A Combined Risk Score Model to Assess Prognostic Value in Patients with Soft Tissue Sarcomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Sarcoma Datasets
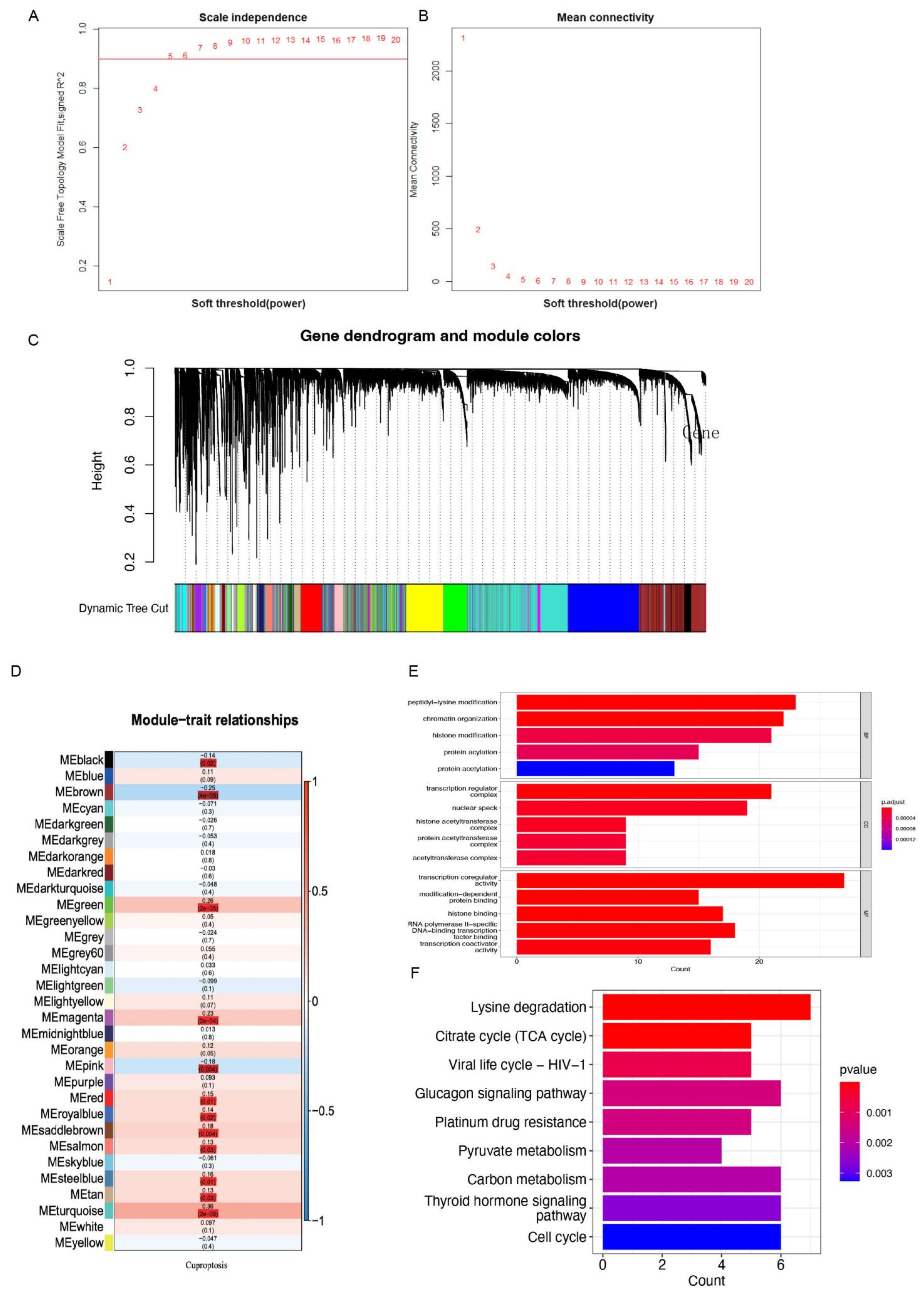
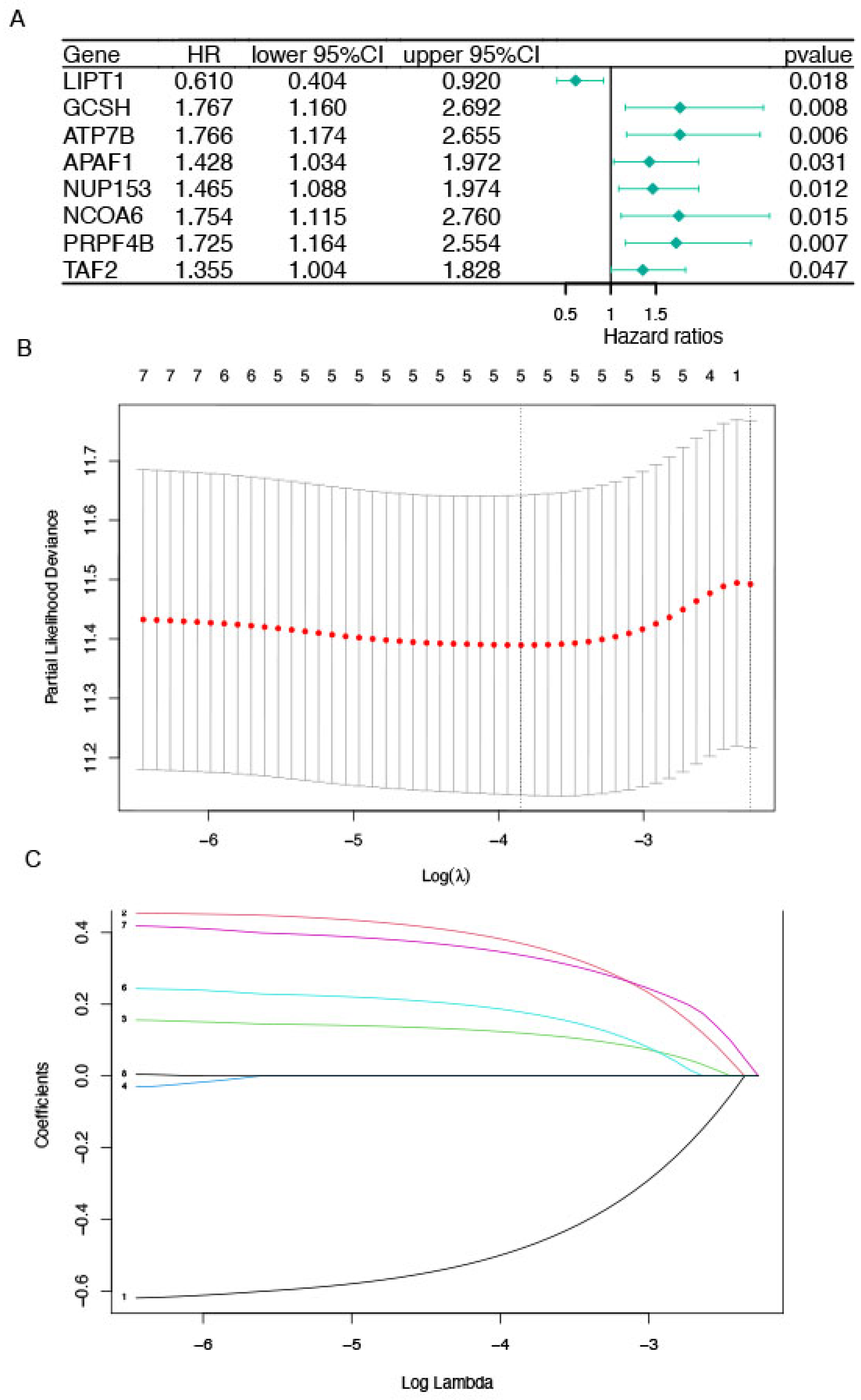
2.2. Identification of Prognostic CRGs
2.3. Functional Analyses and Mechanism Investigation
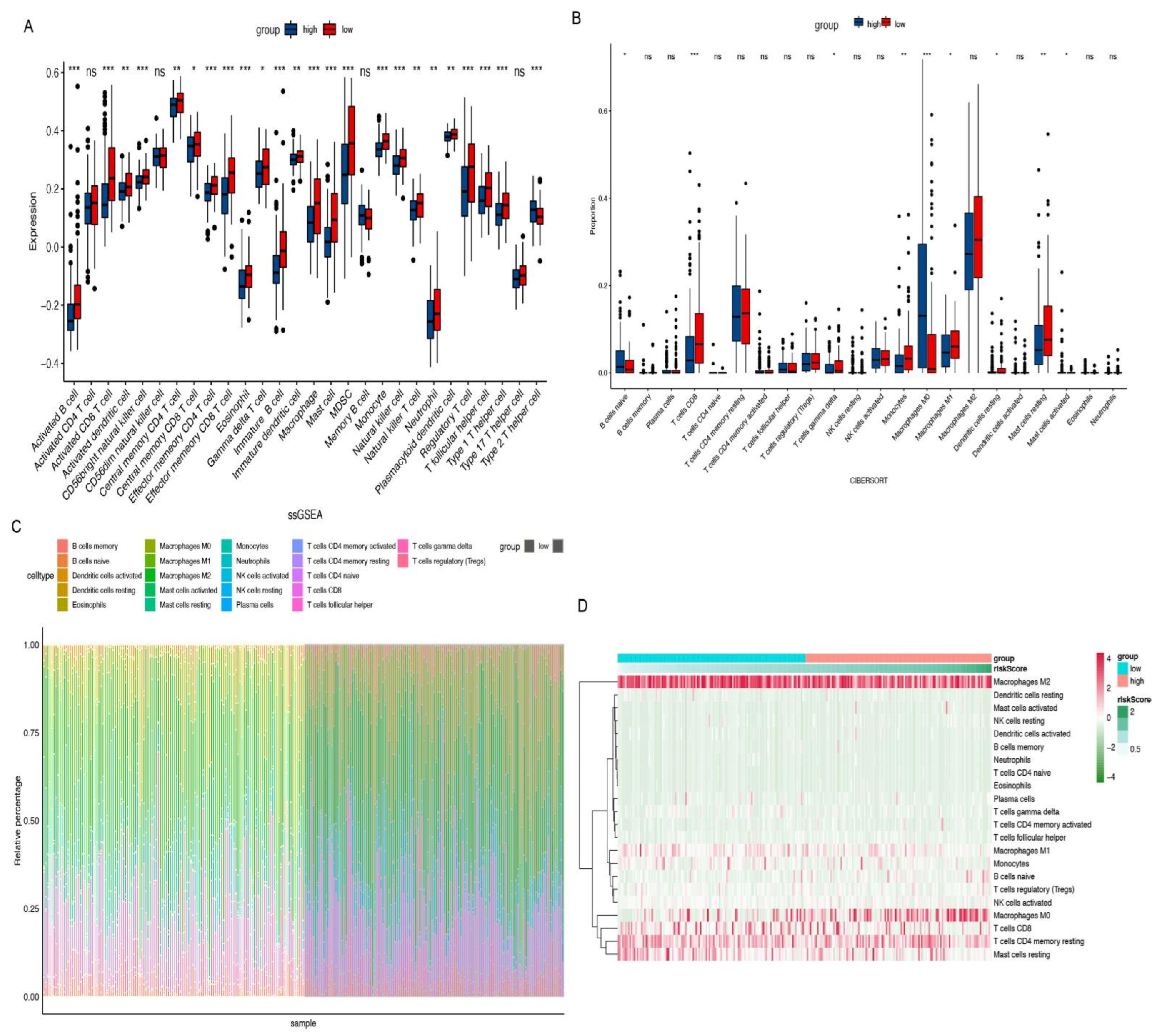
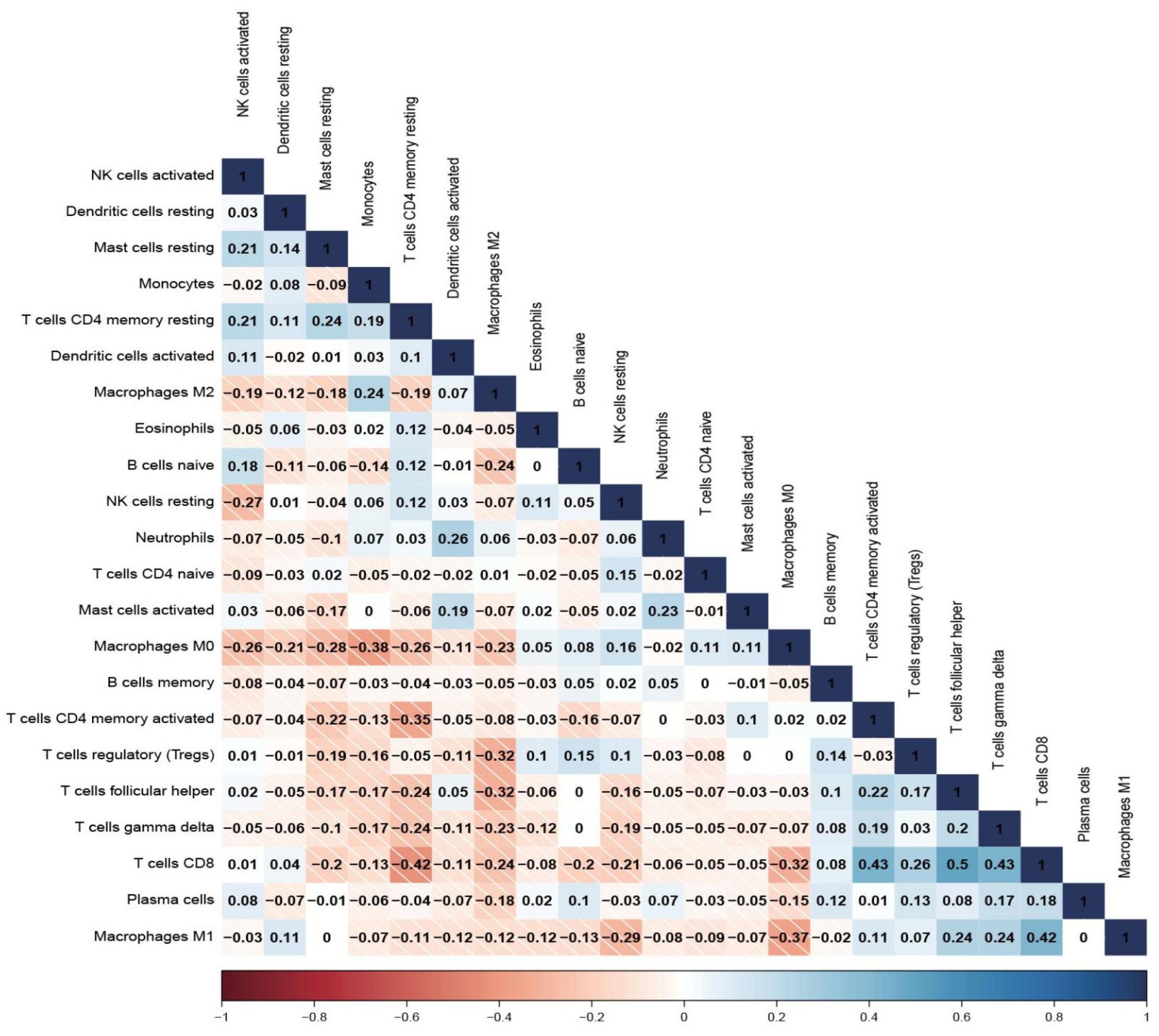
2.4. Immune Infiltration Analysis
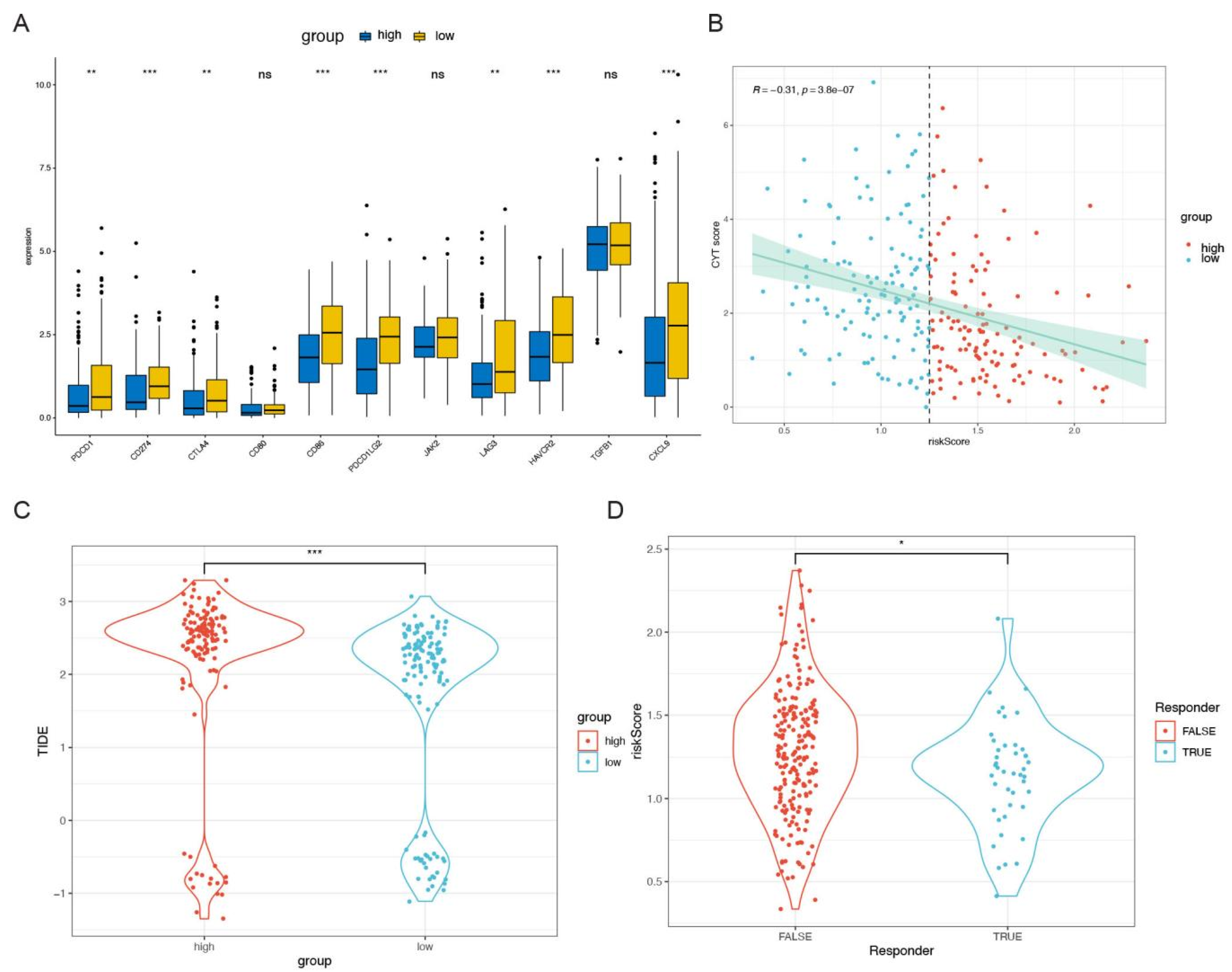
2.5. Exploration the Relationship between TME and Risk Scores
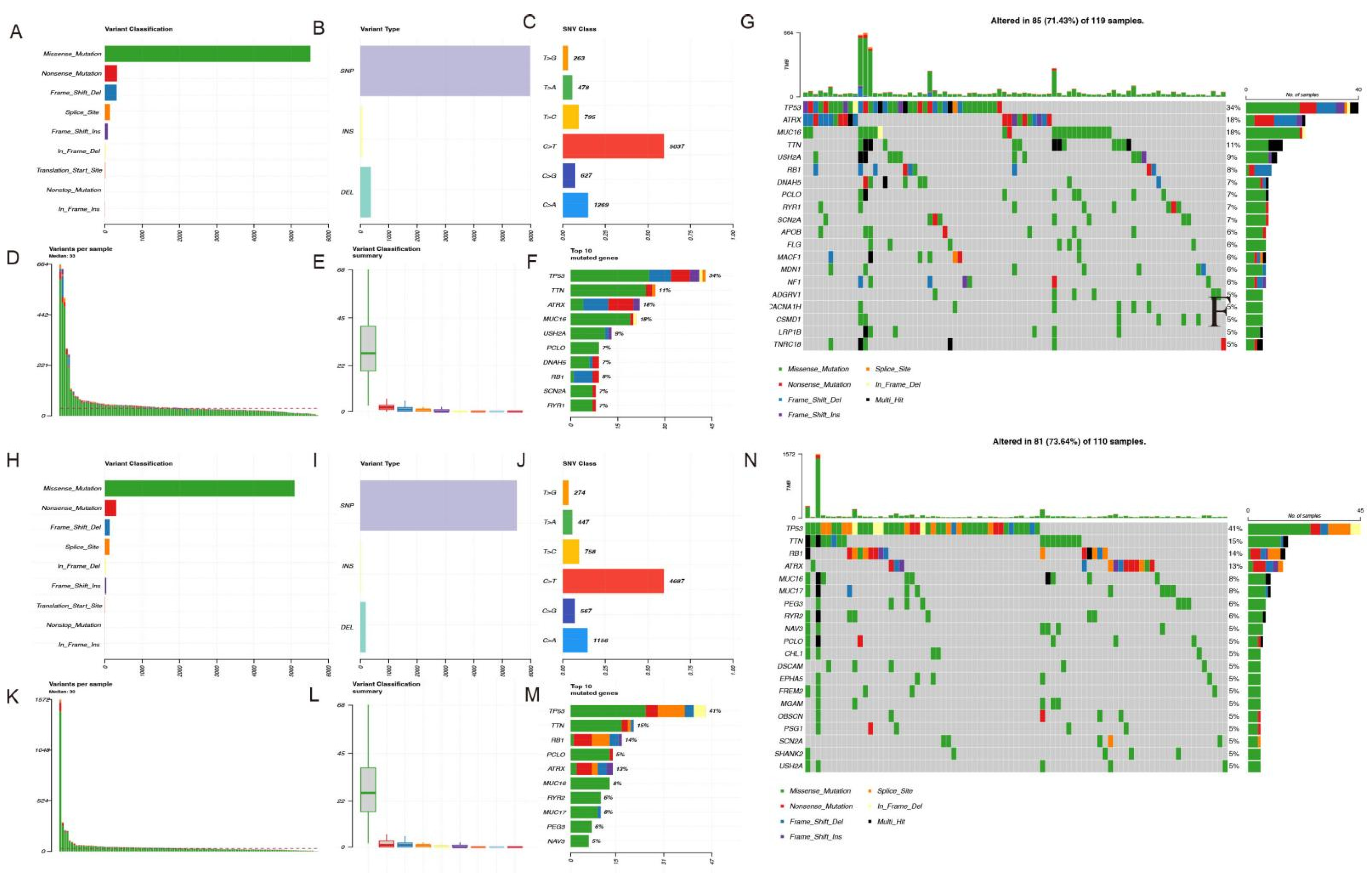
2.6. Mutation Landscape Analysis
2.7. Clinical Specimens
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analyses
3. Result
3.1. Identification of the Cuproptosis Subtypes and Prognostic CRGs
3.2. Determination of CRGs and Validation
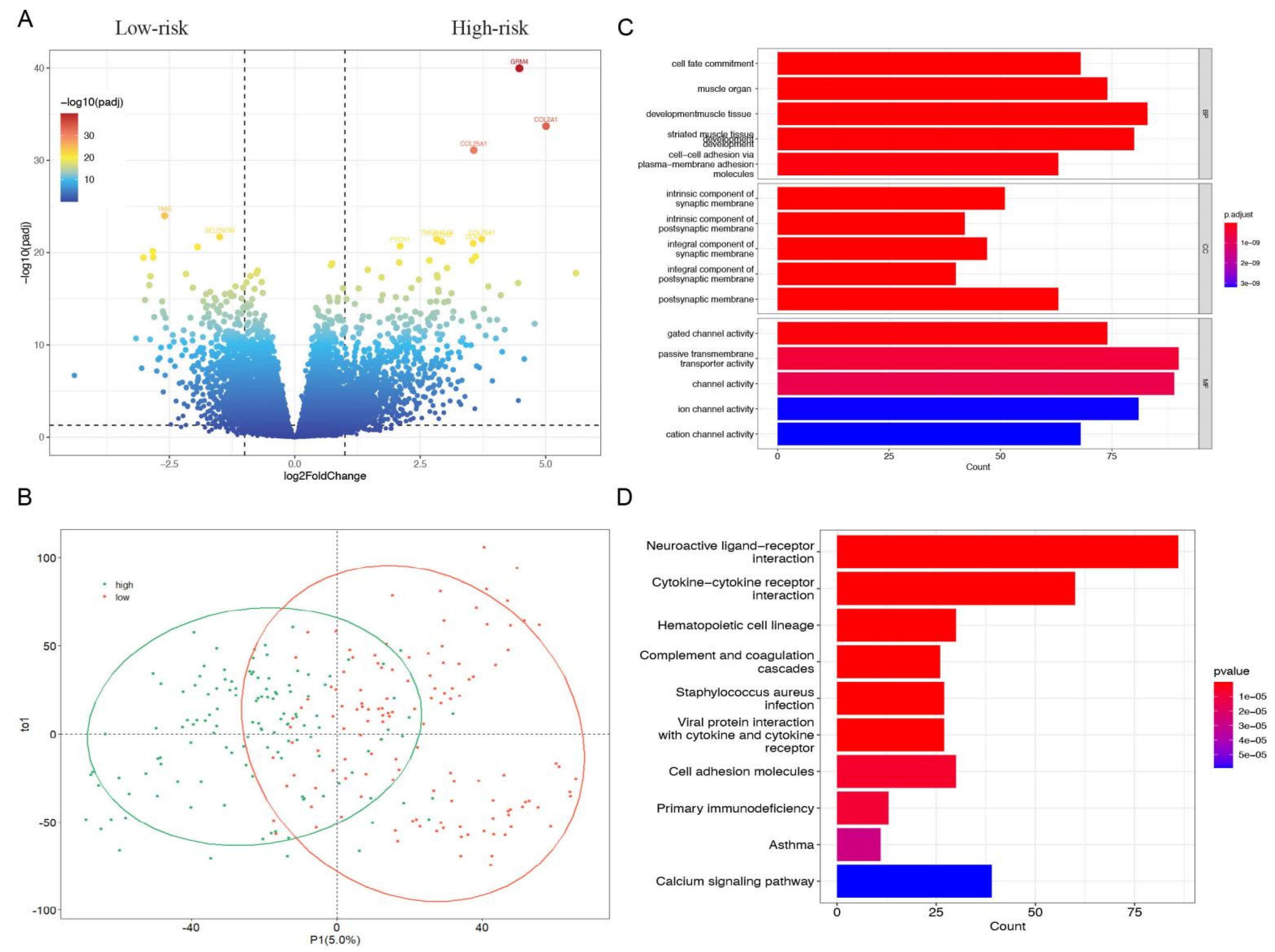
3.3. GO Terms and KEGG Pathway Analysis of the Differentially Expressed Genes in High- and Low-Risk Group
3.4. Biological Characteristics and TME Investigation
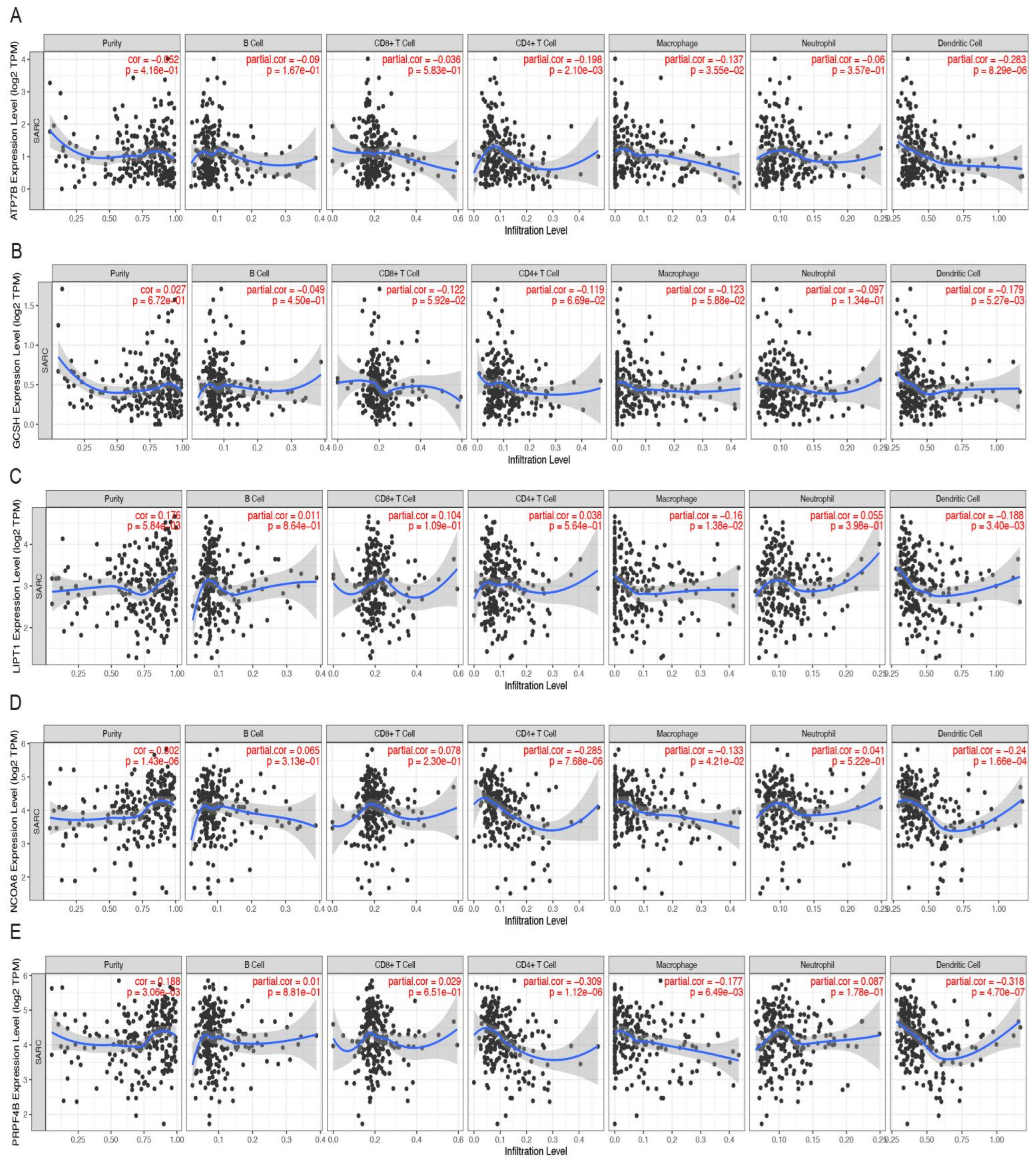
3.5. Hub CRGs Immune Infiltration Analysis
3.6. Correlation between Molecular Features and Risk
3.7. Mutation Landscape Analysis
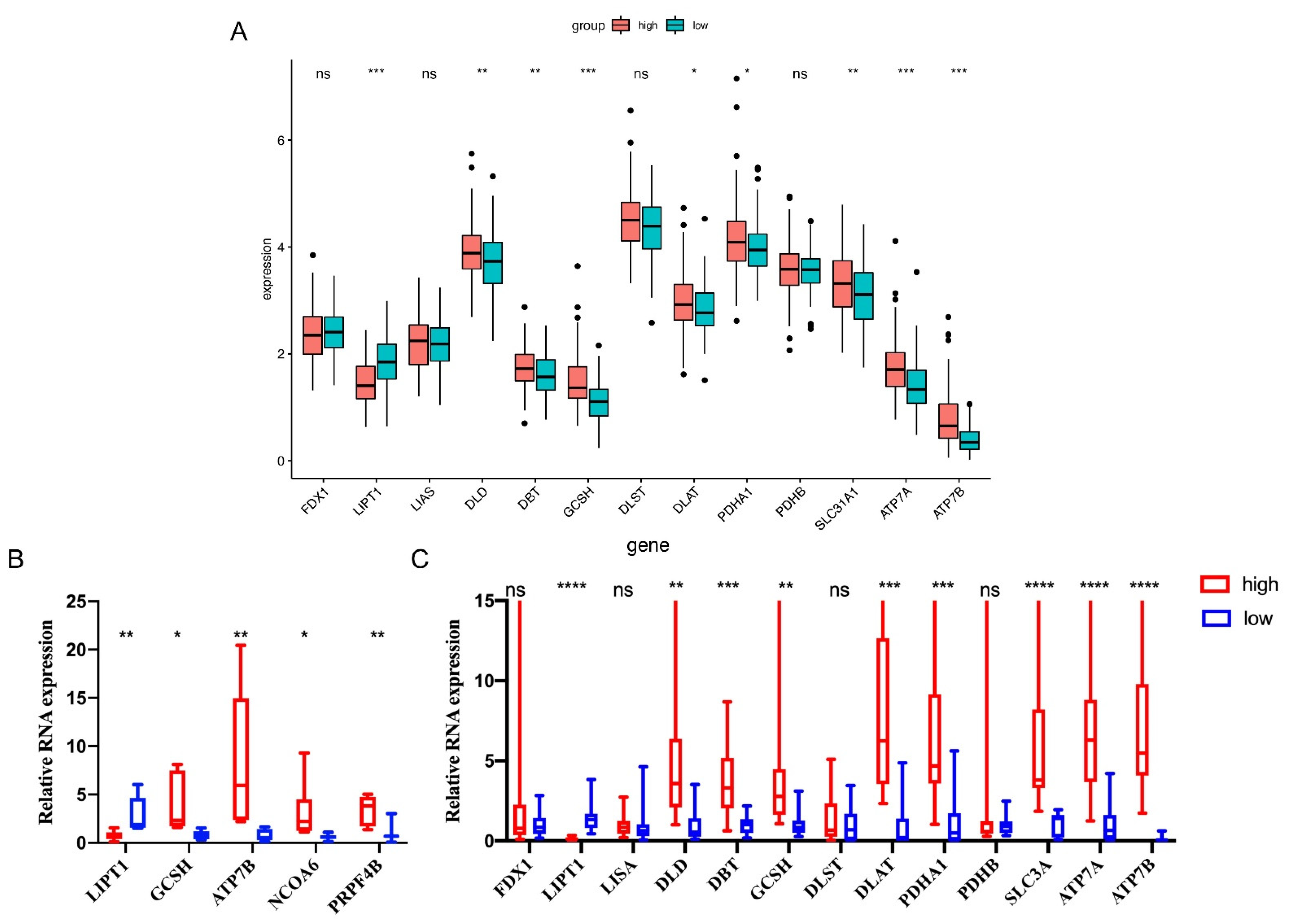
3.8. qRT-PCR Validations in Sarcoma Patients’ Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STS | soft tissue sarcoma |
| TME | tumor microenvironment |
| TIDE | tumor immune dysfunction and exclusion |
| CRGs | cuproptosis-related genes |
| LASSO | least absolute shrinkage and selection operator |
| GO | gene Ontology |
| WGCNA | weighted gene co-expression network analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GSEA | gene set enrichment analysis |
| GSVA | gene set variation analysis |
| TCGA | the caner genome atlas |
| TIMER | tumor immune estimation resource |
| FDR | false discovery rate |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| CC | cellular component |
| BPs | biological processes |
| FDX1 | Ferredoxin 1 |
| LIPT1 | Lipoyltransferase 1 |
| LIAS | Lipoic Acid Synthetase |
| DLD | Dihydrolipoamide Dehydrogenase |
| DBT | Dihydrolipoamide Branched Chain Transacylase E2 |
| GCSH | Glycine Cleavage System Protein H |
| DLST | Dihydrolipoamide S-Succinyltransferase |
| DLAT | Dihydrolipoamide S-Acetyltransferase |
| PDHA1 | Pyruvate Dehydrogenase E1 Subunit Alpha 1 |
| PDHB | Pyruvate Dehydrogenase E1 Subunit Beta |
| SLC31A1 | Solute Carrier Family 31 Member 1 |
| ATP7A | ATPase Copper Transporting Alpha |
| ATP7B | ATPase Copper Transporting Beta |
| PLS-DA | Partial Least-Squares Discriminant Analysis |
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA A Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Casper, E.S.; Conrad, E.U., 3rd; Delaney, T.F.; Ganjoo, K.N.; George, S.; et al. Soft tissue sarcoma, version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA A Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayodele, O.; Razak, A.R.A. Immunotherapy in soft-tissue sarcoma. Curr. Oncol. 2020, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.M.; Cote, G.M.; Hornick, J.L. Contemporary Sarcoma Diagnosis, Genetics, and Genomics. J. Clin. Oncol. 2018, 36, 101–110. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.; Lane, D.J.; Richardson, D.R. Mitochondrial mayhem: The mitochondrion as a modulator of iron metabolism and its role in disease. Antioxid. Redox Signal. 2011, 15, 3003–3019. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Y.G. Variable selection in rank regression for analyzing longitudinal data. Stat. Methods Med. Res. 2018, 27, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yan, G.; Chai, B.; Hou, X. XGBLC: An Improved Survival Prediction Model Based on Xgboost. Bioinformatics 2021, 38, 410–418. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional variable importance for random forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Rice, W.R.; Gaines, S.D. One-way analysis of variance with unequal variances. Proc. Natl. Acad. Sci. USA 1989, 86, 8183–8184. [Google Scholar] [CrossRef] [Green Version]
- Mangone, L.; Mancuso, P.; Braghiroli, M.B.; Bisceglia, I.; Campari, C.; Caroli, S.; Marino, M.; Caldarella, A.; Giorgi Rossi, P.; Pinto, C. Prompt Resumption of Screening Programme Reduced the Impact of COVID-19 on New Breast Cancer Diagnoses in Northern Italy. Cancers 2022, 14, 3029. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef]
- Linch, M.; Miah, A.B.; Thway, K.; Judson, I.R.; Benson, C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014, 11, 187–202. [Google Scholar] [CrossRef]
- Dufresne, A.; Brahmi, M.; Karanian, M.; Blay, J.Y. Using biology to guide the treatment of sarcomas and aggressive connective-tissue tumours. Nat. Rev. Clin. Oncol. 2018, 15, 443–458. [Google Scholar] [CrossRef]
- Reed, D.R.; Hayashi, M.; Wagner, L.; Binitie, O.; Steppan, D.A.; Brohl, A.S.; Shinohara, E.T.; Bridge, J.A.; Loeb, D.M.; Borinstein, S.C.; et al. Treatment pathway of bone sarcoma in children, adolescents, and young adults. Cancer 2017, 123, 2206–2218. [Google Scholar] [CrossRef]
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Lancet 1997, 350, 1647–1654. [Google Scholar] [CrossRef]
- Matushansky, I.; Taub, R.N. Adjuvant chemotherapy in 2011 for patients with soft-tissue sarcoma. Nat. Rev. Clin. Oncol. 2011, 8, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gouw, A.M.; LaGory, E.L.; Guo, S.; Attarwala, N.; Tang, Y.; Qi, J.; Chen, Y.S.; Gao, Z.; Casey, K.M.; et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol. 2021, 39, 357–367. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Muhanhali, D.; Jia, X.; Wu, Z.; Cai, Z.; Ling, Y. Identification of gene co-expression modules and hub genes associated with lymph node metastasis of papillary thyroid cancer. Endocrine 2019, 66, 573–584. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Duan, Z.; Jia, K.; Yao, Y.; Liu, K.; Qiao, Y.; Gao, Q.; Yang, Y.; Li, G.; Shang, A. A Combined Risk Score Model to Assess Prognostic Value in Patients with Soft Tissue Sarcomas. Cells 2022, 11, 4077. https://doi.org/10.3390/cells11244077
Li Z, Duan Z, Jia K, Yao Y, Liu K, Qiao Y, Gao Q, Yang Y, Li G, Shang A. A Combined Risk Score Model to Assess Prognostic Value in Patients with Soft Tissue Sarcomas. Cells. 2022; 11(24):4077. https://doi.org/10.3390/cells11244077
Chicago/Turabian StyleLi, Zihua, Zhengwei Duan, Keyao Jia, Yiwen Yao, Kaiyuan Liu, Yue Qiao, Qiuming Gao, Yunfeng Yang, Guodong Li, and Anquan Shang. 2022. "A Combined Risk Score Model to Assess Prognostic Value in Patients with Soft Tissue Sarcomas" Cells 11, no. 24: 4077. https://doi.org/10.3390/cells11244077
APA StyleLi, Z., Duan, Z., Jia, K., Yao, Y., Liu, K., Qiao, Y., Gao, Q., Yang, Y., Li, G., & Shang, A. (2022). A Combined Risk Score Model to Assess Prognostic Value in Patients with Soft Tissue Sarcomas. Cells, 11(24), 4077. https://doi.org/10.3390/cells11244077







