Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Analysis of Histone Levels in Plasma Samples
2.3. Analysis of H3 Citrullination
2.4. Cell Culture and Experimental Design
2.5. Histone Extraction, Purification, and In Vitro Citrullination
2.6. Cell Viability and Cell Death Determination
2.7. Western Blot
2.8. RNA Isolation and Purification and Quantitative Real-Time PCR Assay (RT-qPCR)
2.9. Statistical Analysis
3. Results
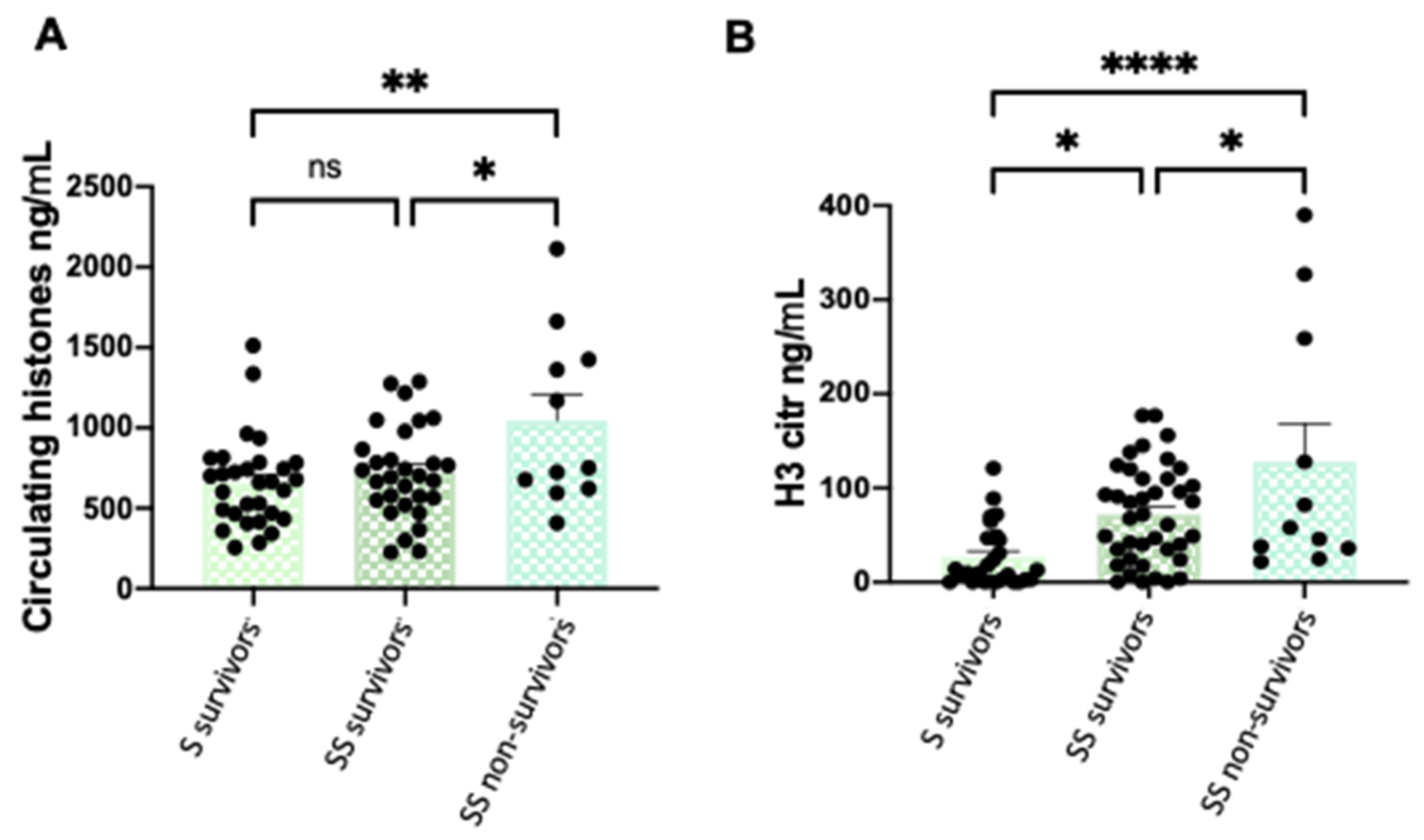
3.1. Circulating and Citrullinated H3 Levels are Higher in Patients with Severe Phenotypes
3.2. Correlations between Clinical Features and Circulating and H3 Citrullinated Histones
3.3. Extracellular Citrullinated Histones Do Not Affect to Cellular Viability in HUVEC
3.4. Native Extracellular Histones Induce Oxidative Stress and Activate Antioxidant Response While Citrullinated Histones Do Not Alter the Oxidative Patterns
3.5. Prostanoid Biosynthesis is Altered by Extracellular Histones in HUVECs
3.6. Extracellular Histones Induce Molecular Pathways of Inflammation and Modulate Pathways of Prostanoidbiosynthesis
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it ? Blood 2013, 122, 2784–2795. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv Prepr. Serv. Health Sci. 2020, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 1, e138999. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, X. The Role of Histones and Heparin in Sepsis: A Review. J. Intensiv. Care Med. 2021, 37, 319–326. [Google Scholar] [CrossRef]
- Zhou, M.; Aziz, M.; Wang, P. Damage-Associated Molecular Patterns as Double-Edged Swords in Sepsis. Antioxidants Redox Signal. 2021, 35, 1308–1323. [Google Scholar] [CrossRef]
- Ginsburg, I.; Koren, E.; Varani, J.; Kohen, R. Nuclear histones: Major virulence factors or just additional early sepsis markers? A comment. Inflammopharmacology 2016, 24, 287–289. [Google Scholar] [CrossRef]
- Bowman, G.D.; Poirier, M.G. Post-Translational Modifications of Histones That Influence Nucleosome Dynamics. Chem. Rev. 2014, 115, 2274–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baka, Z.; György, B.; Géher, P.; Buzás, E.I.; Falus, A.; Nagy, G. Citrullination under physiological and pathological conditions. Jt. Bone Spine 2012, 79, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Radic, M. Knotting the NETs: Analyzing histone modifications in neutrophil extracellular traps. Arthritis Res. Ther. 2012, 14, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Bolt, M.; Guertin, M.J.; Chen, W.; Zhang, S.; Cherrington, B.D.; Slade, D.J.; Dreyton, C.J.; Subramanian, V.; Bicker, K.L.; et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor α target gene activation. Proc. Natl. Acad. Sci. USA 2012, 109, 13331–13336. [Google Scholar] [CrossRef] [Green Version]
- Cherrington, B.D.; Zhang, X.; McElwee, J.L.; Morency, E.; Anguish, L.J.; Coonrod, S.A. Potential Role for PAD2 in Gene Regulation in Breast Cancer Cells. PLoS ONE 2012, 7, e41242. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, T.; Nakashima, K.; Hirano, H.; Senshu, T.; Yamada, M. Deimination of Arginine Residues in Nucleophosmin/B23 and Histones in HL-60 Granulocytes. Biochem. Biophys. Res. Commun. 2002, 290, 979–983. [Google Scholar] [CrossRef]
- Nakashima, K.; Hagiwara, T.; Yamada, M. Nuclear Localization of Peptidylarginine Deiminase V and Histone Deimination in Granulocytes. J. Biol. Chem. 2002, 277, 49562–49568. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, G.L.; Daujat, S.; Snowden, A.W.; Erdjument-Bromage, H.; Hagiwara, T.; Yamada, M.; Schneider, R.; Gregory, P.D.; Tempst, P.; Bannister, A.J.; et al. Histone Deimination Antagonizes Arginine Methylation. Cell 2004, 118, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.-H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 Regulates Histone Arginine Methylation Levels via Demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef]
- Christophorou, M.A.; Castelo-Branco, G.; Halley-Stott, R.P.; Oliveira, C.S.; Loos, R.; Radzisheuskaya, A.; Mowen, K.A.; Bertone, P.; Silva, J.C.R.; Zernicka-Goetz, M.; et al. Citrullination regulates pluripotency and histone H1 binding to chroma-tin. Nature 2014, 507, 104–108. [Google Scholar] [CrossRef]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arter. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2020, 117, 60–73. [Google Scholar] [CrossRef]
- Mena, H.A.; Carestia, A.; Scotti, L.; Parborell, F.; Schattner, M.; Negrotto, S. Extracellular histones reduce survival and an-giogenic responses of late outgrowth progenitor and mature endothelial cells. J. Thromb. Haemost. 2016, 14, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.-P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef] [Green Version]
- Hack, C.E.; Zeerleder, S. The endothelium in sepsis: Source of and a target for inflammation. Crit. Care Med. 2001, 29, S21–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Stöcklin, E.; Wissler, M.; Gouilleux, F.; Groner, B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature 1996, 383, 726–728. [Google Scholar] [CrossRef]
- Chow, J.C.; Ling, P.R.; Qu, Z.; Laviola, L.; Ciccarone, A.; Bistrian, B.R.; Smith, R.J. Growth hormone stimulates tyrosine phosphorylation of JAK2 and STAT5, but not insulin receptor substrate-1 or SHC proteins in liver and skeletal muscle of normal rats in vivo. Endocrinology 1996, 137, 2880–2886. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yang, Y.; Hou, J.; Shu, Q.; Yin, Y.; Fu, W.; Han, F.; Hou, T.; Zeng, C.; Nemeth, E.; et al. Increased gene copy number of DEFA1/DEFA3 worsens sepsis by inducing endothelial pyroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 3161–3170. [Google Scholar] [CrossRef] [Green Version]
- Lüscher, T.F.; Tanner, F.C. Endothelial Regulation of Vascular Tone and Growth. Am. J. Hypertens. 1993, 6, 283S–293S. [Google Scholar] [CrossRef]
- Nakahara, M.; Ito, T.; Kawahara, K.-I.; Yamamoto, M.; Nagasato, T.; Shrestha, B.; Yamada, S.; Miyauchi, T.; Higuchi, K.; Takenaka, T.; et al. Recombinant Thrombomodulin Protects Mice against Histone-Induced Lethal Thromboembolism. PLoS ONE 2013, 8, e75961. [Google Scholar] [CrossRef] [Green Version]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating Histones Are Mediators of Trauma-associated Lung Injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cremades, D.; Bueno-Betí, C.; García-Giménez, J.L.; Ibañez-Cabellos, J.S.; Hermenegildo, C.; Pallardó, F.V.; Novella, S. Extracellular histones disarrange vasoactive mediators release through a COX-NOS interaction in human endothelial cells. J. Cell. Mol. Med. 2017, 21, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Cabellos, J.S.; Aguado, C.; Pérez-Cremades, D.; García-Giménez, J.L.; Bueno-Betí, C.; García-López, E.M.; Romá-Mateo, C.; Novella, S.; Hermenegildo, C.; Pallardó, F.V. Extracellular histones activate autophagy and apoptosis via mTOR signaling in human endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3234–3246. [Google Scholar] [CrossRef]
- Eitel, J.; Meixenberger, K.; Suttorp, N.; Opitz, B. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. Thromb. Haemost. 2009, 102, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Shechter, D.; Dormann, H.L.; Allis, C.D.; Hake, S.B. Extraction, purification and analysis of histones. Nat. Protoc. 2007, 2, 1445–1457. [Google Scholar] [CrossRef]
- Mao, L.; Mostafa, R.; Ibili, E.; Fert-Bober, J. Role of protein deimination in cardiovascular diseases: Potential new avenues for diagnostic and prognostic biomarkers. Expert Rev. Proteom. 2021, 18, 1059–1071. [Google Scholar] [CrossRef]
- Beltran-Garcia, J.; Osca-Verdegal, R.; García-Giménez, J.L.; Pallardó, F.V. Oxidative stress leads to severe phenotypes in sepsis through activation of NLRP3-pyroptosis. Redox Exp. Med. 2022, 2022, R84–R95. [Google Scholar] [CrossRef]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the Role of Nitric Oxide and Its Clinical Applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Kamal, M.A.; Poddar, N. Integrated Pathways of COX-2 and mTOR: Roles in Cell Sensing and Alzheimer’s Disease. Front. Neurosci. 2020, 14, 693. [Google Scholar] [CrossRef] [PubMed]
- Seibert, K.; Masferrer, J.; Zhang, Y.; Gregory, S.; Olson, G.; Häuser, S.; Leahy, K.; Perkins, W.; Isakson, P. Mediation of Inflammation by Cyclooxygenase-2. In Novel Molecular Approaches to Anti-Inflammatory Therapy; Birkhäuser Basel: Basel, Switzerland, 1995; pp. 41–50. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Dayang, E.-Z.; Plantinga, J.; ter Ellen, B.; van Meurs, M.; Molema, G.; Moser, J. Identification of LPS-Activated Endothelial Subpopulations With Distinct Inflammatory Phenotypes and Regulatory Signaling Mechanisms. Front. Immunol. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The role of extracellular histone in organ injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wan, D.; Luo, X.; Song, T.; Wang, Y.; Yu, Q.; Jiang, L.; Liao, R.; Zhao, W.; Su, B. Circulating Histones in Sepsis: Po-tential Outcome Predictors and Therapeutic Targets. Front. Immunol. 2021, 12, 650184. [Google Scholar] [CrossRef] [PubMed]
- Chaput, C.; Zychlinsky, A. Sepsis: The dark side of histones. Nat. Med. 2009, 15, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, C.; Leso, A.; Nefedova, Y. Quantification of Citrullinated Histone H3 Bound DNA for Detection of Neutrophil Extracellular Traps. Cancers 2020, 12, 3424. [Google Scholar] [CrossRef]
- Tsourouktsoglou, T.-D.; Warnatsch, A.; Ioannou, M.; Hoving, D.; Wang, Q.; Papayannopoulos, V. Histones, DNA, and Citrullination Promote Neutrophil Extracellular Trap Inflammation by Regulating the Localization and Activation of TLR4. Cell Rep. 2020, 31, 107602. [Google Scholar] [CrossRef]
- Radic, M. NETosis and ETosis: Incompletely Understood Types of Granulocyte Death and their Proposed Adaptive Benefits and Costs. In Apoptosis and Beyond: The Many Ways Cells Die; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 511–534. [Google Scholar] [CrossRef]
- Tan, C.; Aziz, M.; Wang, P. The vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The changing immune system in sepsis. Virulence 2013, 5, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the adaptive immune response in sepsis. Intensiv. Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Romá-Mateo, C.; Carbonell, N.; Ferreres, J.; Rodríguez, M.; Mulet, S.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic biomarkers for human sepsis and septic shock: Insights from immunosup-pression. Epigenomics 2020, 12, 617–646. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Wardini, A.B.; Pinto-Da-Silva, L.H.; Saraiva, E.M. ETosis: A Microbicidal Mechanism beyond Cell Death. J. Parasitol. Res. 2012, 2012, 929743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogos, C.; Kotsaki, A.; Pelekanou, A.; Giannikopoulos, G.; Vaki, I.; Maravitsa, P.; Adamis, S.; Alexiou, Z.; Andrianopoulos, G.; Antonopoulou, A.; et al. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit. Care 2010, 14, R96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-García, J.; Osca-Verdegal, R.; Jávega, B.; Herrera, G.; O’Connor, J.-E.; García-López, E.; Casabó-Vallés, G.; Rodriguez-Gimillo, M.; Ferreres, J.; Carbonell, N.; et al. Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock. Biomedicines 2022, 10, 525. [Google Scholar] [CrossRef]
- Ekaney, M.L.; Otto, G.P.; Sossdorf, M.; Sponholz, C.; Boehringer, M.; Loesche, W.; Rittirsch, D.; Wilharm, A.; Kurzai, O.; Bauer, M.; et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 2014, 18, 543. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Romá-Mateo, C.; Carbonell, N.; Palacios, L.; Peiró-Chova, L.; García-López, E.; García-Simón, M.; Lahuerta, R.; Gimenez-Garzó, C.; Berenguer-Pascual, E.; et al. A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci. Rep. 2017, 7, 10643. [Google Scholar] [CrossRef]
- van Vught, L.A.; Uhel, F.; Ding, C.; Veer, C.V.; Scicluna, B.P.; Peters-Sengers, H.; Klouwenberg, P.M.C.K.; Nürnberg, P.; Cremer, O.L.; Schultz, M.J.; et al. Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis. J. Thromb. Haemost. 2021, 19, 1049–1063. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ito, T.; Yasuda, T.; Furubeppu, H.; Kamikokuryo, C.; Yamada, S.; Maruyama, I.; Kakihana, Y. Circulating histone H3 levels in septic patients are associated with coagulopathy, multiple organ failure, and death: A single-center observational study. Thromb. J. 2019, 17, 1. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Pérez-Cremades, D.; Novella, S.; Hermenegildo, C.; Pallardó, F.V.; García-Giménez, J.L. Extracellular Histones Activate Endothelial NLRP3 Inflammasome and are Associated with a Severe Sepsis Phenotype. J. Inflamm. Res. 2022, 15, 4217–4238. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xu, J.-W. NETosis as a Pathogenic Factor for Heart Failure. Oxidative Med. Cell. Longev. 2021, 2021, 6687096. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; ten Cate, H.; Hofstra, L.; et al. Elevated Levels of Circulating DNA and Chromatin Are Independently Associated With Severe Coronary Atherosclerosis and a Prothrombotic State. Arter. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varjú, I.; Sorvillo, N.; Cherpokova, D.; Farkas, Z.; Farkas, V.J.; Komorowicz, E.; Feller, T.; Kiss, B.; Kellermayer, M.Z.; Szabó, L.; et al. Citrullinated Fibrinogen Renders Clots Mechanically Less Stable, but Lysis-Resistant. Circ. Res. 2021, 129, 342–344. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eit-zman, D.T.; et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Re-sponses in Murine Models of Atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Martinod, K.; Witsch, T.; Erpenbeck, L.; Savchenko, A.; Hayashi, H.; Cherpokova, D.; Gallant, M.; Mauler, M.; Cifuni, S.M.; Wagner, D.D. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J. Exp. Med. 2016, 214, 439–458. [Google Scholar] [CrossRef]
- Tilvawala, R.; Nemmara, V.V.; Reyes, A.C.; Sorvillo, N.; Salinger, A.J.; Cherpokova, D.; Fukui, S.; Gutch, S.; Wagner, D.; Thompson, P.R. The role of SERPIN citrullination in thrombosis. Cell Chem. Biol. 2021, 28, 1728–1739.e5. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Rubenfeld, G.D. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr. Infect. Dis. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [Green Version]
- Courtright, K.R.; Jordan, L.; Murtaugh, C.M.; Barrón, Y.; Deb, P.; Moore, S.; Bowles, K.H.; Mikkelsen, M.E. Risk Factors for Long-term Mortality and Patterns of End-of-Life Care among Medicare Sepsis Survivors Discharged to Home Health Care. JAMA Netw. Open 2020, 3, e200038. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, N.; Ammollo, C.T.; Semeraro, F.; Colucci, M. SEPSIS-ASSOCIATED DISSEMINATED INTRAVASCULAR COAGULATION AND THROMBOEMBOLIC DISEASE. Mediterr. J. Hematol. Infect. Dis. 2010, 2, e2010024. [Google Scholar] [CrossRef] [PubMed]
- Delabranche, X.; Quenot, J.-P.; Lavigne, T.; Mercier, E.; François, F.; Severac, F.; Grunebaum, L.; Mehdi, M.; Zobairi, F.; Toti, F.; et al. Early Detection of Disseminated Intravascular Coagulation During Septic Shock. Crit. Care Med. 2016, 44, e930–e939. [Google Scholar] [CrossRef] [PubMed]
- Kaplan JM Neutrophil extracelullar traps (NETs):Double-edged swords of innate immunity 1. J. Immunol. 2013, 189, 2689–2695.
- Cheng, Z.; Abrams, S.T.; Alhamdi, Y.; Toh, J.; Yu, W.; Wang, G.; Toh, C.-H. Circulating Histones Are Major Mediators of Multiple Organ Dysfunction Syndrome in Acute Critical Illnesses. Crit. Care Med. 2019, 47, e677–e684. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Hoeksema, M.; van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Futur. Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef]








| Protein | Peptide |
|---|---|
| Histone H2B | LLLPGELAK |
| Histone H3 | STELLIR |
| Histone H4 | DNIQGITKPAIR and VFLENVIR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osca-Verdegal, R.; Beltrán-García, J.; Paes, A.B.; Nacher-Sendra, E.; Novella, S.; Hermenegildo, C.; Carbonell, N.; García-Giménez, J.L.; Pallardó, F.V. Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation. Cells 2022, 11, 4070. https://doi.org/10.3390/cells11244070
Osca-Verdegal R, Beltrán-García J, Paes AB, Nacher-Sendra E, Novella S, Hermenegildo C, Carbonell N, García-Giménez JL, Pallardó FV. Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation. Cells. 2022; 11(24):4070. https://doi.org/10.3390/cells11244070
Chicago/Turabian StyleOsca-Verdegal, Rebeca, Jesús Beltrán-García, Ana B. Paes, Elena Nacher-Sendra, Susana Novella, Carlos Hermenegildo, Nieves Carbonell, José Luis García-Giménez, and Federico V. Pallardó. 2022. "Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation" Cells 11, no. 24: 4070. https://doi.org/10.3390/cells11244070
APA StyleOsca-Verdegal, R., Beltrán-García, J., Paes, A. B., Nacher-Sendra, E., Novella, S., Hermenegildo, C., Carbonell, N., García-Giménez, J. L., & Pallardó, F. V. (2022). Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation. Cells, 11(24), 4070. https://doi.org/10.3390/cells11244070








