Expression of Steroid Receptor RNA Activator 1 (SRA1) in the Adipose Tissue Is Associated with TLRs and IRFs in Diabesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Anthropometry
2.2. Collection of Subcutaneous Adipose Tissue Samples
2.3. Measurement of Metabolic Markers
2.4. Quantitative, Real-Time, Reverse-Transcription Polymerase Chain Reaction (RT-qPCR)
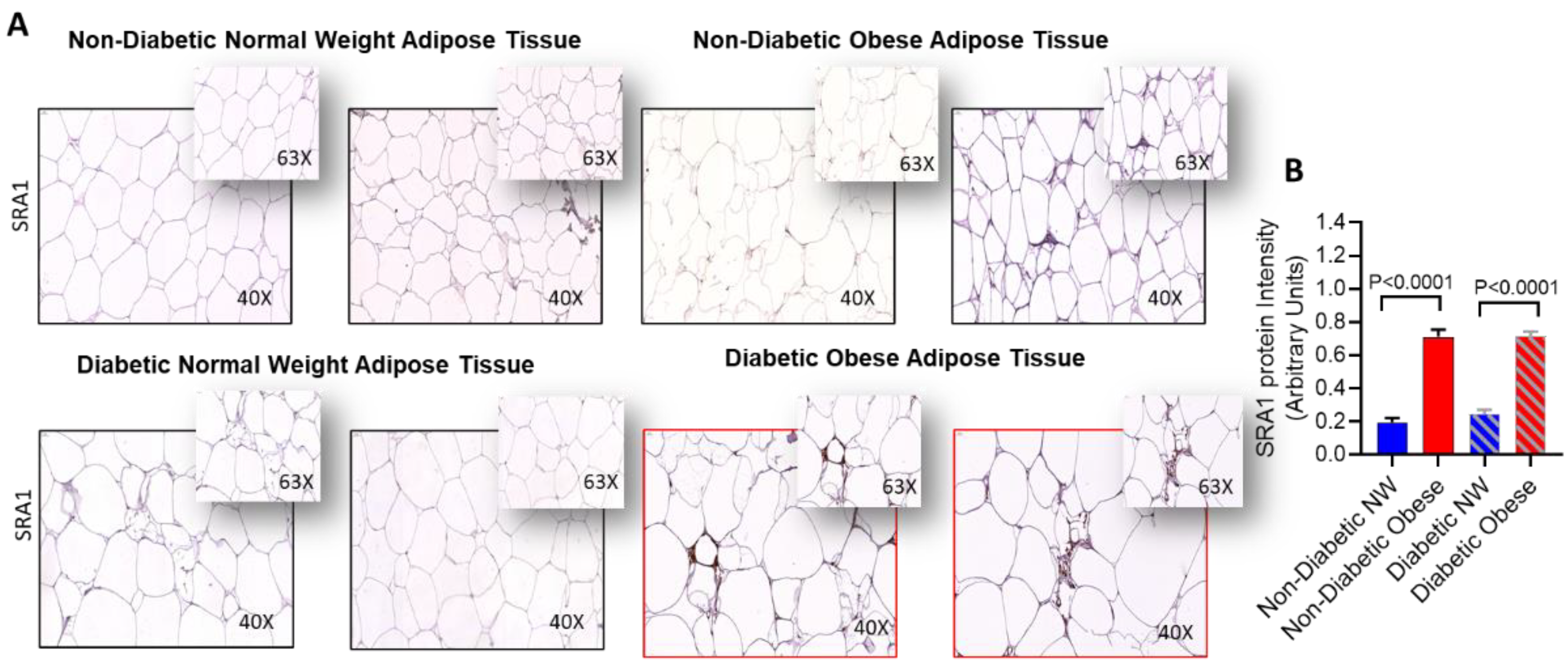
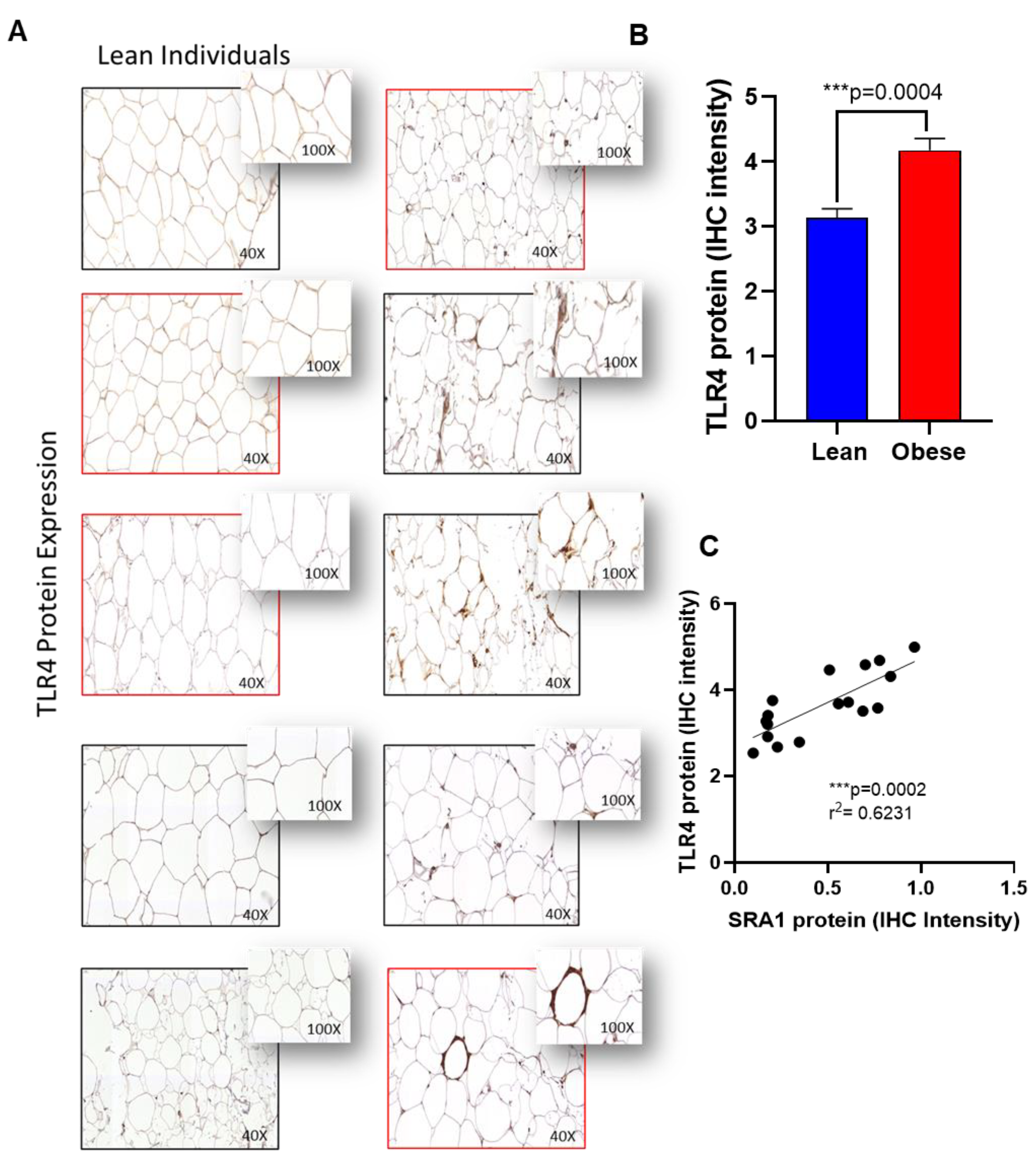
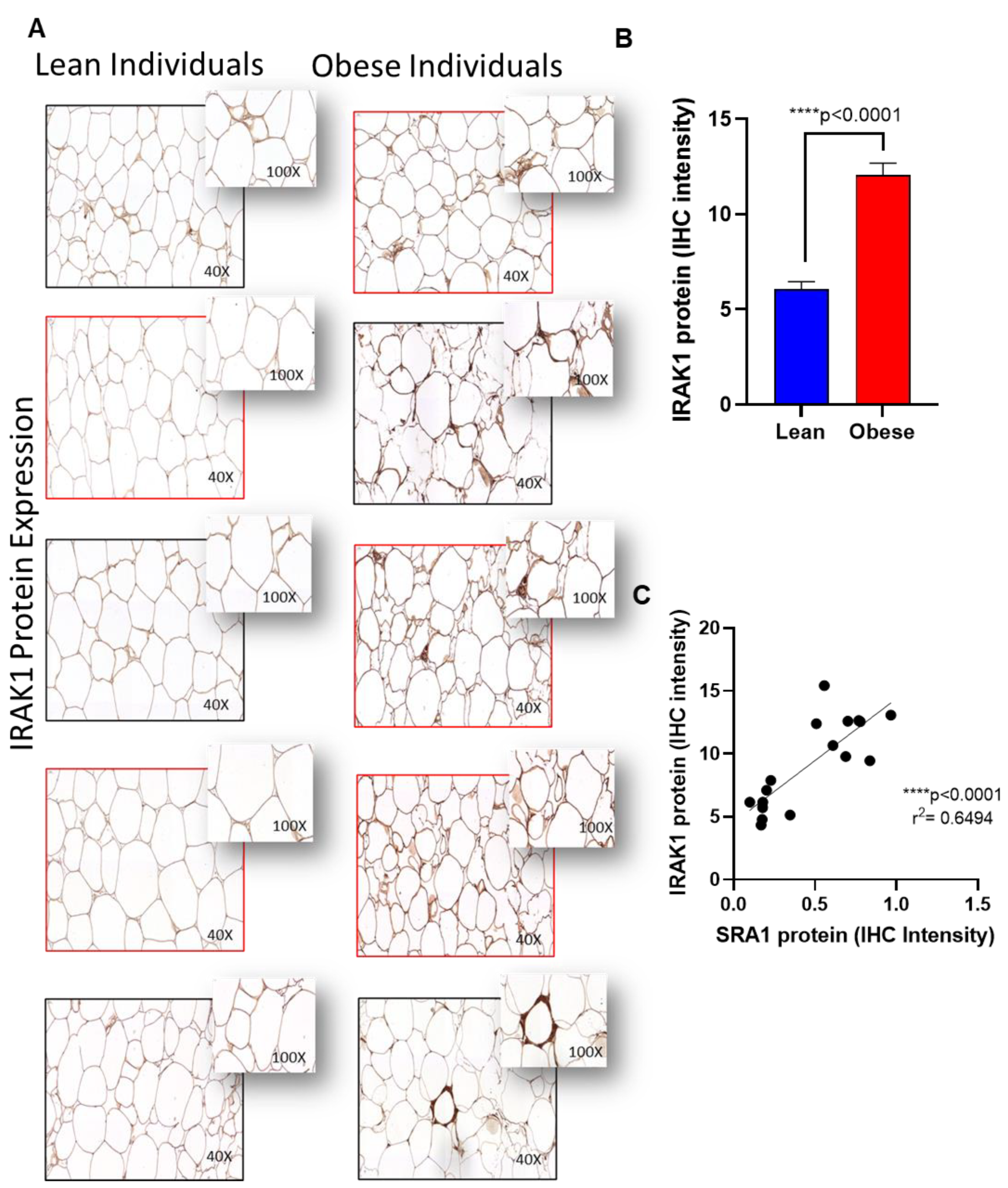
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
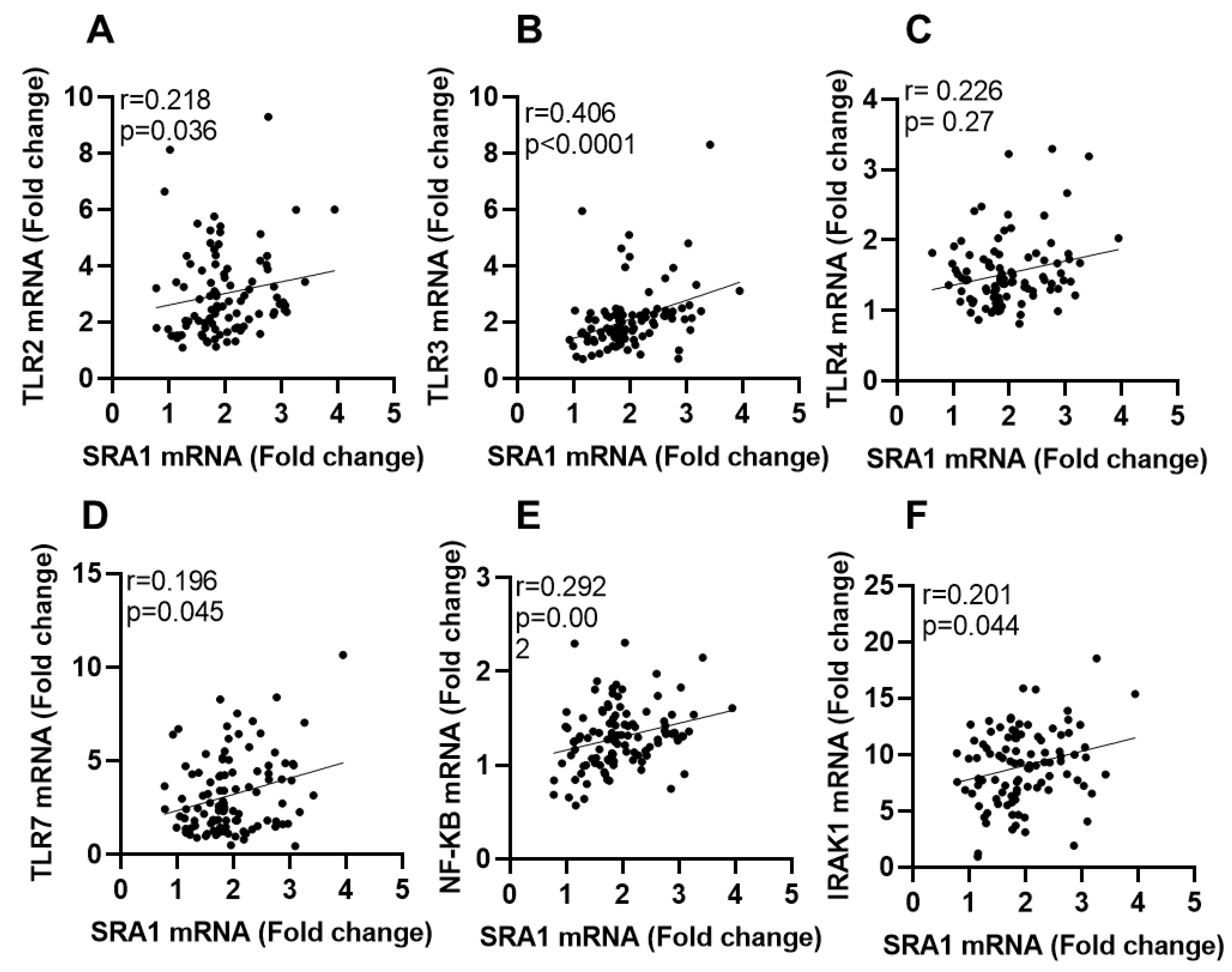
3.1. SRA1 Adipose Expression and Its Association with TLRs, Downstream Signaling Mediators and IRFs Expression in the Study Population
3.2. Association of Adipose SRA1 Expression (mRNA) with TLRs, Their Signaling Mediators, and IRFs in Individuals Classified as Those with NW, Overweight, and Obesity
3.3. Association of Adipose SRA1 Expression (mRNA) with TLRs, Their Signaling Mediators, and IRFs in Individuals with/without T2D
3.4. Analysis of the Independent Associations between SRA1 and TLRs, Their Signaling Mediators, and IRFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Khaodhiar, L.; Ling, P.R.; Blackburn, G.L.; Bistrian, B.R. Serum levels of interleukin-6 and c-reactive protein correlate with body mass index across the broad range of obesity. JPEN J. Parenter. Enter. Nutr. 2004, 28, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef]
- Lyon, C.J.; Law, R.E.; Hsueh, W.A. Minireview: Adiposity, inflammation, and atherogenesis. Endocrinology 2003, 144, 2195–2200. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461s–465s. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Curat, C.A.; Miranville, A.; Sengenès, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumié, A. From blood monocytes to adipose tissue-resident macrophages. Induction Diapedesis Hum. Matur. Adipocytes 2004, 53, 1285–1292. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Farb, M.G.; Mott, M.M.; Hess, D.T.; Carmine, B.; Fiscale, A.; Joseph, L.; Apovian, C.M.; Gokce, N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr. Diabetes 2012, 2, e30. [Google Scholar] [CrossRef]
- Morris, D.L.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages: Phenotypic plasticity and diversity in lean and obese states. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 341–346. [Google Scholar] [CrossRef]
- Sell, H.; Eckel, J. Adipose tissue inflammation: Novel insight into the role of macrophages and lymphocytes. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 366–370. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Trudler, D.; Farfara, D.; Frenkel, D. Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: Towards future therapeutic application. Mediat. Inflamm. 2010, 2010, 497987. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Burns, K.; Martinon, F.; Esslinger, C.; Pahl, H.; Schneider, P.; Bodmer, J.L.; Di Marco, F.; French, L.; Tschopp, J. Myd88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 1998, 273, 12203–12209. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. Tlrs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef]
- Lee, S.M.; Kok, K.H.; Jaume, M.; Cheung, T.K.; Yip, T.F.; Lai, J.C.; Guan, Y.; Webster, R.G.; Jin, D.Y.; Peiris, J.S. Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3793–3798. [Google Scholar] [CrossRef]
- O’Neill, L.A. How toll-like receptors signal: What we know and what we don’t know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef]
- Narayanankutty, A. Toll-like receptors as a novel therapeutic target for natural products against chronic diseases. Curr. Drug Targets 2019, 20, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, Y.; Choi, Y.H.; Park, T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (tlr) activation and tlr ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Yin, J.; Peng, Y.; Wu, J.; Wang, Y.; Yao, L. Toll-like receptor 2/4 links to free fatty acid-induced inflammation and β-cell dysfunction. J. Leukoc. Biol. 2014, 95, 47–52. [Google Scholar] [CrossRef]
- Könner, A.C.; Brüning, J.C. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol. Metab. TEM 2011, 22, 16–23. [Google Scholar] [CrossRef]
- Wong, F.S.; Wen, L. Toll-like receptors and diabetes. Ann. N. Y. Acad. Sci. 2008, 1150, 123–132. [Google Scholar] [CrossRef]
- Curtiss, L.K.; Tobias, P.S. Emerging role of toll-like receptors in atherosclerosis. J. Lipid Res. 2009, 50, S340–S345. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (tlr) 2 and tlr4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; Defronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher f, M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Kaur, H.; Chien, A.; Jialal, I. Hyperglycemia induces toll like receptor 4 expression and activity in mouse mesangial cells: Relevance to diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2012, 303, F1145–F1150. [Google Scholar] [CrossRef][Green Version]
- Tencerová, M.; Kračmerová, J.; Krauzová, E.; Mališová, L.; Kováčová, Z.; Wedellová, Z.; Šiklová, M.; Štich, V.; Rossmeislová, L. Experimental hyperglycemia induces an increase of monocyte and t-lymphocyte content in adipose tissue of healthy obese women. PLoS ONE 2015, 10, e0122872. [Google Scholar] [CrossRef]
- Kim, J.J.; Sears, D.D. Tlr4 and insulin resistance. Gastroenterol. Res. Pract. 2010, 2010, 212563. [Google Scholar] [CrossRef]
- Lanz, R.B.; McKenna, N.J.; Onate, S.A.; Albrecht, U.; Wong, J.; Tsai, S.Y.; Tsai, M.-J.; O’Malley, B.W. A steroid receptor coactivator, sra, functions as an rna and is present in an src-1 complex. Cell 1999, 97, 17–27. [Google Scholar] [CrossRef]
- Colley, S.M.; Leedman, P.J. Steroid receptor rna activator—A nuclear receptor coregulator with multiple partners: Insights and challenges. Biochimie 2011, 93, 1966–1972. [Google Scholar] [CrossRef]
- Beato, M.; Vicent, G.P. A new role for an old player: Steroid receptor rna activator (sra) represses hormone inducible genes. Transcription 2013, 4, 167–171. [Google Scholar] [CrossRef]
- Leygue, E. Steroid receptor rna activator (sra1): Unusual bifaceted gene products with suspected relevance to breast cancer. Nucl. Recept. Signal. 2007, 5, e006. [Google Scholar] [CrossRef]
- Liu, S.; Sheng, L.; Miao, H.; Saunders, T.L.; MacDougald, O.A.; Koenig, R.J.; Xu, B. Sra gene knockout protects against diet-induced obesity and improves glucose tolerance*. J. Biol. Chem. 2014, 289, 13000–13009. [Google Scholar] [CrossRef]
- Xu, B.; Gerin, I.; Miao, H.; Vu-Phan, D.; Johnson, C.N.; Xu, R.; Chen, X.W.; Cawthorn, W.P.; MacDougald, O.A.; Koenig, R.J. Multiple roles for the non-coding rna sra in regulation of adipogenesis and insulin sensitivity. PLoS ONE 2010, 5, e14199. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Arefanian, H.; Sindhu, S.; Shenouda, S.; Thomas, R.; Al-Mulla, F.; Tuomilehto, J.; Ahmad, R. Adipose tissue steroid receptor rna activator 1 (sra1) expression is associated with obesity, insulin resistance, and inflammation. Cells 2021, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Levy, J.C.; Matthews, D.R. Expansion of the homeostasis model assessment of β-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: Ihoma2. Diabetes Care 2013, 36, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Thomas, R.; Kochumon, S.; Wilson, A.; Abu-Farha, M.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Increased adipose tissue expression of interferon regulatory factor (irf)-5 in obesity: Association with metabolic inflammation. Cells 2019, 8, 1418. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of ccl19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar] [CrossRef]
- Kochumon, S.; Jacob, T.; Koshy, M.; Al-Rashed, F.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Rosen, E.D.; Ahmad, R. Palmitate potentiates lipopolysaccharide-induced il-6 production via coordinated acetylation of h3k9/h3k18, p300, and rna polymerase ii. J. Immunol. 2022, 209, 731–741. [Google Scholar]
- Khadir, A.; Tiss, A.; Abubaker, J.; Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; John, J.; Kavalakatt, S.; Warsame, S.; Al-Madhoun, A.; et al. Map kinase phosphatase dusp1 is overexpressed in obese humans and modulated by physical exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E71–E83. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of osteopontin with il-18 in obese individuals: Implications for insulin resistance. PLoS ONE 2013, 8, e63944. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Kochumon, S.; Al-Rashed, F.; Sindhu, S.; Thomas, R.; Miranda, L.; Al-Mulla, F.; Ahmad, R. Dectin-1 as a potential inflammatory biomarker for metabolic inflammation in adipose tissue of individuals with obesity. Cells 2022, 11, 2879. [Google Scholar] [CrossRef]
- Haider, M.J.A.; Albaqsumi, Z.; Al-Mulla, F.; Ahmad, R.; Al-Rashed, F. Socs3 regulates dectin-2-induced inflammation in pbmcs of diabetic patients. Cells 2022, 11, 2670. [Google Scholar] [CrossRef]
- Akhter, N.; Kochumon, S.; Hasan, A.; Wilson, A.; Nizam, R.; Al Madhoun, A.; Al-Rashed, F.; Arefanian, H.; Alzaid, F.; Sindhu, S.; et al. Ifn-γ and lps induce synergistic expression of ccl2 in monocytic cells via h3k27 acetylation. J. Inflamm. Res. 2022, 15, 4291–4302. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Roub, A.; Kochumon, S.; Akther, N.; Thomas, R.; Kumari, M.; Koshy, M.S.; Tiss, A.; Hannun, Y.A.; Tuomilehto, J.; et al. The synergy between palmitate and tnf-alpha for ccl2 production is dependent on the trif/irf3 pathway: Implications for metabolic inflammation. J. Immunol. 2018, 200, 3599–3611. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Thomas, R.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Enhanced adipose expression of interferon regulatory factor (irf)-5 associates with the signatures of metabolic inflammation in diabetic obese patients. Cells 2020, 9, 730. [Google Scholar] [CrossRef]
- Burns, K.; Clatworthy, J.; Martin, L.; Martinon, F.; Plumpton, C.; Maschera, B.; Lewis, A.; Ray, K.; Tschopp, J.; Volpe, F. Tollip, a new component of the il-1ri pathway, links irak to the il-1 receptor. Nat. Cell Biol. 2000, 2, 346–351. [Google Scholar] [CrossRef]
- Huang, J.; Gao, X.; Li, S.; Cao, Z. Recruitment of irak to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc. Natl. Acad. Sci. USA 1997, 94, 12829–12832. [Google Scholar] [CrossRef]
- Qian, Y.; Commane, M.; Ninomiya-Tsuji, J.; Matsumoto, K.; Li, X. Irak-mediated translocation of traf6 and tab2 in the interleukin-1-induced activation of nfkappa b. J. Biol. Chem. 2001, 276, 41661–41667. [Google Scholar] [CrossRef]
- Takaesu, G.; Kishida, S.; Hiyama, A.; Yamaguchi, K.; Shibuya, H.; Irie, K.; Ninomiya-Tsuji, J.; Matsumoto, K. Tab2, a novel adaptor protein, mediates activation of tak1 mapkkk by linking tak1 to traf6 in the il-1 signal transduction pathway. Mol. Cell 2000, 5, 649–658. [Google Scholar] [CrossRef]
- Baccala, R.; Hoebe, K.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. Tlr-dependent and tlr-independent pathways of type i interferon induction in systemic autoimmunity. Nat. Med. 2007, 13, 543–551. [Google Scholar] [CrossRef]
- Ahmad, R.; Shihab, P.K.; Thomas, R.; Alghanim, M.; Hasan, A.; Sindhu, S.; Behbehani, K. Increased expression of the interleukin-1 receptor-associated kinase (irak)-1 is associated with adipose tissue inflammatory state in obesity. Diabetol. Metab. Syndr. 2015, 7, 71. [Google Scholar] [CrossRef]
- Walther, K.; Schulte, L.N. The role of lncrnas in innate immunity and inflammation. RNA Biol. 2021, 18, 587–603. [Google Scholar] [CrossRef]
- Yarani, R.; Mirza, A.H.; Kaur, S.; Pociot, F. The emerging role of lncrnas in inflammatory bowel disease. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Emberley, E.; Huang, G.-J.; Hamedani, M.K.; Czosnek, A.; Ali, D.; Grolla, A.; Lu, B.; Watson, P.H.; Murphy, L.C.; Leygue, E. Identification of new human coding steroid receptor rna activator isoforms. Biochem. Biophys. Res. Commun. 2003, 301, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, F.; Zugck, C.; Rauch, G.J.; Ivandic, B.; Weichenhan, D.; Müller-Bardorff, M.; Meder, B.; Mokhtari, N.E.E.; Regitz-Zagrosek, V.; Hetzer, R.; et al. Hbegf, sra1, and ik: Three cosegregating genes as determinants of cardiomyopathy. Genome Res. 2009, 19, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ye, L.; Zhang, D.; Cawthorn, W.P.; Xu, B. New insights into the long non-coding rna sra: Physiological functions and mechanisms of action. Front. Med. 2018, 5, 244. [Google Scholar] [CrossRef]
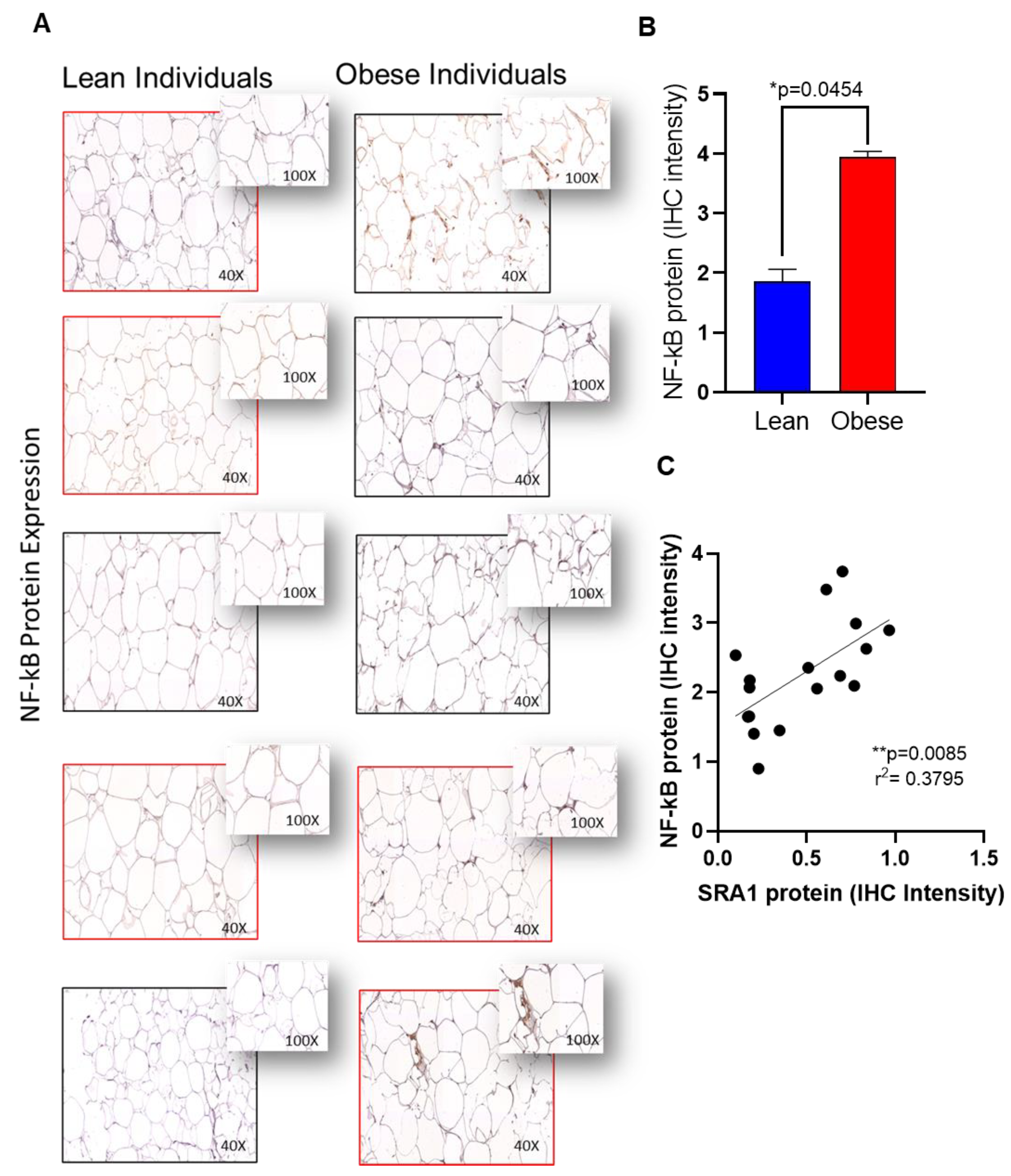

| Inflammatory Marker | Total Participants (N = 108) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without T2D (N = 55) | With T2D (N = 53) | ||||||||||
| Normal Weight (N = 8) | Overweight (N = 19) | Obese (N = 28) | Normal Weight vs. Overweight (p Value) # | Normal Weight vs. Obese (p Value) # | Normal Weight (N = 4) | Overweight (N = 13) | Obese (N = 36) | Normal Weight vs. Overweight (p Value) # | Normal Weight vs. Obese (p Value) # | Without T2D (N = 55) vs. With T2D (N = 53) (p Value) $ | |
| SRA1 | 1.7 ± 0.24 | 1.8 ± 0.61 | 2.2 ± 0.62 | 0.335 | 0.025 * | 2.3 ± 0.95 | 1.7 ± 0.55 | 1.9 ± 1.17 | 0.175 | 0.436 | 0.373 |
| TLR2 | 1.95 ± 1.04 | 2.88 ± 1.96 | 3.20 ± 1.31 | 0.197 | 0.018 * | 2.18 ± 0.76 | 2.82 ± 1.17 | 3.27 ± 1.66 | 0.465 | 0.272 | 0.305 |
| TLR3 | 1.49 ± 0.30 | 1.79 ± 1.17 | 2.57 ± 1.50 | 0.629 | 0.019 * | 3.24 ± 2.20 | 1.74 ± 1.06 | 2.07 ± 0.59 | 0.168 | 0.616 | 0.618 |
| TLR4 | 1.31 ± 0.23 | 1.42 ± 0.41 | 1.67 ± 0.65 | 0.661 | 0.267 | 2.04 ± 0.89 | 1.33 ± 0.31 | 1.57 ±0.45 | 0.144 | 0.441 | 0.609 |
| TLR7 | 2.06 ± 0.83 | 3.15 ± 2.27 | 3.38 ± 2.17 | 0.502 | 0.141 | 2.89 ± 1.51 | 1.92 ± 1.36 | 3.78 ± 2.04 | 0.387 | 0.638 | 0.546 |
| TLR8 | 1.68 ± 0.69 | 3.51 ± 2.90 | 3.31 ± 1.96 | 0.117 | 0.061 * | 2.85 ± 0.75 | 2.71 ± 1.60 | 3.94 ± 2.78 | 0.776 | 0.655 | 0.202 |
| TLR9 | 8.51 ± 3.91 | 7.85 ± 3.37 | 7.32 ± 3.87 | 0.803 | 0.292 | 7.02 ± 5.62 | 7.63 ± 2.54 | 7.91 ± 3.47 | 0.379 | 0.357 | 0.852 |
| TLR10 | 21.88 ± 22.28 | 23.04 ± 22.15 | 29.88 ± 37.15 | 0.719 | 0.549 | 24.96 ± 15.52 | 23.97 ± 24.62 | 23.05 ± 20.07 | 0.625 | 0.65 | 0.976 |
| NF-κB | 1.18 ± 0.20 | 1.28 ± 0.33 | 1.38 ± 0.28 | 0.476 | 0.053 | 1.47 ± 0.29 | 1.28 ± 0.42 | 1.26 ± 0.37 | 0.342 | 0.282 | 0.559 |
| MyD88 | 1.62 ± 0.47 | 1.86 ± 0.73 | 1.85 ± 0.57 | 0.421 | 0.346 | 2.71 ± 0.77 | 1.96 ± 0.57 | 1.92 ± 0.59 | 0.166 | 0.112 | 0.240 |
| IRAK1 | 6.14 ± 2.83 | 8.19 ± 3.37 | 9.12 ± 3.28 | 0.119 | 0.042 * | 8.01 ± 1.11 | 9.45 ± 3.46 | 9.69 ± 3.23 | 0.432 | 0.353 | 0.116 |
| TRAF6 | 2.49 ± 0.35 | 2.26 ± 1.18 | 2.36 ± 0.98 | 0.113 | 0.255 | 2.12 ± 0.80 | 2.26 ± 0.75 | 2.33 ± 0.64 | 0.922 | 0.712 | 0.544 |
| IRF3 | 1.81 ± 0.57 | 2.31 ± 0.68 | 2.54 ± 0.61 | 0.152 | 0.065 | 2.45 ± 0.80 | 2.73 ± 0.56 | 2.65 ± 0.59 | 0.4542 | 0.675 | 0.047 * |
| IRF4 | 2.21 ± 0.84 | 3.61 ± 2.18 | 3.40 ± 1.99 | 0.228 | 0.169 | 3.74 ± 3.41 | 4.32 ± 2.10 | 3.95 ±1.46 | 0.702 | 0.828 | 0.020 * |
| IRF5 | 1.53 ± 0.33 | 2.82 ± 1.44 | 2.73 ± 1.08 | 0.027 * | 0.016 * | 2.72 ± 0.93 | 2.90 ± 1.31 | 3.13 ±1.13 | 0.879 | 0.584 | 0.039 * |
| All participants (N = 108) | ||
|---|---|---|
| Adipose Marker | r | p Value |
| TLR2 | 0.218 | 0.036 * |
| TLR3 | 0.406 | <0.0001 **** |
| TLR4 | 0.226 | 0.027 * |
| TLR7 | 0.196 | 0.045 * |
| TLR8 | 0.071 | 0.481 |
| TLR9 | 0.080 | 0.411 |
| TLR10 | 0.074 | 0.459 |
| NF-κB | 0.297 | 0.002 ** |
| MyD88 | 0.149 | 0.134 |
| IRAK1 | 0.201 | 0.044 * |
| TRAF6 | 0.032 | 0.746 |
| IRF3 | 0.139 | 0.187 |
| IRF4 | −0.009 | 0.931 |
| IRF5 | 0.175 | 0.080 |
| Obesity Level | Normal Weight | Overweight | Obese | |||
|---|---|---|---|---|---|---|
| (N = 12) | (N = 32) | (N = 64) | ||||
| Adipose Marker | r | p Value | r | p Value | r | p Value |
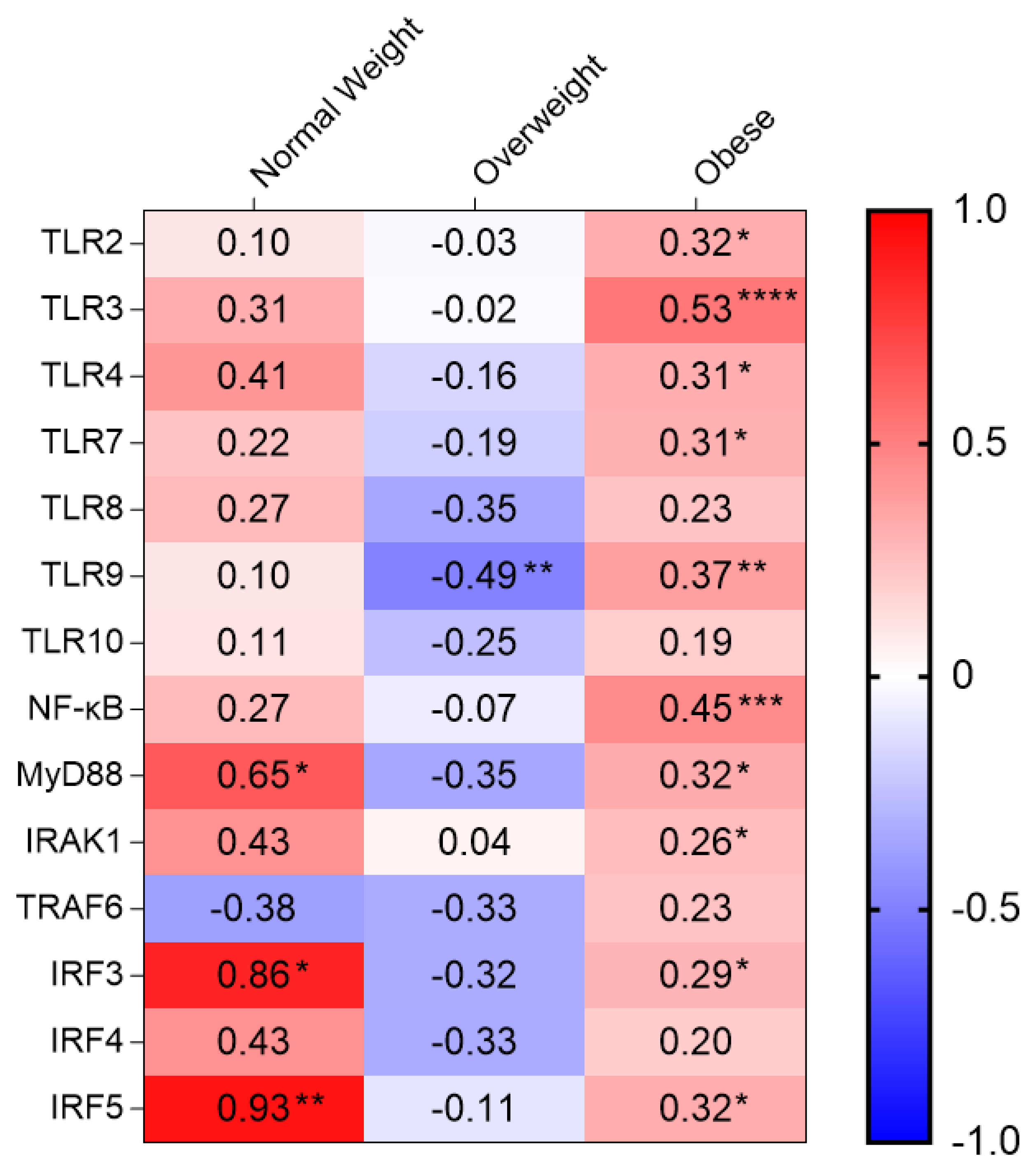
| TLR2 | 0.095 | 0.823 | −0.026 | 0.895 | 0.317 | 0.017 * |
| TLR3 | 0.310 | 0.456 | −0.016 | 0.934 | 0.531 | <0.0001 **** |
| TLR4 | 0.405 | 0.320 | −0.164 | 0.386 | 0.311 | 0.022 * |
| TLR7 | 0.224 | 0.533 | −0.190 | 0.297 | 0.305 | 0.015 * |
| TLR8 | 0.267 | 0.488 | −0.347 | 0.060 | 0.229 | 0.071 |
| TLR9 | 0.098 | 0.762 | −0.489 | 0.005 ** | 0.374 | 0.002 ** |
| TLR10 | 0.105 | 0.746 | −0.248 | 0.194 | 0.192 | 0.134 |
| NF-κB | 0.266 | 0.404 | −0.071 | 0.699 | 0.454 | <0.001 *** |
| MyD88 | 0.648 | 0.043 * | −0.347 | 0.060 | 0.324 | 0.010 * |
| IRAK1 | 0.429 | 0.289 | 0.044 | 0.818 | 0.255 | 0.044 * |
| TRAF6 | −0.378 | 0.226 | −0.327 | 0.073 | 0.233 | 0.064 |
| IRF3 | 0.857 | 0.014 * | −0.322 | 0.088 | 0.290 | 0.030 * |
| IRF4 | 0.429 | 0.397 | −0.330 | 0.075 | 0.195 | 0.136 |
| IRF5 | 0.929 | 0.003 ** | −0.105 | 0.575 | 0.321 | 0.010 * |
| Diabetes Status | Without T2D | With T2D | ||
|---|---|---|---|---|
| (N = 55) | (N = 53) | |||
| Adipose Marker | r | p Value | r | p Value |
| TLR2 | 0.206 | 0.165 | 0.223 | 0.136 |
| TLR3 | 0.247 | 0.080 | 0.555 | <0.0001 **** |
| TLR4 | 0.096 | 0.522 | 0.302 | 0.044 * |
| TLR7 | 0.140 | 0.309 | 0.292 | 0.040 * |
| TLR8 | 0.028 | 0.843 | 0.180 | 0.210 |
| TLR9 | −0.290 | 0.034 * | 0.398 | 0.003 ** |
| TLR10 | 0.062 | 0.667 | 0.084 | 0.556 |
| NF-κB | 0.191 | 0.162 | 0.381 | 0.005 ** |
| MyD88 | 0.014 | 0.918 | 0.311 | 0.030 * |
| IRAK1 | 0.220 | 0.117 | 0.239 | 0.099 |
| TRAF6 | −0.318 | 0.019 * | 0.286 | 0.038 * |
| IRF3 | 0.051 | 0.739 | 0.220 | 0.137 |
| IRF4 | 0.066 | 0.649 | −0.017 | 0.909 |
| IRF5 | 0.167 | 0.243 | 0.288 | 0.043 * |
| All participants (N = 108) | ANOVA | r2 = 0.21 | p value < 0.0001 | |
| Predictor Variable | TLR3 | β value = 0.392 | p value < 0.0001 | |
| IRAK1 | β value = 0.279 | p value = 0004 | ||
| With T2D (N = 53) | ANOVA | r2= 0.35 | p value < 0.0001 | |
| Predictor Variable | TLR3 | β value = 0.499 | p value < 0.001 | |
| TLR9 | β value = 0.329 | p value = 0.010 | ||
| Normal Weight (N = 12) | ANOVA | r2= 0.64 | p value = 0.005 | |
| Predictor Variable | MyD88 | β value = 0.801 | p value = 0.005 | |
| Overweight (N = 32) | ANOVA | r2= 0.25 | p value = 0.004 | |
| Predictor Variable | TLR9 | β value = −0.499 | p value = 0.004 | |
| Obese (N = 64) | ANOVA | r2= 0.38 | p value < 0.0001 | |
| Predictor Variable | TLR3 | β value = 0.464 | p value < 0.0001 | |
| IRAK1 | β value = 0.317 | p value = 0.005 | ||
| TLR7 | β value = 0.246 | p value = 0.027 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochumon, S.; Arefanian, H.; Sindhu, S.; Thomas, R.; Jacob, T.; Al-Sayyar, A.; Shenouda, S.; Al-Rashed, F.; Koistinen, H.A.; Al-Mulla, F.; et al. Expression of Steroid Receptor RNA Activator 1 (SRA1) in the Adipose Tissue Is Associated with TLRs and IRFs in Diabesity. Cells 2022, 11, 4007. https://doi.org/10.3390/cells11244007
Kochumon S, Arefanian H, Sindhu S, Thomas R, Jacob T, Al-Sayyar A, Shenouda S, Al-Rashed F, Koistinen HA, Al-Mulla F, et al. Expression of Steroid Receptor RNA Activator 1 (SRA1) in the Adipose Tissue Is Associated with TLRs and IRFs in Diabesity. Cells. 2022; 11(24):4007. https://doi.org/10.3390/cells11244007
Chicago/Turabian StyleKochumon, Shihab, Hossein Arefanian, Sardar Sindhu, Reeby Thomas, Texy Jacob, Amnah Al-Sayyar, Steve Shenouda, Fatema Al-Rashed, Heikki A. Koistinen, Fahd Al-Mulla, and et al. 2022. "Expression of Steroid Receptor RNA Activator 1 (SRA1) in the Adipose Tissue Is Associated with TLRs and IRFs in Diabesity" Cells 11, no. 24: 4007. https://doi.org/10.3390/cells11244007
APA StyleKochumon, S., Arefanian, H., Sindhu, S., Thomas, R., Jacob, T., Al-Sayyar, A., Shenouda, S., Al-Rashed, F., Koistinen, H. A., Al-Mulla, F., Tuomilehto, J., & Ahmad, R. (2022). Expression of Steroid Receptor RNA Activator 1 (SRA1) in the Adipose Tissue Is Associated with TLRs and IRFs in Diabesity. Cells, 11(24), 4007. https://doi.org/10.3390/cells11244007










