Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia
Abstract
:1. Introduction
2. Methods
2.1. Tetrahymena Cells Strains and Culture Conditions
2.2. Gene Knock-Outs and Knock-Ins
2.3. Phenotypic Analyses
2.4. Immunofluorescence and Ultrastructural Studies
2.5. Western Blot and 2D-Electrophoresis
2.6. Proximity Labeling (BioID) and Pull-Down Assays
2.7. Total Label-Free Cilia Proteome Analyses
2.8. MS/MS Data Analysis
2.9. Quantitative Real-Time PCR and RT-PCR
3. Results
3.1. Identification of Proteins Positioned in Close Proximity to Tetrahymena Cfap91
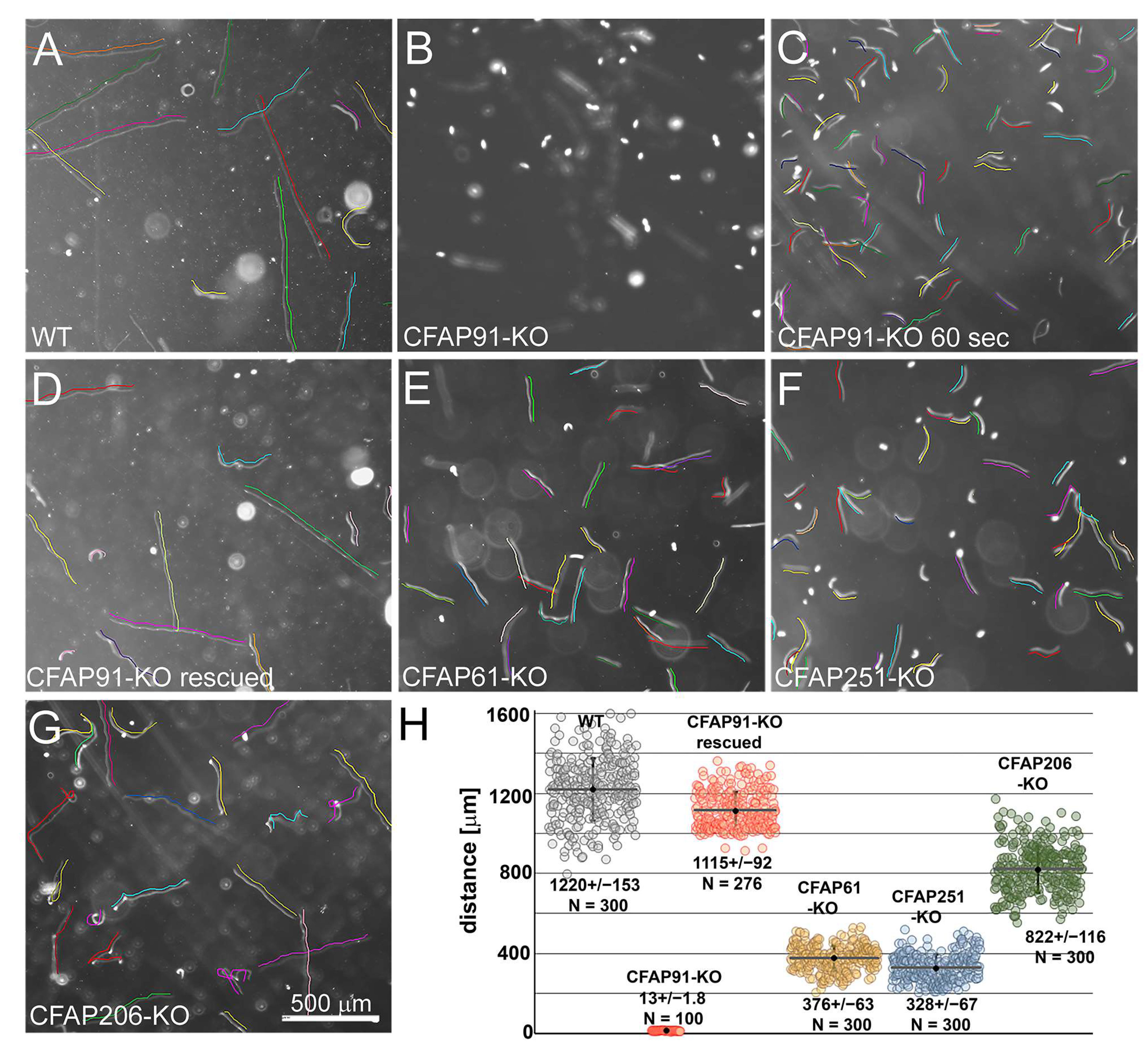
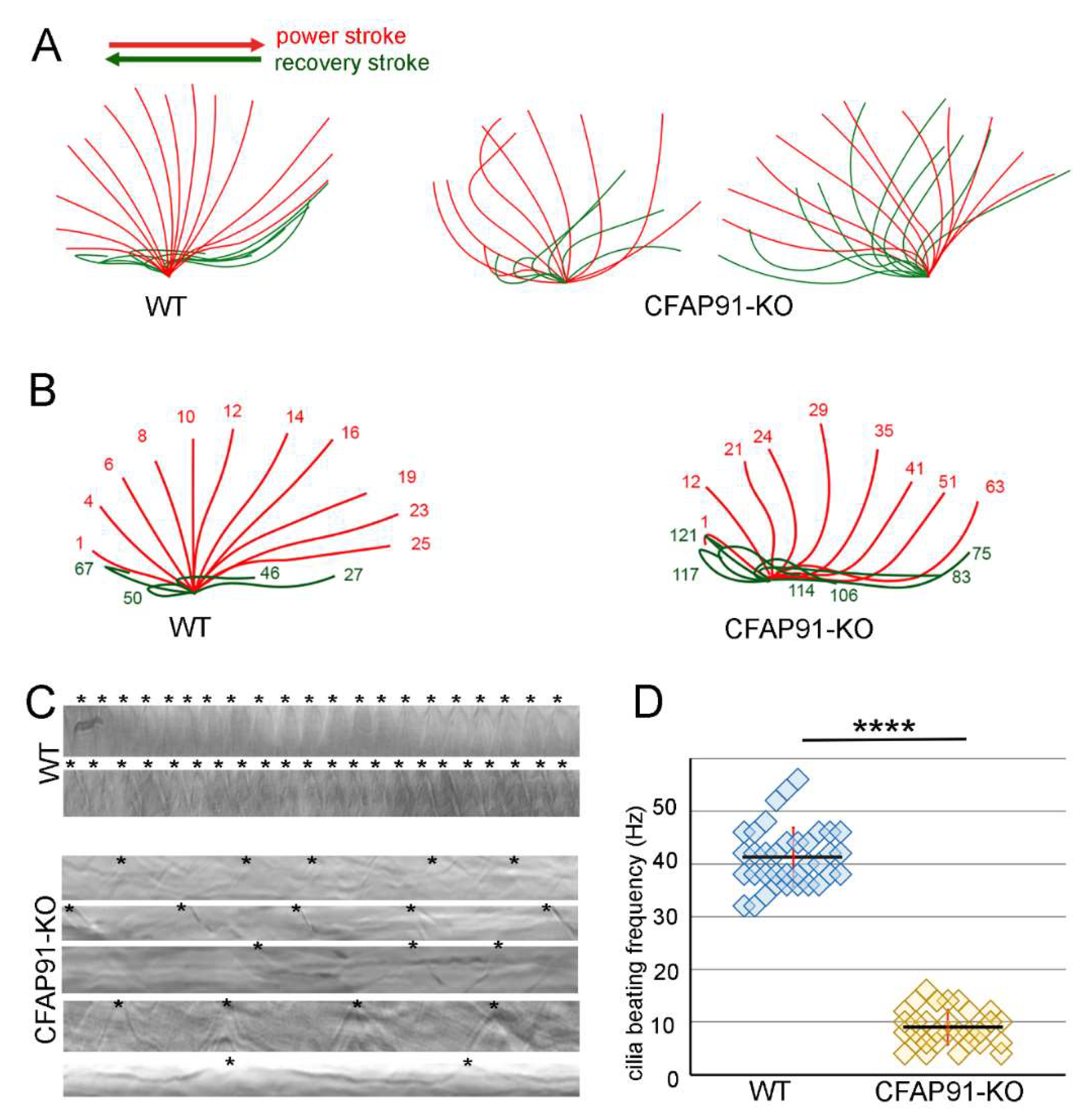
3.2. Knockout of CFAP91 Dramatically Reduces Tetrahymena Cell Motility
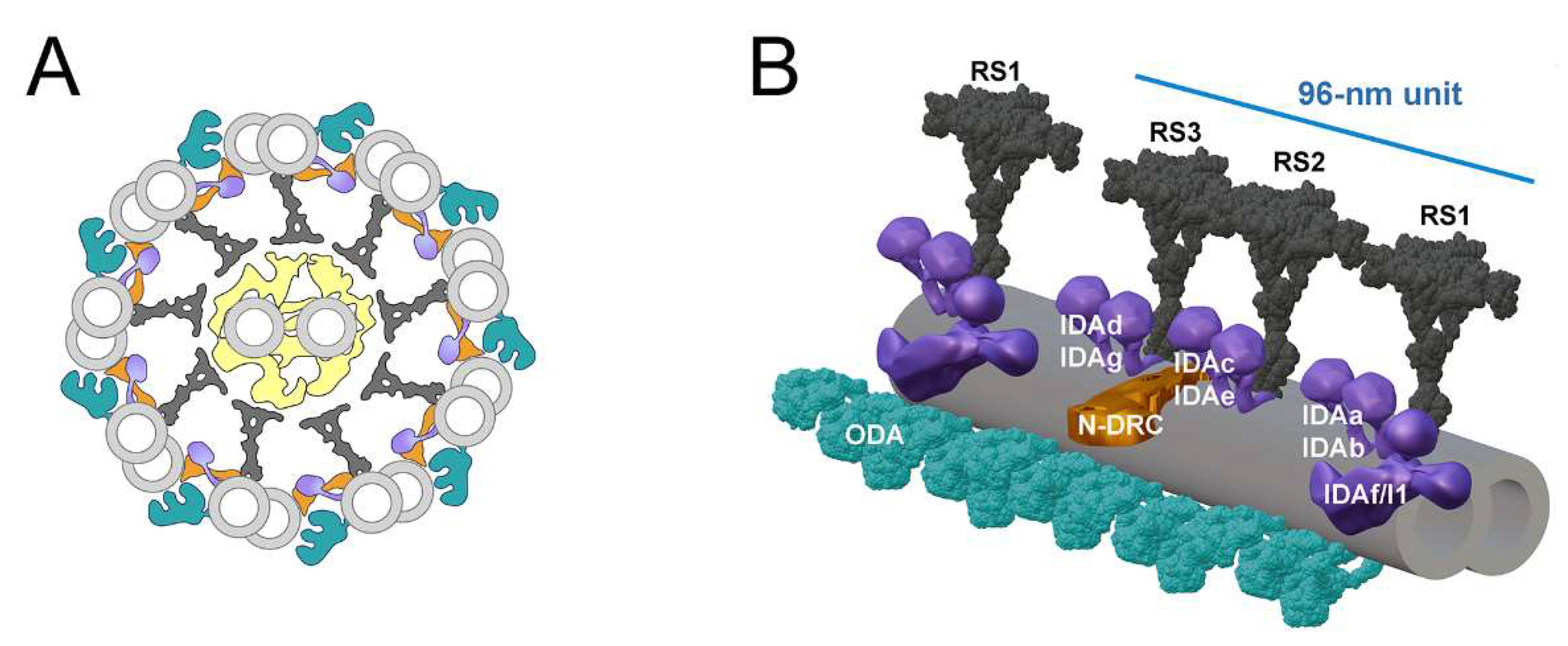
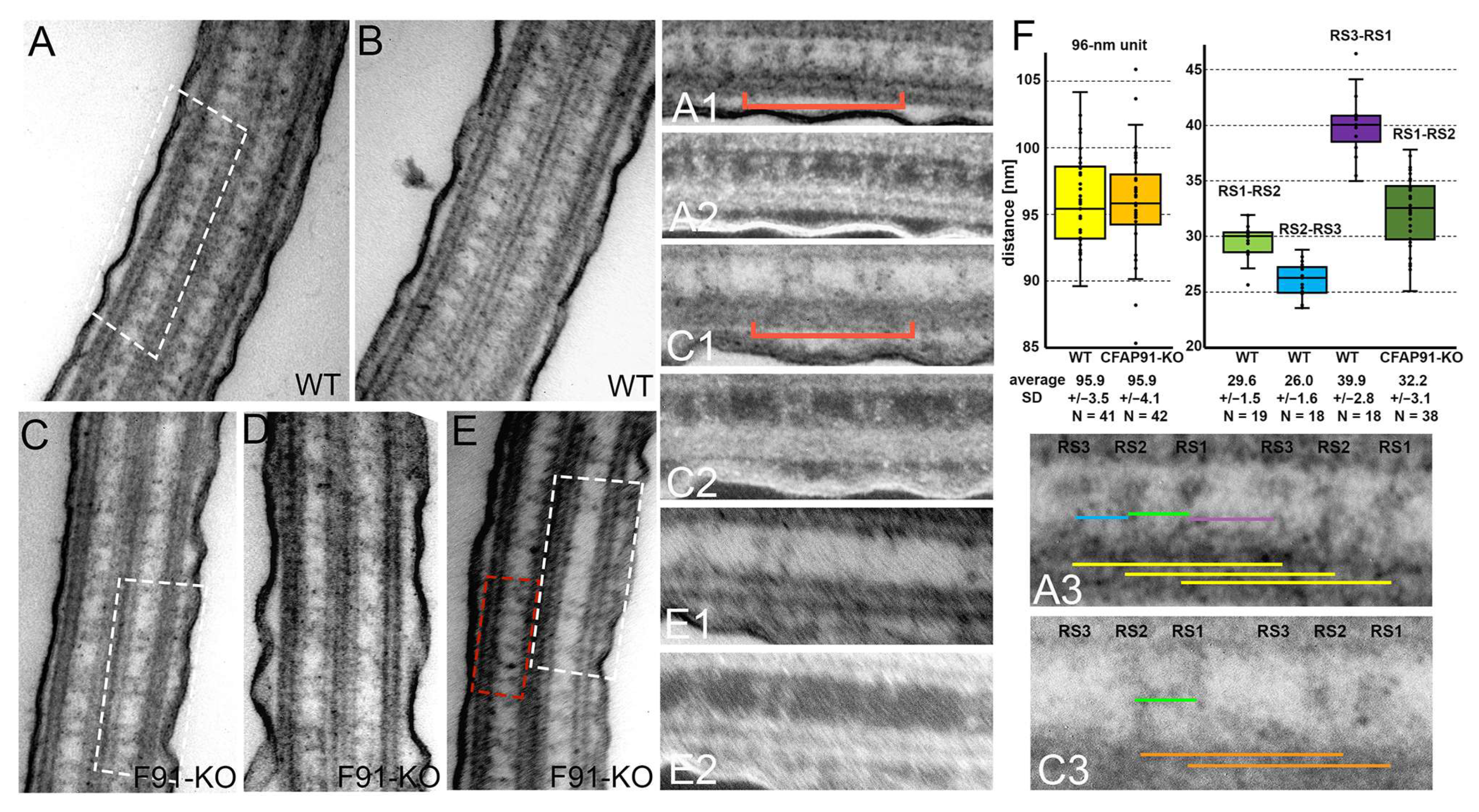
3.3. Cilia of Cfap91 Knockout Mutant Lack Some Radial Spokes and Inner Dynein Arms
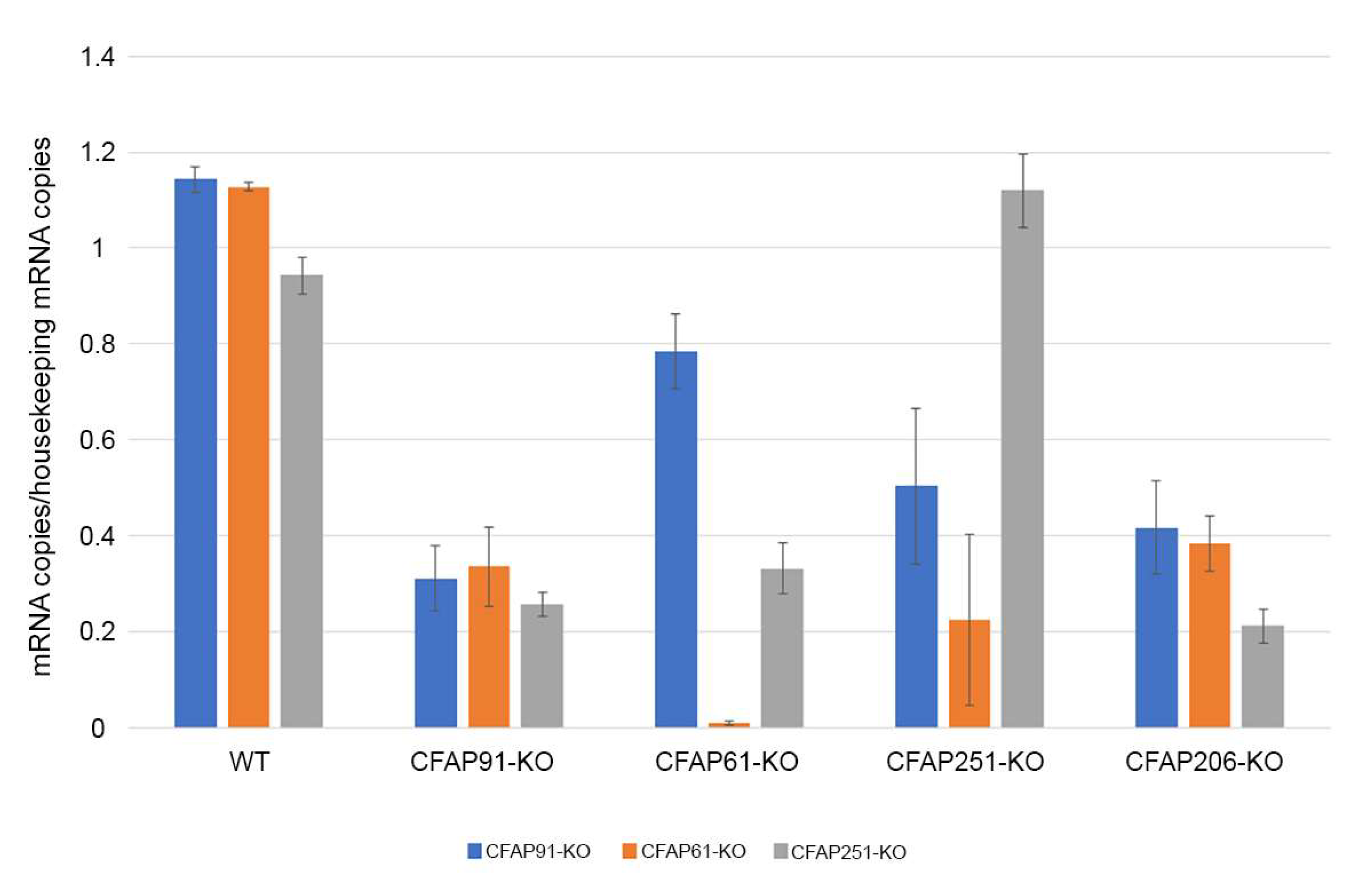
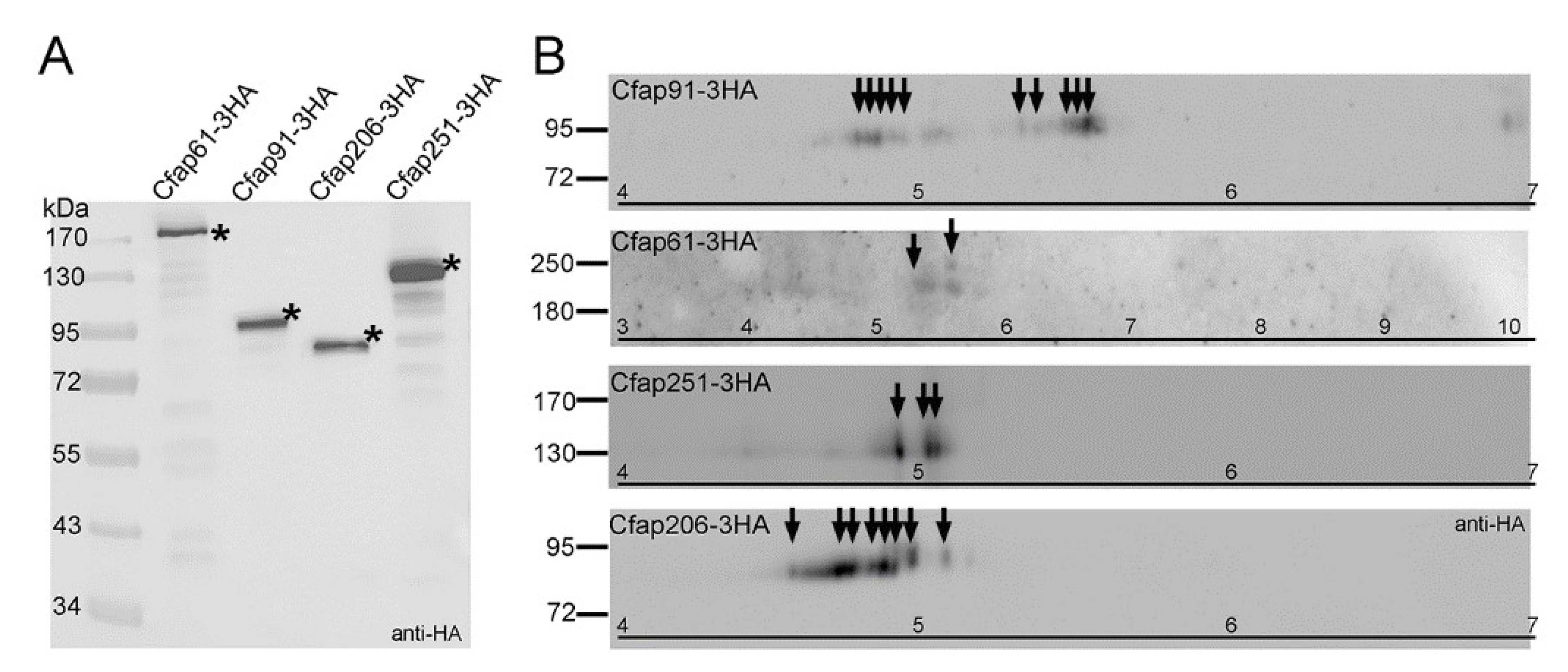
3.4. Regulation of Cfap61, Cfap91, and Cfap251 Proteins in Tetrahymena
3.5. Cfap91 Interacts Directly with Molecular Ruler Protein, Ccdc39
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, W.; Tang, Z.; Chen, Y.; Wang, C.; Tan, C.; Liao, J.; Tong, L.; Xiao, G. Ependymal Cilia: Physiology and Role in Hydrocephalus. Front. Mol. Neurosci. 2022, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef]
- Wang, S.; Burton, J.C.; Behringer, R.R.; Larina, I.V. In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct. Sci. Rep. 2015, 5, 13216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoque, M.; Kim, E.N.; Chen, D.; Li, F.-Q.; Takemaru, K.-I. Essential Roles of Efferent Duct Multicilia in Male Fertility. Cells 2022, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, H. Molecular and cellular basis of left–right asymmetry in vertebrates. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 273–296. [Google Scholar] [CrossRef]
- Little, R.B.; Norris, D.P. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021, 110, 11–18. [Google Scholar] [CrossRef]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Prim. 2020, 6, 1–29. [Google Scholar] [CrossRef]
- Osinka, A.; Poprzeczko, M.; Zielińska, M.M.; Fabczak, H.; Joachimiak, E.; Wloga, D. Ciliary Proteins: Filling the Gaps. Recent Advances in Deciphering the Protein Composition of Motile Ciliary Complexes. Cells 2019, 8, 730. [Google Scholar] [CrossRef] [Green Version]
- Samsel, Z.; Sekretarska, J.; Osinka, A.; Wloga, D.; Joachimiak, E. Central Apparatus, the Molecular Kickstarter of Ciliary and Flagellar Nanomachines. Int. J. Mol. Sci. 2021, 22, 3013. [Google Scholar] [CrossRef]
- Rao, Q.; Han, L.; Wang, Y.; Chai, P.; Kuo, Y.-W.; Yang, R.; Hu, F.; Yang, Y.; Howard, J.; Zhang, K. Structures of outer-arm dynein array on microtubule doublet reveal a motor coordination mechanism. Nat. Struct. Mol. Biol. 2021, 28, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Ma, M.; Sze-Tu, E.; Wang, X.; Koh, F.; Zhong, E.D.; Berger, B.; Davis, J.H.; Dutcher, S.K.; Zhang, R.; et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 2021, 28, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dymek, E.E.; Smith, E.F. A conserved CaM- and radial spoke–associated complex mediates regulation of flagellar dynein activity. J. Cell Biol. 2007, 179, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dymek, E.E.; Heuser, T.; Nicastro, D.; Smith, E.F. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol. Biol. Cell 2011, 22, 2520–2531. [Google Scholar] [CrossRef]
- Barber, C.F.; Heuser, T.; Carbajal-González, B.I.; Botchkarev, V.V.; Nicastro, D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell 2012, 23, 111–120. [Google Scholar] [CrossRef]
- Lin, J.; Heuser, T.; Carbajal-González, B.I.; Song, K.; Nicastro, D. The structural heterogeneity of radial spokes in cilia and flagella is conserved. Cytoskeleton 2012, 69, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Pigino, G.; Bui, K.H.; Maheshwari, A.; Lupetti, P.; Diener, D.; Ishikawa, T. Cryoelectron tomography of radial spokes in cilia and flagella. J. Cell Biol. 2011, 195, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Heuser, T.; Dymek, E.E.; Lin, J.; Smith, E.F.; Nicastro, D. The CSC connects three major axonemal complexes involved in dynein regulation. Mol. Biol. Cell 2012, 23, 3143–3155. [Google Scholar] [CrossRef]
- Martinez, G.; Beurois, J.; Dacheux, D.; Cazin, C.; Bidart, M.; Kherraf, Z.-E.; Robinson, D.R.; Satre, V.; Le Gac, G.; Ka, C.; et al. Biallelic variants in MAATS1 encoding CFAP91, a calmodulin-associated and spoke-associated complex protein, cause severe astheno-teratozoospermia and male infertility. J. Med Genet. 2020, 57, 708–716. [Google Scholar] [CrossRef]
- Kherraf, Z.-E.; Amiri-Yekta, A.; Dacheux, D.; Karaouzène, T.; Coutton, C.; Christou-Kent, M.; Martinez, G.; Landrein, N.; Le Tanno, P.; Ben Mustapha, S.F.; et al. A Homozygous Ancestral SVA-Insertion-Mediated Deletion in WDR66 Induces Multiple Morphological Abnormalities of the Sperm Flagellum and Male Infertility. Am. J. Hum. Genet. 2018, 103, 400–412. [Google Scholar] [CrossRef]
- Urbanska, P.; Song, K.; Joachimiak, E.; Krzemien-Ojak, L.; Koprowski, P.; Hennessey, T.; Jerka-Dziadosz, M.; Fabczak, H.; Gaertig, J.; Nicastro, D.; et al. The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia. Mol. Biol. Cell 2015, 26, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Meng, L.; Tan, C.; Luo, C.; He, W.-B.; Tu, C.; Zhang, H.; Du, J.; Nie, H.; Lu, G.-X.; et al. Biallelic CFAP61 variants cause male infertility in humans and mice with severe oligoasthenoteratozoospermia. J. Med Genet. 2022, 59, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Kherraf, Z.E.; Sun, S.; Zhang, X.; Cazin, C.; Coutton, C.; Zouari, R.; Zhao, S.; Hu, F.; et al. CFAP61 is required for sperm flagellum formation and male fertility in human and mouse. Development 2021, 148, dev199805. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zeb, A.; Ali, I.; Zhao, D.; Khan, A.; Zhang, B.; Zhou, J.; Khan, R.; Zhang, H.; Zhang, Y.; et al. Biallelic Variants in CFAP61 Cause Multiple Morphological Abnormalities of the Flagella and Male Infertility. Front. Cell Dev. Biol. 2021, 9, 803818. [Google Scholar] [CrossRef] [PubMed]
- Auguste, Y.; Delague, V.; Desvignes, J.-P.; Longepied, G.; Gnisci, A.; Besnier, P.; Levy, N.; Beroud, C.; Megarbane, A.; Metzler-Guillemain, C.; et al. Loss of Calmodulin- and Radial-Spoke-Associated Complex Protein CFAP251 Leads to Immotile Spermatozoa Lacking Mitochondria and Infertility in Men. Am. J. Hum. Genet. 2018, 103, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; He, X.; Yang, S.; Liu, C.; Wu, H.; Liu, W.; Lv, M.; Tang, D.; Tan, J.; Tang, S.; et al. Biallelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J. Hum. Genet. 2019, 64, 49–54. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Tang, H.; Zheng, A.; Li, H.; Yang, S.; Xiang, J. Successful Results of Intracytoplasmic Sperm Injection of a Chinese Patient With Multiple Morphological Abnormalities of Sperm Flagella Caused by a Novel Splicing Mutation in CFAP251. Front. Genet. 2021, 12, 783790. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Hallström, B.M.; Lindskog, C.; Mardinoglu, A.; Pontén, F.; Nielsen, J. Transcriptomics resources of human tissues and organs. Mol. Syst. Biol. 2016, 12, 862. [Google Scholar] [CrossRef]
- Gorovsky, M.A.; Yao, M.-C.; Keevert, J.B.; Pleger, G.L. Chapter 16 Isolation of Micro- and Macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975, 9, 311–327. [Google Scholar] [CrossRef]
- Orias, E.; Rasmussen, L. Dual capacity for nutrient uptake in Tetrahymena: IV. Growth without food vacuoles and its implications. Exp. Cell Res. 1976, 102, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, K.K.; Song, K.; Alford, L.M.; Sale, W.S.; Dymek, E.E.; Smith, E.F.; Hennessey, T.; Joachimiak, E.; Urbanska, P.; Wloga, D.; et al. FAP206 is a microtubule-docking adapter for ciliary radial spoke 2 and dynein c. Mol. Biol. Cell 2015, 26, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Hanley, D.; Bowen, J.; Lee, J.H.; Cole, E.; VerPlank, L.A.; Gaertig, J.; Gorovsky, M.A.; Bruns, P.J. Germline and Somatic Transformation of Mating Tetrahymena thermophila by Particle Bombardment. Genetics 1997, 146, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Wloga, D.; Gaertig, J. Manipulating Ciliary Protein-Encoding Genes in Tetrahymena thermophila. Methods Cell Biol. 2009, 93, 1–20. [Google Scholar] [CrossRef]
- Mochizuki, K. High efficiency transformation of Tetrahymena using a codon-optimized neomycin resistance gene. Gene 2008, 425, 79–83. [Google Scholar] [CrossRef]
- Bregier, C.; KrzemieŃ-Ojak, L.; Włoga, D.; Jerka-Dziadosz, M.; Joachimiak, E.; Batko, K.; Filipiuk, I.; Śmietanka, U.; Gaertig, J.; Fabczak, S.; et al. PHLP2 is essential and plays a role in ciliogenesis and microtubule assembly in Tetrahymena thermophila. J. Cell. Physiol. 2013, 228, 2175–2189. [Google Scholar] [CrossRef]
- Urbanska, P.; Joachimiak, E.; Bazan, R.; Fu, G.; Poprzeczko, M.; Fabczak, H.; Nicastro, D.; Wloga, D. Ciliary proteins Fap43 and Fap44 interact with each other and are essential for proper cilia and flagella beating. Cell. Mol. Life Sci. 2018, 75, 4479–4493. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Mori, C.; Hiraoka, Y.; Haraguchi, T. Puromycin resistance gene as an effective selection marker for ciliate Tetrahymena. Gene 2014, 534, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Joachimiak, E.; Osinka, A.; Farahat, H.; Świderska, B.; Sitkiewicz, E.; Poprzeczko, M.; Fabczak, H.; Wloga, D. Composition and function of the C1b/C1f region in the ciliary central apparatus. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Duan, J.; Gorovsky, M.A. Both Carboxy-Terminal Tails of α- and β-Tubulin Are Essential, but Either One Will Suffice. Curr. Biol. 2002, 12, 313–316. [Google Scholar] [CrossRef]
- Wloga, D.; Rogowski, K.; Sharma, N.; Van Dijk, J.; Janke, C.; Eddé, B.; Bré, M.-H.; Levilliers, N.; Redeker, V.; Duan, J.; et al. Glutamylation on α-Tubulin Is Not Essential but Affects the Assembly and Functions of a Subset of Microtubules in Tetrahymena thermophila. Eukaryot. Cell 2008, 7, 1362–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpiński, A.A.; Elguera, J.C.T.; Sanner, A.; Konopka, W.; Kaczmarek, L.; Winter, D.; Konopka, A.; Bulska, E. Study on Tissue Homogenization Buffer Composition for Brain Mass Spectrometry-Based Proteomics. Biomedicines 2022, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Bekker-Jensen, D.B.; Martínez-Val, A.; Steigerwald, S.; Rüther, P.; Fort, K.L.; Arrey, T.N.; Harder, A.; Makarov, A.; Olsen, J.V. A Compact Quadrupole-Orbitrap Mass Spectrometer with FAIMS Interface Improves Proteome Coverage in Short LC Gradients. Mol. Cell. Proteom. 2020, 19, 716–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, G.K.; Sundermann, C.A. Doublet cells inTetrahymena as indicators of culture media composition. Biol. Trace Element Res. 1995, 50, 181–191. [Google Scholar] [CrossRef]
- Brown, J.M.; Hardin, C.; Gaertig, J. Rotokinesis, a Novel Phenomenon of Cell Locomotion-Assisted Cytokinesis in the Ciliate Tetrahymena Thermophila. Cell Biol. Int. 1999, 23, 841–848. [Google Scholar] [CrossRef]
- Gaertig, J.; Thatcher, T.H.; Gu, L.; Gorovsky, M.A. Electroporation-mediated replacement of a positively and negatively selectable beta-tubulin gene in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 1994, 91, 4549–4553. [Google Scholar] [CrossRef] [Green Version]
- Pigino, G.; Ishikawa, T. Axonemal Radial Spokes: 3D Structure, Function and Assembly. Bioarchitecture 2012, 2, 50–58. [Google Scholar] [CrossRef]
- Oda, T.; Yanagisawa, H.; Kamiya, R.; Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 2014, 346, 857–860. [Google Scholar] [CrossRef]
- Yagi, T.; Uematsu, K.; Liu, Z.; Kamiya, R. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 2009, 122, 1306–1314. [Google Scholar] [CrossRef]
- Wilkes, D.E.; Watson, H.E.; Mitchell, D.R.; Asai, D.J. Twenty-five dyneins in Tetrahymena: A re-examination of the multidynein hypothesis. Cell Motil. Cytoskelet. 2008, 65, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Kabi, A.; Gray, S.F.; Pennock, D. p28 dynein light chains and ciliary motility in Tetrahymena thermophila. Cytoskeleton 2016, 73, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Bazan, R.; Schröfel, A.; Joachimiak, E.; Poprzeczko, M.; Pigino, G.; Wloga, D. Ccdc113/Ccdc96 complex, a novel regulator of ciliary beating that connects radial spoke 3 to dynein g and the nexin link. PLOS Genet. 2021, 17, e1009388. [Google Scholar] [CrossRef] [PubMed]
- Ralston, K.S.; Lerner, A.G.; Diener, D.R.; Hill, K.L. Flagellar Motility Contributes to Cytokinesis in Trypanosoma brucei and Is Modulated by an Evolutionarily Conserved Dynein Regulatory System. Eukaryot. Cell 2006, 5, 696–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuser, T.; Raytchev, M.; Krell, J.; Porter, M.E.; Nicastro, D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 2009, 187, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996, 12, 543–548. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B. GeneDoc: Analysis and visualization of genetic variation. Embnew. News. 1997, 4, 14. [Google Scholar]



| Protein Name | Localization | Number in TGD | Mw (kDa) | Number of Identified Peptides | |||||
|---|---|---|---|---|---|---|---|---|---|
| WT | Cfap91-HA-BirA* | ||||||||
| Exp 1 | Exp 2 | Exp 3 | Exp 1 | Exp 2 | Exp 3 | ||||
| Cfap91 | RS base | TTHERM_00578560 | 76 | 0/0 | 0/0 | 0/0 | 11/10 | 7/6 | 11/10 |
| Cfap206 | RS2 base | TTHERM_00820660 | 74 | 0/0 | 0/0 | 0/0 | 9/9 | 5/5 | 12/12 |
| Cfap61 | RS3 stalk | TTHERM_00641200 | 199 | 0/0 | 0/0 | 0/0 | 26/24 | 13/13 | 22/22 |
| Cfap251 | RS3 base | TTHERM_01262850 | 115 | 0/0 | 0/0 | 0/0 | 22/22 | 12/12 | 12/11 |
| Rsp3B | RS stalk | TTHERM_00566810 | 80 | 0/0 | 0/0 | 0/0 | 7/7 | 4/4 | 13/11 |
| Rsp3C | RS stalk | TTHERM_00418270 | 111 | 0/0 | 0/0 | 0/0 | 15/15 | 9/9 | 9/7 |
| Rsp8 | RS stalk | TTHERM_00313520 | 65 | 0/0 | 0/0 | 0/0 | 6/6 | 4/4 | 8/7 |
| EF-hand | unknown | TTHERM_00492840 | 67 | 0/0 | 0/0 | 0/0 | 9/9 | 5/3 | 7/7 |
| Hcfc1-like | unknown | TTHERM_00760390 | 48 | 0/0 | 0/0 | 0/0 | 4/4 | 2/2 | 3/3 |
| Ak8 | unknown | TTHERM_00227800 | 50 | 0/0 | 0/0 | 0/0 | 20/18 | 11/11 | 1/1 |
| Gk1 | unknown | TTHERM_00781030 | 31 | 0/0 | 0/0 | 0/0 | 6/5 | 2/2 | 3/3 |
| 𝛼-tubulin | MT | TTHERM_00558620 | 50 | 4/4 | 21/11 | 3/3 | 14/13 | 10/9 | 5/5 |
| β-tubulin | MT | TTHERM_00348510 | 50 | 4/4 | 21/9 | 4/4 | 16/14 | 11/11 | 8/7 |
| Name. | TGD IDTTHERM_ | Subunit of the Structure | Number of Peptides | Ratio KO/WT | Mean Number of Peptides from 3 Replicates | Ratio KO/WT | ||
|---|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | |||||||
| WT | 91-KO | WT | 91-KO | |||||
| Cfap91 | 00578560 | RS2/RS3 base | 33 | 0 | 0 | 32 | 0 | 0 |
| Cfap206 | 00820660 | RS2 base | 31 | 1 | 0.032 | 27.7 | 0.3 | 0.01 |
| Cfap61 | 00641200 | RS3 stalk | 61 | 10 | 0.164 | 60 | 8.7 | 0.14 |
| Cfap251 | 01262850 | arch at the RS3 base | 51 | 3 | 0.059 | 47.7 | 0.3 | 0.007 |
| Outer Dynein Arms (ODA) Heavy Chains | ||||||||
| Dyh3 | 01276420 | ODA | 284 | 258 | 0.908 | 314 | 263 | 0.86 |
| Dyh4 | 00499300 | ODA | 302 | 272 | 0.9 | 335 | 290 | 0.86 |
| Dyh5 | 00486600 | ODA | 252 | 244 | 0.968 | 278 | 221 | 0.79 |
| Two-Headed Inner Dynein Arm (IDAf/I1) Heavy Chains | ||||||||
| Dyh6 | 00688470 | IDA f/I1 | 170 | 145 | 0.853 | 158 | 157 | 0.99 |
| Dyh7 | 00912290 | IDA f/I1 | 166 | 136 | 0.819 | 148 | 152 | 1.02 |
| Single-Headed Inner Dynein Arms (IDA) Heavy Chains | ||||||||
| Dyh8 | 00531870 | ? | 13 | 8 | 0.615 | 14.3 | 8.7 | 0.6 |
| Dyh9 | 00947430 | ? | 29 | 28 | 0.966 | 23.7 | 37.3 | 1.58 |
| Dyh10 | 00420340 | dynein c | 8 | 1 | 0.125 | 12 | 3 | 0.25 |
| Dyh11 | 00252430 | ? | 121 | 83 | 0.686 | 124 | 89 | 0.72 |
| Dyh12 | 00919540 | dynein c | 56 | 4 | 0.071 | 46.7 | 3 | 0.06 |
| Dyh13 | 01104900 | ? | 0 | 0 | - | 12 | 2.7 | 0.22 |
| Dyh14 | 00492830 | ? | 53 | 58 | 1.094 | 38.7 | 49 | 1.26 |
| Dyh15 | 00433800 | dynein g | 150 | 129 | 0.86 | 152 | 110 | 0.72 |
| Dyh16 | 00558640 | dynein d | 122 | 49 | 0.402 | 93.7 | 45 | 0.48 |
| Dyh17 | 00850620 | ? | 0 | 0 | - | 0 | 0 | - |
| Dyh18 | 00047540 | ? | 3 | 3 | 1 | 0 | 0 | - |
| Dyh19 | 01027670 | ? | 112 | 81 | 0.723 | 92.3 | 91.7 | 0.99 |
| Dyh20 | 00821980 | dynein d | 23 | 14 | 0.609 | 19 | 16 | 0.84 |
| Dyh22 | 00565600 | dynein g | 130 | 44 | 0.338 | 113 | 55 | 0.49 |
| Dyh23 | 00355100 | dynein g | 5 | 2 | 0.4 | 7.3 | 3.7 | 0.5 |
| Dyh24 | 00193520 | dynein g | 95 | 27 | 0.284 | 85.7 | 22.7 | 0.26 |
| Dyh25 | 00774810 | dynein c | 61 | 4 | 0.066 | 58.3 | 10.3 | 0.18 |
| p28A | 00841210 | IDA | 19 | 9 | 0.47 | 17 | 14.3 | 0.84 |
| p28B | 00319990 | IDA | 8 | 3 | 0.38 | 17.3 | 6.3 | 0.37 |
| p28C | 01129720 | IDA | 14 | 11 | 0.78 | 15.7 | 8.7 | 0.55 |
| Central Pair (CP) and Tether/Tether Head (T/TH) Proteins | ||||||||
| Pf16 | 000157929 | CP | 24 | 20 | 0.833 | 37.7 | 32.7 | 0.87 |
| Pf20 | 00134890 | CP | 33 | 35 | 1.060 | 29.3 | 23.7 | 0.81 |
| Hydin | 00551040 | CP | 183 | 202 | 1.103 | 159.7 | 169.3 | 1.06 |
| Cfap43 | 00196190 | T/TH | 80 | 70 | 0.87 | 65.3 | 69.7 | 1.07 |
| Cfap44 | 00498220 | T/TH | 90 | 81 | 0.9 | 82 | 72.7 | 0.88 |
| Calculated pI/Mw: | Cfap91 | Cfap61 | Cfap251 | Cfap206 |
|---|---|---|---|---|
| without a 3HA tag | 7.25/76 kDa | 5.41/198 kDa | 4.75/114 kDa | 6.03/73 kDa |
| with a 3HA tag | 6.23/79.5 kDa | 5.32/202 kDa | 4.68/118 kDa | 5.48/77 kDa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicka, M.; Joachimiak, E.; Urbanska, P.; Osinka, A.; Konopka, A.; Bulska, E.; Wloga, D. Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia. Cells 2022, 11, 4048. https://doi.org/10.3390/cells11244048
Bicka M, Joachimiak E, Urbanska P, Osinka A, Konopka A, Bulska E, Wloga D. Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia. Cells. 2022; 11(24):4048. https://doi.org/10.3390/cells11244048
Chicago/Turabian StyleBicka, Marta, Ewa Joachimiak, Paulina Urbanska, Anna Osinka, Anna Konopka, Ewa Bulska, and Dorota Wloga. 2022. "Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia" Cells 11, no. 24: 4048. https://doi.org/10.3390/cells11244048
APA StyleBicka, M., Joachimiak, E., Urbanska, P., Osinka, A., Konopka, A., Bulska, E., & Wloga, D. (2022). Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia. Cells, 11(24), 4048. https://doi.org/10.3390/cells11244048







