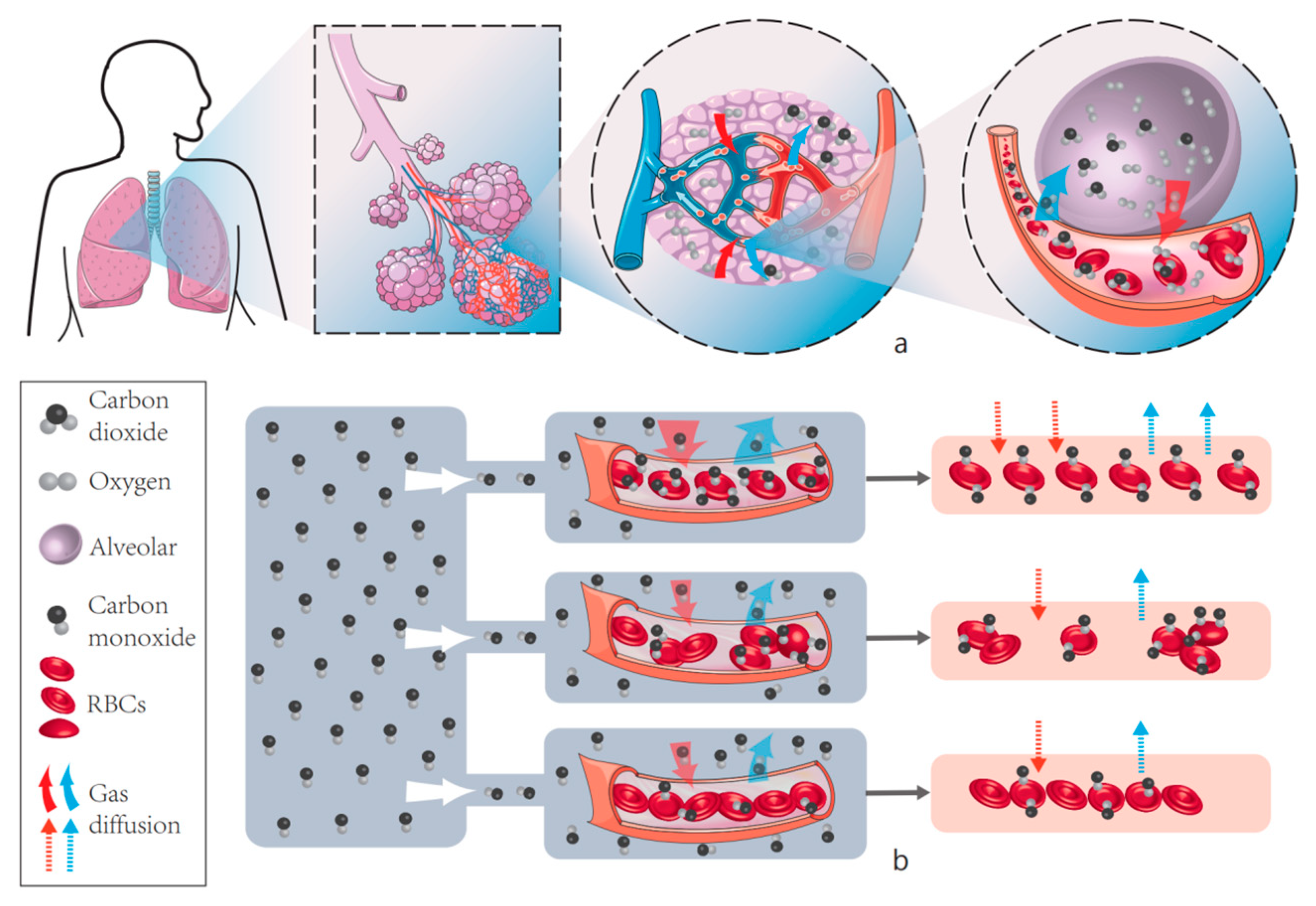
3.2. CO Diffusion and Absorption in Capillary Networks
4. Effect of RBC Deformation on CO Absorption Rate
5. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Betticher, D.C.; Reinhart, W.H.; Geiser, J. Effect of RBC shape and deformability on pulmonary O2 diffusing capacity and resistance to flow in rabbit lungs. J. Appl. Physiol. 1995, 78, 778–783. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Motion, Flow, Stress, and Growth; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Presson, R.G.; Graham, J.A.; Hanger, C.C.; Bodbex, P.S.; Gobb, S.A.; Sidner, R.A.; Glenny, R.W.; Wagner, W., Jr. Distribution of pulmonary capillary redblood cell transit times. J. Appl. Physiol. 1995, 79, 382–388. [Google Scholar] [CrossRef]
- Whiteley, J.P.; Gavaghan, D.J.; Hahn, C.E. Mathematical modelling of pulmonary gas transport. J. Math. Biol. 2003, 47, 79–99. [Google Scholar] [CrossRef]
- Frank, A.O.; Chuong, C.J.C.; Johnson, R.L. A finite-element model of oxygen diffusion in the pulmonary capillaries. J. Appl. Physiol. 1997, 82, 2036–2044. [Google Scholar] [CrossRef]
- Borland, C.D.R.; Hughes, J.M.B. Lung Diffusing Capacities (DL) for Nitric Oxide (NO) and Carbon Monoxide (CO): The Evolving Story. Compr. Physiol. 2019, 10, 73–97. [Google Scholar]
- Zhang, L.; Gerstenberger, A.; Wang, X.; Liu, W.K. Immersed finite element method. Comput. Methods Appl. Mech. Eng. 2004, 193, 2051–2067. [Google Scholar] [CrossRef]
- Liu, Y.; Chung, J.-H.; Liu, A.W.K.; Ruoff, R.S. Dielectrophoretic Assembly of Nanowires. J. Phys. Chem. B 2006, 110, 14098–14106. [Google Scholar]
- Liu, W.K.; Liu, Y.; Farrell, D.; Zhang, L.; Wang, X.S.; Fukui, Y.; Patankar, N.; Zhang, Y.; Bajaj, C.; Lee, J.; et al. Immersed finite element method and its applications to biological systems. Comput. Methods Appl. Mech. Eng. 2006, 195, 1722–1749. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.K. Rheology of red blood cell aggregation by computer simulation. J. Comput. Phys. 2006, 220, 139–154. [Google Scholar]
- Liu, Y.; Zhang, L.; Wang, X.; Liu, W.K. Coupling of Navier-Stokes equations with protein molecular dynamics and its application to hemodynamics. Int. J. Numer. Methods Fluids 2004, 46, 1237–1252. [Google Scholar] [CrossRef]
- Lee, T.-R.; Chang, Y.-S.; Choi, J.-B.; Kim, D.W.; Liu, W.K.; Kim, Y.-J. Immersed finite element method for rigid body motions in the incompressible Navier–Stokes flow. Comput. Methods Appl. Mech. Eng. 2008, 197, 2305–2316. [Google Scholar]
- Tan, J.; Thomas, A.; Liu, Y. Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter 2011, 8, 1934–1946. [Google Scholar] [PubMed]
- Saad, Y.; Schultz, M.H. GMRES: A Generalized Minimal Residual Algorithm for Solving Nonsymmetric Linear Systems. SIAM J. Sci. Stat. Comput. 1986, 7, 856–869. [Google Scholar] [CrossRef]
- Zhang, L.; Wagner, G.J.; Liu, W.K. A parallized meshfree method with boundary enrichment for large-scale CFD. J. Comput. Phys. 2002, 176, 483–506. [Google Scholar] [CrossRef]
- Evans, E.; Fung, Y.-C. Improved measurements of the erythrocyte geometry. Microvasc. Res. 1972, 4, 335–347. [Google Scholar]
- Wang, X.; Liu, W.K. Extended immersed boundary method using FEM and RKPM. Comput. Methods Appl. Mech. Eng. 2004, 193, 1305–1321. [Google Scholar]
- Eggleton, C.D.; Popel, A. Large deformation of red blood cell ghosts in a simple shear flow. Phys. Fluids 1998, 10, 1834–1845. [Google Scholar] [CrossRef]
- Pozrikidis, C. Numerical Simulation of the Flow-Induced Deformation of Red Blood Cells. Ann. Biomed. Eng. 2003, 31, 1194–1205. [Google Scholar]
- Hsia, C.C.; Johnson, R.L., Jr.; Shah, D. Red cell distribution and the recruitment of pulmonary diffusing capacity. J. Appl. Physiol. 1999, 86, 1460–1467. [Google Scholar] [CrossRef]
- Bates, D.V.; Pearce, J.F. The pulmonary diffusing capacity; a comparison of methods of measurement and a study of the effect of body position. J. Physiol. 1956, 132, 232–238. [Google Scholar] [CrossRef]
- Krogh, M. The diffusion of gases through the lungs of man. J. Physiol. 1915, 49, 271–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cho, Y.; Cheng, X.; Yang, S.; Liu, Y.; Liu, Y. Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells. Micromachines 2019, 10, 698. [Google Scholar] [CrossRef]
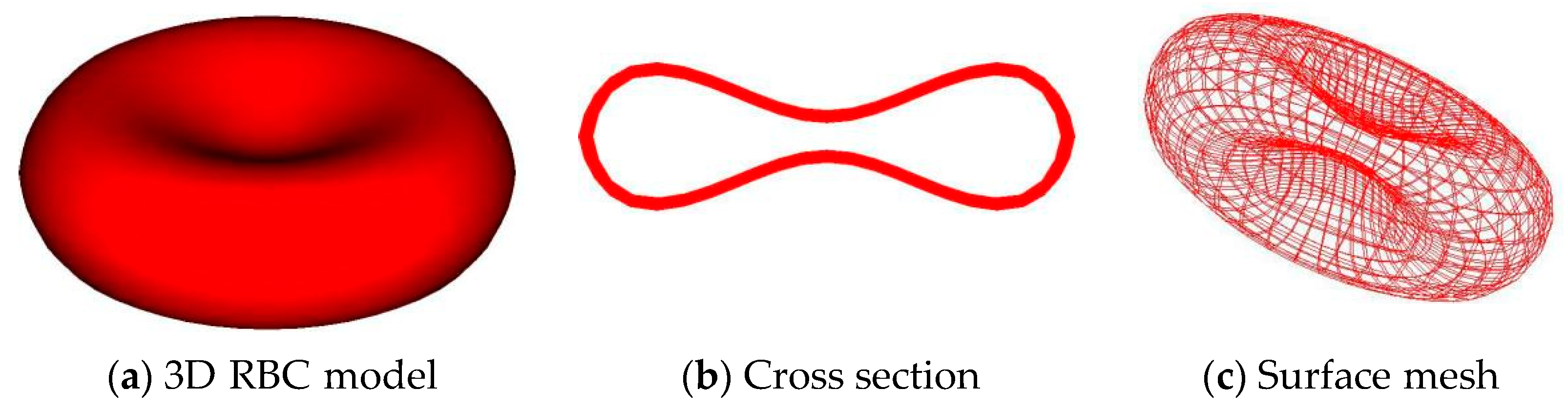








| Fluid Domain (Ω) | Solid Domain (Ωs) | |
|---|---|---|
| spatial coordinate | x | xs |
| displacement | - | us = xs − Xs |
| velocity | v | vs = dus/dt = ús |
| acceleration | - | as = d2us/dt2 = üs |
| Capillary diameter | 10.0 µm |
| RBC diameter | 7.5 µm |
| Alveolar P | 1.0 Torr (1 Torr = 1 mmHg) |
| D in air | 2.413 × 107 µm2/s |
| D in Tissue and plasma | 2.453 × 103 µm2/s |
| α | 2.363 × 10−5 Torr−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.; Ji, F.; Yin, Y.; Liu, Y.; Zhou, C. Modeling of Red Blood Cells in Capillary Flow Using Fluid–Structure Interaction and Gas Diffusion. Cells 2022, 11, 3987. https://doi.org/10.3390/cells11243987
An L, Ji F, Yin Y, Liu Y, Zhou C. Modeling of Red Blood Cells in Capillary Flow Using Fluid–Structure Interaction and Gas Diffusion. Cells. 2022; 11(24):3987. https://doi.org/10.3390/cells11243987
Chicago/Turabian StyleAn, Ling, Fenglong Ji, Yueming Yin, Yi Liu, and Chichun Zhou. 2022. "Modeling of Red Blood Cells in Capillary Flow Using Fluid–Structure Interaction and Gas Diffusion" Cells 11, no. 24: 3987. https://doi.org/10.3390/cells11243987
APA StyleAn, L., Ji, F., Yin, Y., Liu, Y., & Zhou, C. (2022). Modeling of Red Blood Cells in Capillary Flow Using Fluid–Structure Interaction and Gas Diffusion. Cells, 11(24), 3987. https://doi.org/10.3390/cells11243987





