Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions
Abstract
1. Introduction
2. The Role of Astrocytic microRNAs in Multiple Pathways in AD
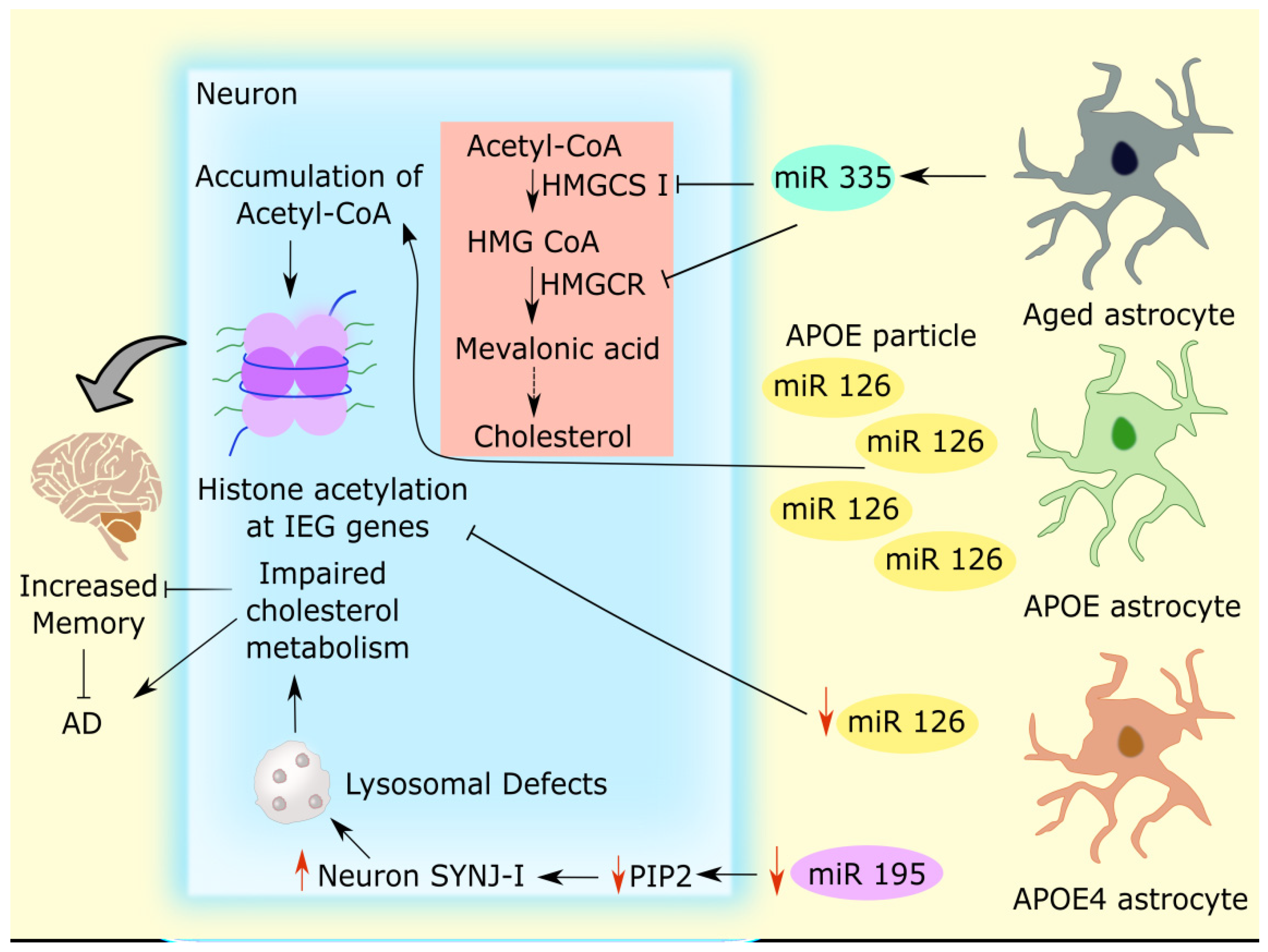
2.1. Astrocytic microRNAs Mediate Cholesterol Metabolism in Alzheimer’s Disease
2.2. Astrocytic microRNAs in Dysregulated Glutamate Uptake in Alzheimer’s Disease
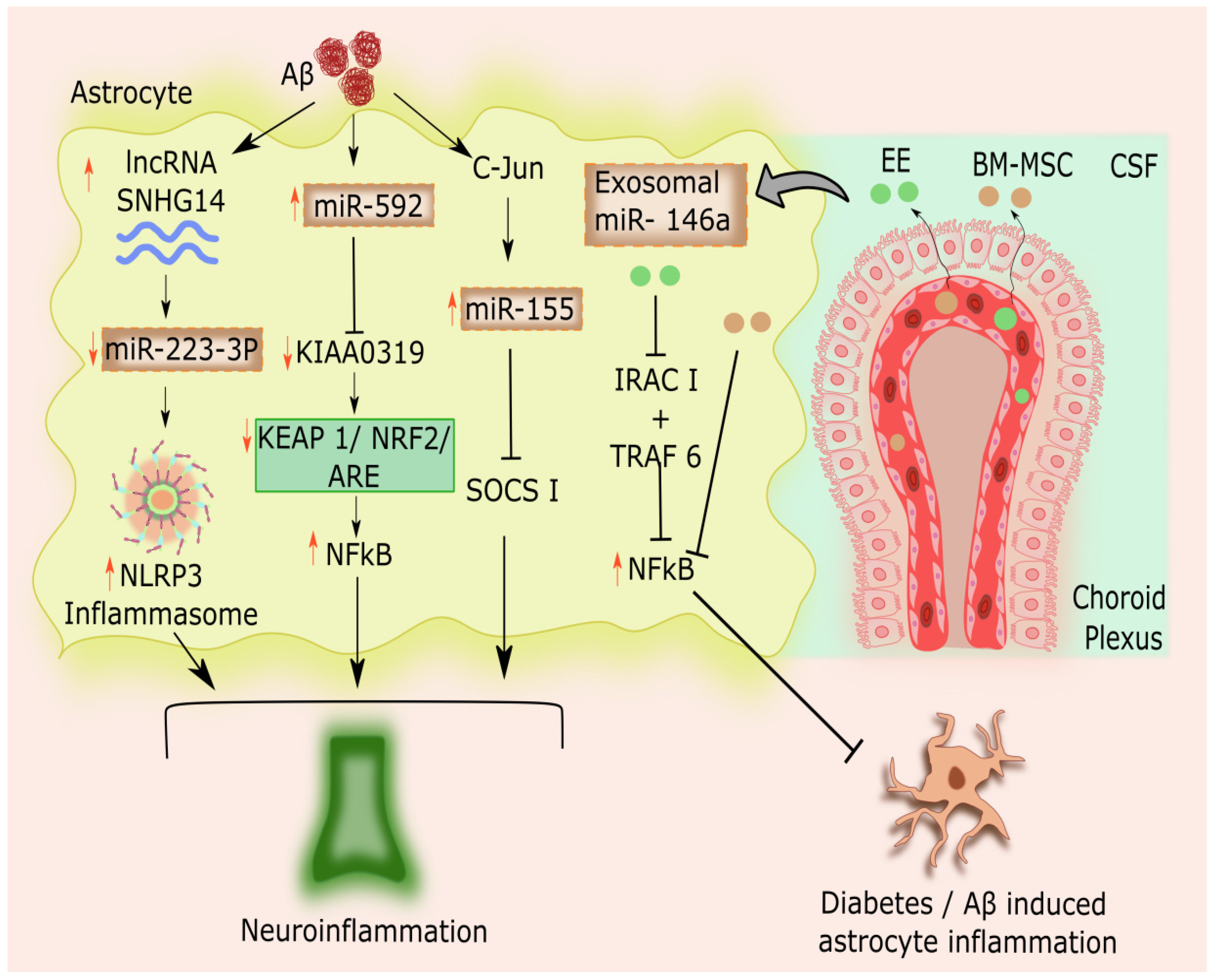
2.3. Astrocytic microRNAs Modulating Inflammation in Alzheimer’s Disease
2.4. Astrocytic microRNAs- Long Non-coding RNA Interconnection in AD-linked Astrocytic Inflammation
3. Astrocyte-Specific Critical Transcription Factors in Alzheimer’s Disease
3.1. STAT3
3.2. PAX6
3.3. TFEB
3.4. TFAM
3.5. NFIA
3.6. CEBPβ
3.7. YY-1
4. Epigenetic Astrocyte Transcription Factors in Neurodegeneration
4.1. HDAC
4.2. CREB
4.3. Nurr1
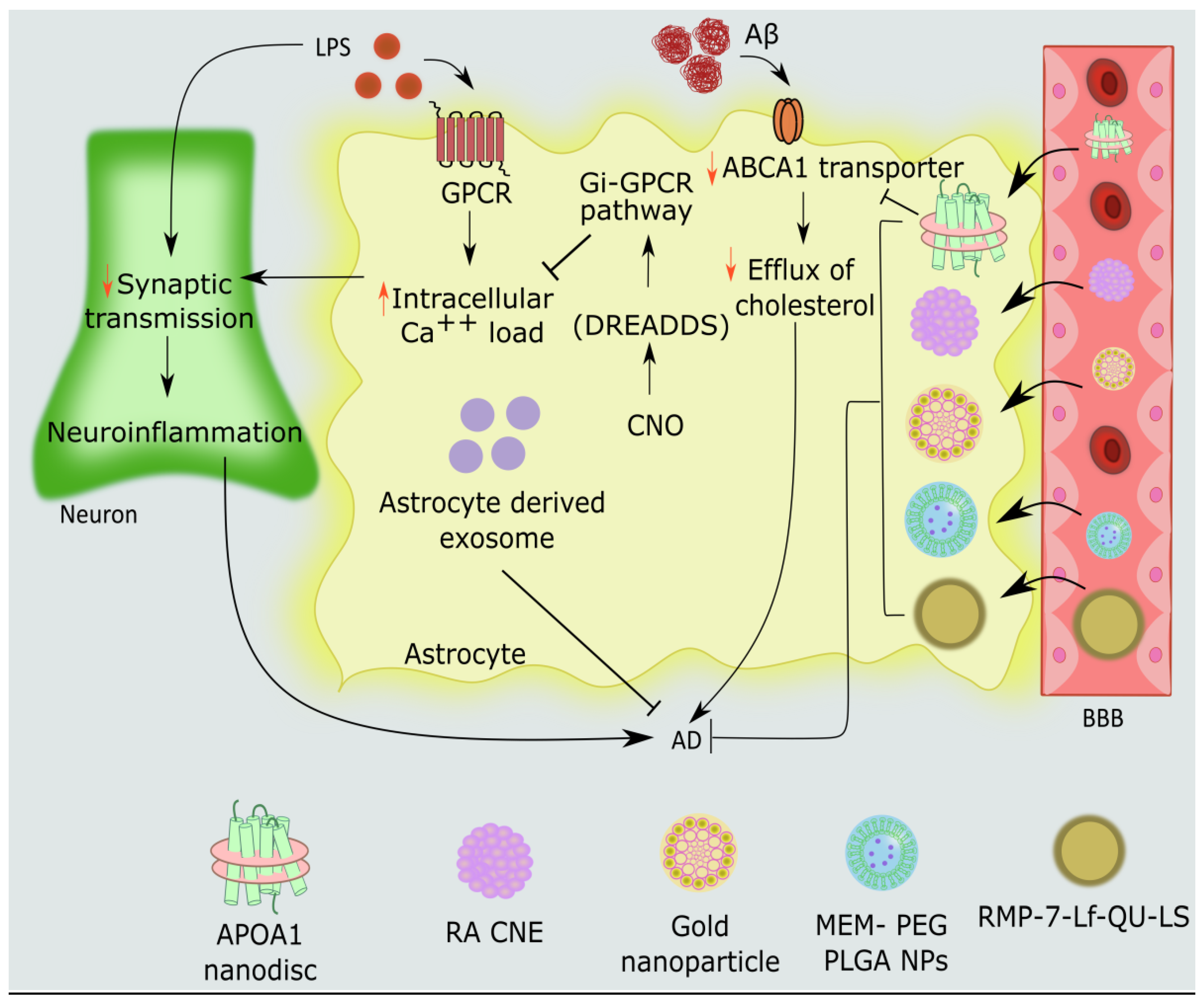
5. Novel Therapeutic Strategies to Target Astrocyte Dysfunction in Alzheimer’s Disease
5.1. APOA1 Nanodiscs in Astrocytes
5.2. Engineered G Protein-Coupled Receptors (GPCRs) in Astrocytes- Chemogenetic Approaches
5.3. Extracellular Vesicles
5.4. Nanoparticles
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kieran, N.W.; Suresh, R.; Dorion, M.-F.; MacDonald, A.; Blain, M.; Wen, D.; Fuh, S.-C.; Ryan, F.; Diaz, R.J.; Stratton, J.A.; et al. MicroRNA-210 Regulates the Metabolic and Inflammatory Status of Primary Human Astrocytes. J. Neuroinflamm. 2022, 19, 10. [Google Scholar] [CrossRef]
- Ouyang, Y.-B.; Xu, L.; Lu, Y.; Sun, X.; Yue, S.; Xiong, X.-X.; Giffard, R.G. Astrocyte-Enriched MiR-29a Targets PUMA and Reduces Neuronal Vulnerability to Forebrain Ischemia. Glia 2013, 61, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Su, X.; Piao, L.; Jin, Z.; Jin, R. Involvement of Astrocytes and MicroRNA Dysregulation in Neurodegenerative Diseases: From Pathogenesis to Therapeutic Potential. Front. Mol. Neurosci. 2021, 14, 556215. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Gong, G.; Wang, Y.; Bian, M.; Yu, L.; Wei, C. MiR-124 Acts as a Target for Alzheimer’s Disease by Regulating BACE1. Oncotarget 2017, 8, 114065–114071. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; Sierksma, A.; Vanden Eynden, E.; De Strooper, B. MiR-132 Loss De-represses ITPKB and Aggravates Amyloid and TAU Pathology in Alzheimer’s Brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Zhou, Y.; Liu, L.; Liu, Y.; Wang, D.; Zhang, S.; Yang, M. MiR-9 Regulates the Expression of BACE1 in Dementia Induced by Chronic Brain Hypoperfusion in Rats. Cell. Physiol. Biochem. 2017, 42, 1213–1226. [Google Scholar] [CrossRef]
- Wang, R.; Lahiri, D.K. Effects of MicroRNA-298 on APP and BACE1 Translation Differ According to Cell Type and 3′-UTR Variation. Sci. Rep. 2022, 12, 3074. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; Pereira de Almeida, L.; Pedroso de Lima, M.C. MiR-155 Modulates Microglia-Mediated Immune Response by down-Regulating SOCS-1 and Promoting Cytokine and Nitric Oxide Production. Immunology 2012, 135, 73–88. [Google Scholar] [CrossRef]
- Hutchison, E.R.; Kawamoto, E.M.; Taub, D.D.; Lal, A.; Abdelmohsen, K.; Zhang, Y.; Wood, W.H.; Lehrmann, E.; Camandola, S.; Becker, K.G.; et al. Evidence for MiR-181 Involvement in Neuroinflammatory Responses of Astrocytes. Glia 2013, 61, 1018–1028. [Google Scholar] [CrossRef]
- Raihan, O.; Brishti, A.; Molla, M.R.; Li, W.; Zhang, Q.; Xu, P.; Khan, M.I.; Zhang, J.; Liu, Q. The Age-Dependent Elevation of MiR-335-3p Leads to Reduced Cholesterol and Impaired Memory in Brain. Neuroscience 2018, 390, 160–173. [Google Scholar] [CrossRef]
- Hauser, P.S.; Narayanaswami, V.; Ryan, R.O. Apolipoprotein E: From Lipid Transport to Neurobiology. Prog. Lipid Res. 2011, 50, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Rao, R. Amyloid Clearance Defect in ApoE4 Astrocytes Is Reversed by Epigenetic Correction of Endosomal PH. Proc. Natl. Acad. Sci. 2018, 115, E6640–E6649. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.-C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science (80-.) 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- van Deijk, A.-L.F.; Broersen, L.M.; Verkuyl, J.M.; Smit, A.B.; Verheijen, M.H.G. High Content Analysis of Hippocampal Neuron-Astrocyte Co-Cultures Shows a Positive Effect of Fortasyn Connect on Neuronal Survival and Postsynaptic Maturation. Front. Neurosci. 2017, 11, 440. [Google Scholar] [CrossRef]
- van Deijk, A.-L.F.; Camargo, N.; Timmerman, J.; Heistek, T.; Brouwers, J.F.; Mogavero, F.; Mansvelder, H.D.; Smit, A.B.; Verheijen, M.H.G. Astrocyte Lipid Metabolism Is Critical for Synapse Development and Function in Vivo. Glia 2017, 65, 670–682. [Google Scholar] [CrossRef]
- Mitsis, T.; Efthimiadou, A.; Bacopoulou, F.; Vlachakis, D.; Chrousos, G.P.; Eliopoulos, E. Transcription Factors and Evolution: An Integral Part of Gene Expression (Review). World Acad. Sci. J. 2020, 2, 3–8. [Google Scholar] [CrossRef]
- Santos-Terra, J.; Deckmann, I.; Fontes-Dutra, M.; Schwingel, G.B.; Bambini-Junior, V.; Gottfried, C. Transcription Factors in Neurodevelopmental and Associated Psychiatric Disorders: A Potential Convergence for Genetic and Environmental Risk Factors. Int. J. Dev. Neurosci. 2021, 81, 545–578. [Google Scholar] [CrossRef]
- Li, P.; Spolski, R.; Liao, W.; Leonard, W.J. Complex Interactions of Transcription Factors in Mediating Cytokine Biology in T Cells. Immunol. Rev. 2014, 261, 141–156. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The Role of Astrocytic Glutamate Transporters GLT-1 and GLAST in Neurological Disorders: Potential Targets for Neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.-M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A Central Element in Neurological Diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and Apolipoprotein E Receptors: Normal Biology and Roles in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Avila-Muñoz, E.; Arias, C. Cholesterol-Induced Astrocyte Activation Is Associated with Increased Amyloid Precursor Protein Expression and Processing. Glia 2015, 63, 2010–2022. [Google Scholar] [CrossRef]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of Beta-Amyloid Production in Neurons by Astrocyte-Derived Cholesterol. Proc. Natl. Acad. Sci. 2021, 118, e2102191118. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol Metabolism and Homeostasis in the Brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef]
- de Chaves, E.P.; Narayanaswami, V.; Christoffersen, C.; Nielsen, L.B. Apolipoprotein E and Cholesterol in Aging and Disease in the Brain. Future Lipidol. 2008, 3, 505–530. [Google Scholar] [CrossRef]
- Qian, L.; Chai, A.B.; Gelissen, I.C.; Brown, A.J. Balancing Cholesterol in the Brain: From Synthesis to Disposal. Explor. Neuroprotective Ther. 2022, 2, 1–27. [Google Scholar] [CrossRef]
- Mao, S.; Sun, Q.; Xiao, H.; Zhang, C.; Li, L. Secreted MiR-34a in Astrocytic Shedding Vesicles Enhanced the Vulnerability of Dopaminergic Neurons to Neurotoxins by Targeting Bcl-2. Protein Cell 2015, 6, 529–540. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Li, D.; He, C.; He, K.; Xue, T.; Wan, L.; Zhang, C.; Liu, Q. Astrocytic ApoE Reprograms Neuronal Cholesterol Metabolism and Histone-Acetylation-Mediated Memory. Neuron 2021, 109, 957–970.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Yang, J.; Lin, S.; Chen, L. MiRNA-Based Signature to Predict the Development of Alzheimer’s Disease. Comb. Chem. High Throughput Screen. 2022, 25, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Depp, C.; Sasmita, A.O.; Vasileva, M.H.; Scholz, P.; Zhao, Y.; Krueger-Burg, D.; et al. Neuronal Cholesterol Synthesis Is Essential for Repair of Chronically Demyelinated Lesions in Mice. Cell Rep. 2021, 37, 109889. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular Cholesterol Trafficking and Compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Fagan, A.M.; Holtzman, D.M. Astrocyte Lipoproteins, Effects of ApoE on Neuronal Function, and Role of ApoE in Amyloid-? Deposition in Vivo. Microsc. Res. Tech. 2000, 50, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Savini, M.; Folick, A.; Lee, Y.-T.; Jin, F.; Cuevas, A.; Tillman, M.C.; Duffy, J.D.; Zhao, Q.; Neve, I.A.; Hu, P.-W.; et al. Lysosome Lipid Signalling from the Periphery to Neurons Regulates Longevity. Nat. Cell Biol. 2022, 24, 906–916. [Google Scholar] [CrossRef]
- Thelen, A.M.; Zoncu, R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, M.; Guo, L.; Zhu, L.; Hou, J.; Zhang, L.; Pero, A.; Ng, S.; El Gaamouch, F.; Elder, G.; et al. MicroRNA-195 Rescues ApoE4-Induced Cognitive Deficits and Lysosomal Defects in Alzheimer’s Disease Pathogenesis. Mol. Psychiatry 2021, 26, 4687–4701. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.C.; Hille, B. Regulation of Ion Channels by Phosphatidylinositol 4,5-Bisphosphate. Curr. Opin. Neurobiol. 2005, 15, 370–378. [Google Scholar] [CrossRef]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in Cell Regulation and Membrane Dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, M.; Elder, G.A.; Sano, M.; Holtzman, D.M.; Gandy, S.; Cardozo, C.; Haroutunian, V.; Robakis, N.K.; Cai, D. Phospholipid Dysregulation Contributes to Apoe4-Associated Cognitive Deficits in Alzheimer’s Disease Pathogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 11965–11970. [Google Scholar] [CrossRef] [PubMed]
- Cossec, J.C.; Lavaur, J.; Berman, D.E.; Rivals, I.; Hoischen, A.; Stora, S.; Ripoll, C.; Mircher, C.; Grattau, Y.; Olivomarin, J.C.; et al. Trisomy for Synaptojanin1 in down Syndrome Is Functionally Linked to the Enlargement of Early Endosomes. Hum. Mol. Genet. 2012, 21, 3156–3172. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, M.; Zhao, J.; Rhee, H.; Caesar, I.; Knight, E.M.; Volpicelli-Daley, L.; Bustos, V.; Netzer, W.; Liu, L.; et al. Reduction of Synaptojanin 1 Accelerates Aβ Clearance and Attenuates Cognitive Deterioration in an Alzheimer Mouse Model. J. Biol. Chem. 2013, 288, 32050–32063. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Shin, S.; Kim, Y.; Cho, H.; Park, M.K.; Kim, T.W.; Voronov, S.V.; Di Paolo, G.; Suh, B.C.; Chung, S. Cholesterol Modulates Ion Channels via Down-Regulation of Phosphatidylinositol 4,5-Bisphosphate. J. Neurochem. 2010, 112, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Bojja, S.L.; Gurram, P.C.; Mudgal, J.; Arora, D.; Nampoothiri, M. Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders. Cells 2022, 11, 1139. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Martin, L.; Levey, A.I.; Dykes-Hoberg, M.; Jin, L.; Wu, D.; Nash, N.; Kuncl, R.W. Localization of Neuronal and Glial Glutamate Transporters. Neuron 1994, 13, 713–725. [Google Scholar] [CrossRef]
- Marttinen, M.; Takalo, M.; Natunen, T.; Wittrahm, R.; Gabbouj, S.; Kemppainen, S.; Leinonen, V.; Tanila, H.; Haapasalo, A.; Hiltunen, M. Molecular Mechanisms of Synaptotoxicity and Neuroinflammation in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 963. [Google Scholar] [CrossRef]
- Mookherjee, P.; Green, P.S.; Watson, G.S.; Marques, M.A.; Tanaka, K.; Meeker, K.D.; Meabon, J.S.; Li, N.; Zhu, P.; Olson, V.G.; et al. GLT-1 Loss Accelerates Cognitive Deficit Onset in an Alzheimer’s Disease Animal Model. J. Alzheimer’s Dis. 2011, 26, 447–455. [Google Scholar] [CrossRef]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A Uniform System for MicroRNA Annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef]
- Dostie, J.; Mourelatos, Z.; Yang, M.; Sharma, A.; Dreyfuss, G. Numerous MicroRNPs in Neuronal Cells Containing Novel MicroRNAs. RNA 2003, 9, 180–186. [Google Scholar] [CrossRef]
- Kasashima, K.; Nakamura, Y.; Kozu, T. Altered Expression Profiles of MicroRNAs during TPA-Induced Differentiation of HL-60 Cells. Biochem. Biophys. Res. Commun. 2004, 322, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian MicroRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated Astrogliosis Ameliorates Pathology in an Alzheimer’s Disease Model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-Derived Exosomes-Transmitted MiR-124-3p Protect Traumatically Injured Spinal Cord by Suppressing the Activation of Neurotoxic Microglia and Astrocytes. J. Nanobiotechnolo. 2020, 18, 105. [Google Scholar] [CrossRef]
- Zumkehr, J.; Rodriguez-Ortiz, C.J.; Cheng, D.; Kieu, Z.; Wai, T.; Hawkins, C.; Kilian, J.; Lim, S.L.; Medeiros, R.; Kitazawa, M. Ceftriaxone Ameliorates Tau Pathology and Cognitive Decline via Restoration of Glial Glutamate Transporter in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2015, 36, 2260–2271. [Google Scholar] [CrossRef]
- Abdul, H.M.; Sama, M.A.; Furman, J.L.; Mathis, D.M.; Beckett, T.L.; Weidner, A.M.; Patel, E.S.; Baig, I.; Murphy, M.P.; LeVine, H.; et al. Cognitive Decline in Alzheimer’s Disease Is Associated with Selective Changes in Calcineurin/NFAT Signaling. J. Neurosci. 2009, 29, 12957–12969. [Google Scholar] [CrossRef]
- Scimemi, A.; Meabon, J.S.; Woltjer, R.L.; Sullivan, J.M.; Diamond, J.S.; Cook, D.G. Amyloid- 1-42 Slows Clearance of Synaptically Released Glutamate by Mislocalizing Astrocytic GLT-1. J. Neurosci. 2013, 33, 5312–5318. [Google Scholar] [CrossRef]
- Park, K.; Lee, S.J. Deciphering the Star Codings: Astrocyte Manipulation Alters Mouse Behavior. Exp. Mol. Med. 2020, 52, 1028–1038. [Google Scholar] [CrossRef]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome Reporter Mice Reveal the Involvement of Exosomes in Mediating Neuron to Astroglia Communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef]
- Morel, L.; Regan, M.; Higashimori, H.; Ng, S.K.; Esau, C.; Vidensky, S.; Rothstein, J.; Yang, Y. Neuronal Exosomal Mirna-Dependent Translational Regulation of Astroglial Glutamate Transporter Glt1. J. Biol. Chem. 2013, 288, 7105–7116. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, Y.; Xiao, Y.; Peng, M.; Mai, W.; Hu, B.; Jia, Y.; Chen, H.; Yang, Y.; Xiang, Q.; et al. Extracellular Vesicles Derived from Astrocyte-Treated with HaFGF 14-154 Attenuate Alzheimer Phenotype in AD Mice. Theranostics 2022, 12, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.; Dabin, L.C.; Tate, M.D.; Kim, J. MicroRNAs on the Move: MicroRNAs in Astrocyte-Derived ApoE Particles Regulate Neuronal Function. Neuron 2021, 109, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Abuelezz, N.Z.; Nasr, F.E.; AbdulKader, M.A.; Bassiouny, A.R.; Zaky, A. MicroRNAs as Potential Orchestrators of Alzheimer’s Disease-Related Pathologies: Insights on Current Status and Future Possibilities. Front. Aging Neurosci. 2021, 13, 743573. [Google Scholar] [CrossRef]
- Lukiw, W.J. MicroRNA-146a Signaling in Alzheimer’s Disease (AD) and Prion Disease (PrD). Front. Neurol. 2020, 11, 462. [Google Scholar] [CrossRef]
- Chithanathan, K.; Somelar, K.; Jürgenson, M.; Žarkovskaja, T.; Periyasamy, K.; Yan, L.; Magilnick, N.; Boldin, M.P.; Rebane, A.; Tian, L.; et al. Enhanced Cognition and Neurogenesis in MiR-146b Deficient Mice. Cells 2022, 11, 2002. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, D.; Zhao, Y.Y.; Wang, Y.; Zhao, Y.Y.; Wen, C. Inhibition of MicroRNA-155 Alleviates Cognitive Impairment in Alzheimer’s Disease and Involvement of Neuroinflammation. Curr. Alzheimer Res. 2019, 16, 473–482. [Google Scholar] [CrossRef]
- Mor, E.; Cabilly, Y.; Goldshmit, Y.; Zalts, H.; Modai, S.; Edry, L.; Elroy-Stein, O.; Shomron, N. Species-Specific MicroRNA Roles Elucidated Following Astrocyte Activation. Nucleic Acids Res. 2011, 39, 3710–3723. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021, 13, 654931. [Google Scholar] [CrossRef]
- Li, M.; Long, C.; Yang, L. Hippocampal-Prefrontal Circuit and Disrupted Functional Connectivity in Psychiatric and Neurodegenerative Disorders. Biomed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Li, Y.-C.; Gao, W.-J. Norepinephrine versus Dopamine and Their Interaction in Modulating Synaptic Function in the Prefrontal Cortex. Brain Res. 2016, 1641, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of Astrocytes in Hippocampus Decreases Fear Memory through Adenosine A1 Receptors. Elife 2020, 9, e57155. [Google Scholar] [CrossRef]
- Walker, D.G.; Whetzel, A.M.; Lue, L.-F. Expression of Suppressor of Cytokine Signaling Genes in Human Elderly and Alzheimer’s Disease Brains and Human Microglia. Neuroscience 2015, 302, 121–137. [Google Scholar] [CrossRef]
- Mudgal, J.; Basu Mallik, S.; Nampoothiri, M.; Kinra, M.; Hall, S.; Grant, G.D.; Anoopkumar-Dukie, S.; Davey, A.K.; Rao, C.M.; Arora, D. Effect of Coffee Constituents, Caffeine and Caffeic Acid on Anxiety and Lipopolysaccharide-Induced Sickness Behavior in Mice. J. Funct. Foods 2020, 64, 103638. [Google Scholar] [CrossRef]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, J.; Zhao, L.; Pan, D. MicroRNA-146a Inhibition Promotes Total Neurite Outgrowth and Suppresses Cell Apoptosis, Inflammation, and STAT1/MYC Pathway in PC12 and Cortical Neuron Cellular Alzheimer’s Disease Models. Brazilian J. Med. Biol. Res. 2021, 54, e9665. [Google Scholar] [CrossRef]
- Gião, T.; Teixeira, T.; Almeida, M.R.; Cardoso, I. Choroid Plexus in Alzheimer’s Disease—The Current State of Knowledge. Biomedicines 2022, 10, 224. [Google Scholar] [CrossRef]
- Grapp, M.; Wrede, A.; Schweizer, M.; Hüwel, S.; Galla, H.J.; Snaidero, N.; Simons, M.; Bückers, J.; Low, P.S.; Urlaub, H.; et al. Choroid Plexus Transcytosis and Exosome Shuttling Deliver Folate into Brain Parenchyma. Nat. Commun. 2013, 4, 2123. [Google Scholar] [CrossRef] [PubMed]
- Prado Lima, M.G.; Schimidt, H.L.; Garcia, A.; Daré, L.R.; Carpes, F.P.; Izquierdo, I.; Mello-Carpes, P.B. Environmental Enrichment and Exercise Are Better than Social Enrichment to Reduce Memory Deficits in Amyloid Beta Neurotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2403–E2409. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kubota, K.; Hashizume, S.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Fujimiya, M. An Enriched Environment Prevents Cognitive Impairment in an Alzheimer’s Disease Model by Enhancing the Secretion of Exosomal MicroRNA-146a from the Choroid Plexus. Brain, Behav. Immun. - Heal. 2020, 9, 100149. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.M.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A Key Regulator of Astrocyte-Mediated Inflammatory Response. PLoS ONE 2012, 7, e44789. [Google Scholar] [CrossRef]
- Kinra, M.; Joseph, A.; Nampoothiri, M.; Arora, D.; Mudgal, J. Inhibition of NLRP3-Inflammasome Mediated IL-1β Release by Phenylpropanoic Acid Derivatives: In-Silico and in-Vitro Approach. Eur. J. Pharm. Sci. 2021, 157, 105637. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Wang, K. Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer’s Disease: Inhibition of Neuroinflammation. J. Inflamm. Res. 2021, 14, 4761–4775. [Google Scholar] [CrossRef]
- Kubota, K.; Nakano, M.; Kobayashi, E.; Mizue, Y.; Chikenji, T.; Otani, M.; Nagaishi, K.; Fujimiya, M. An Enriched Environment Prevents Diabetes-Induced Cognitive Impairment in Rats by Enhancing Exosomal MiR-146a Secretion from Endogenous Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2018, 13, e0204252. [Google Scholar] [CrossRef]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetes-Induced Cognitive Impairment by Exosome Transfer into Damaged Neurons and Astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, Y.; Zhang, Y.-L.; Zhao, B.; Zhang, X.-L.; Zhang, W.-T.; Lu, Y.-J.; Lu, A.; Zhang, J.J.; Zhang, J.J. Loss of Neurodevelopmental-Associated MiR-592 Impairs Neurogenesis and Causes Social Interaction Deficits. Cell Death Dis. 2022, 13, 292. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 Defines a Physiologically Important Stress Response Mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Uruno, A.; Motohashi, H. The Keap1-Nrf2 System as an in Vivo Sensor for Electrophiles. Nitric Oxide - Biol. Chem. 2011, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Turpaev, K.T. Keap1-Nrf2 Signaling Pathway: Mechanisms of Regulation and Role in Protection of Cells against Toxicity Caused by Xenobiotics and Electrophiles. Biochem. 2013, 78, 111–126. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal Stem Cell-Derived Exosomes as a Nanotherapeutic Agent for Amelioration of Inflammation-Induced Astrocyte Alterations in Mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 Expression Protects Spinal Cord from Oxidative Stress Following Spinal Cord Injury in a Male Mouse Model. J. Neuroinflamm. 2022, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Draheim, T.; Liessem, A.; Scheld, M.; Wilms, F.; Weißflog, M.; Denecke, B.; Kensler, T.W.; Zendedel, A.; Beyer, C.; Kipp, M.; et al. Activation of the Astrocytic Nrf2/ARE System Ameliorates the Formation of Demyelinating Lesions in a Multiple Sclerosis Animal Model. Glia 2016, 64, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- De Wu, G.; Li, Z.H.; Li, X.; Zheng, T.; Zhang, D.K. MicroRNA-592 Blockade Inhibits Oxidative Stress Injury in Alzheimer’s Disease Astrocytes via the KIAA0319-Mediated Keap1/Nrf2/ARE Signaling Pathway. Exp. Neurol. 2020, 324, 113128. [Google Scholar] [CrossRef]
- Liu, X.; Hao, L.; Li, D.; Zhu, L.; Hu, S. Long Non-Coding RNAs and Their Biological Roles in Plants. Genomics. Proteom. Bioinforma. 2015, 13, 137–147. [Google Scholar] [CrossRef]
- Lyu, Y.; Bai, L.; Qin, C. Long Noncoding RNAs in Neurodevelopment and Parkinson’s Disease. Anim. Model. Exp. Med. 2019, 2, 239–251. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Ferdowsi, S.; Asadi, A.; Panahi, Y. Long Non-Coding RNAs Associated with Brain Disorders: A Literature Review. Gene Cell Tissue 2021, 8, e111802. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, Z.; Xu, J.; Lu, Y.; Meng, Q.; Wang, C.; Yang, Y.; Xin, X.; Li, X.; Pu, H.; et al. Long Noncoding RNA MEG3 Suppresses Liver Cancer Cells Growth through Inhibiting β-Catenin by Activating PKM2 and Inactivating PTEN. Cell Death Dis. 2018, 9, 253. [Google Scholar] [CrossRef]
- Meng, J.; Ding, T.; Chen, Y.; Long, T.; Xu, Q.; Lian, W.; Liu, W. LncRNA-Meg3 Promotes Nlrp3-Mediated Microglial Inflammation by Targeting MiR-7a-5p. Int. Immunopharmacol. 2021, 90, 107141. [Google Scholar] [CrossRef] [PubMed]
- Kinra, M.; Nampoothiri, M.; Arora, D.; Mudgal, J. Reviewing the Importance of TLR-NLRP3-pyroptosis Pathway and Mechanism of Experimental NLRP3 Inflammasome Inhibitors. Scand. J. Immunol. 2022, 95, e13124. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Chen, B.; Yao, X.; Lei, Y.; Ou, F.; Huang, F. Upregulation of the LncRNA MEG3 Improves Cognitive Impairment, Alleviates Neuronal Damage, and Inhibits Activation of Astrocytes in Hippocampus Tissues in Alzheimer’s Disease through Inactivating the PI3K/Akt Signaling Pathway. J. Cell. Biochem. 2019, 120, 18053–18065. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Wang, S.Y.; Wei, B.; Deng, Y.; Fu, X.X.; Gong, P.Y.; Yan, E.; Sun, X.J.; Cao, H.M.; Shi, J.Q.; et al. Angiotensin-(1–7) Analogue AVE0991 Modulates Astrocyte-Mediated Neuroinflammation via LncRNA SNHG14/MiR-223-3p/NLRP3 Pathway and Offers Neuroprotection in a Transgenic Mouse Model of Alzheimer’s Disease. J. Inflamm. Res. 2021, 14, 7007–7019. [Google Scholar] [CrossRef]
- Zhang, M.; He, P.; Bian, Z. Long Noncoding RNAs in Neurodegenerative Diseases: Pathogenesis and Potential Implications as Clinical Biomarkers. Front. Mol. Neurosci. 2021, 14, 685143. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, Q.; Zhang, C.; Wang, M. Homoharringtonine Inhibits Alzheimer’s Disease Progression by Reducing Neuroinflammation via STAT3 Signaling in APP/PS1 Mice. Neurodegener. Dis. 2022, 21, 93–102. [Google Scholar] [CrossRef]
- Wan, J.; Fu, A.K.Y.; Ip, F.C.F.; Ng, H.; Hugon, J.; Wang, J.H.; Lai, K.; Wu, Z.; Ip, N.Y. Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: Implications in Alzheimer’s disease. J. Neurosci. 2010, 30, 6873–6881. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.; Lei, M.; Che, J.; Zhang, S.; Zhang, T.; Hu, Y.; Shi, L.; Cui, L.; Liu, J.; et al. Daphnetin Ameliorates Aβ Pathogenesis via STAT3/GFAP Signaling in an APP/PS1 Double-Transgenic Mouse Model of Alzheimer’s Disease. Pharmacol. Res. 2022, 180, 106227. [Google Scholar] [CrossRef]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive Roles for Reactive Astrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef]
- Neal, M.; Richardson, J.R. Epigenetic Regulation of Astrocyte Function in Neuroinflammation and Neurodegeneration. Biochim. Biophys. Acta - Mol. Basis Dis. 2018, 1864, 432–443. [Google Scholar] [CrossRef]
- Mishra, S.; Maurya, S.K.; Srivastava, K.; Shukla, S.; Mishra, R. Pax6 Influences Expression Patterns of Genes Involved in Neuro-Degeneration. Ann. Neurosci. 2015, 22, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Vamanu, E. Therapeutic Potential of Vital Transcription Factors in Alzheimer’s and Parkinson’s Disease With Particular Emphasis on Transcription Factor EB Mediated Autophagy. Front. Neurosci. 2021, 15, 777347. [Google Scholar] [CrossRef]
- La Spina, M.; Contreras, P.S.; Rissone, A.; Meena, N.K.; Jeong, E.; Martina, J.A. MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front. Cell Dev. Biol. 2021, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-rueda, D.; Guerra-ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Ortega, A.; Mauricio, D.; Vila, J.M.; Valles, S.L. Astrocytes Protect Neurons from A β 1-42 Peptide- Induced Neurotoxicity Increasing TFAM and PGC-1 and Decreasing PPAR- γ and SIRT-1. Int. J. Med. Sci. 2015, 12, 48–56. [Google Scholar] [CrossRef]
- Li, Q. Overseeing Memory Circuits by NFIA: New Face In Astrocytes. Neuron 2020, 106, 878–880. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gong, K.; Liu, X.; Zhang, Z.; Sun, X.; Wei, Z.Z.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Johnson, P.F.; et al. Author Correction: C/EBPβ Regulates Delta-Secretase Expression and Mediates Pathogenesis in Mouse Models of Alzheimer’s Disease. Nat. Commun. 2019, 10, 41467. [Google Scholar] [CrossRef]
- Nowak, K.; Lange-Dohna, C.; Zeitschel, U.; Günther, A.; Lüscher, B.; Robitzki, A.; Perez-Polo, R.; Roßner, S. The Transcription Factor Yin Yang 1 Is an Activator of BACE1 Expression. J. Neurochem. 2006, 96, 1696–1707. [Google Scholar] [CrossRef]
- Chen, Z.S.; Chan, H.Y.E. Transcriptional Dysregulation in Neurodegenerative Diseases: Who Tipped the Balance of Yin Yang 1 in the Brain? Neural Regen. Res. 2019, 14, 1148–1151. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, A.; Ma, W. Dexmedetomidine Attenuates the Toxicity of β-Amyloid on Neurons and Astrocytes by Increasing BDNF Production under the Regulation of HDAC2 and HDAC5. Mol. Med. Rep. 2019, 19, 533–540. [Google Scholar] [CrossRef]
- Sen, T.; Sen, N. Isoflurane-Induced Inactivation of CREB through Histone Deacetylase 4 Is Responsible for Cognitive Impairment in Developing Brain. Neurobiol. Dis. 2016, 96, 12–21. [Google Scholar] [CrossRef]
- Jeon, S.G.; Yoo, A.; Chun, D.W.; Hong, S.B.; Chung, H.; Kim, J.-I.; Moon, M. The Critical Role of Nurr1 as a Mediator and Therapeutic Target in Alzheimer’s Disease-Related Pathogenesis. Aging Dis. 2020, 11, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Yi-Bin, W.; Xiang, L.; Bing, Y.; Qi, Z.; Fei-Tong, J.; Minghong, W.; Xiangxiang, Z.; Le, K.; Yan, L.; Ping, S.; et al. Inhibition of the CEBPβ-NFκB Interaction by Nanocarrier-Packaged Carnosic Acid Ameliorates Glia-Mediated Neuroinflammation and Improves Cognitive Function in an Alzheimer’s Disease Model. Cell Death Dis. 2022, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- 123. Delgado-Morales, R.; Agís-Balboa, R.C.; Esteller, M.; Berdasco, M. Epigenetic Mechanisms during Ageing and Neurogenesis as Novel Therapeutic Avenues in Human Brain Disorder. Clin Epigenetics 2017, 29, 67. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M.; Kandel, E.R. The Regulation of Transcription in Memory Consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021741. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, E.; Giannelli, S.; Testa, G.; Sottero, B.; Leonarduzzi, G.; Gamba, P. Cholesterol Dysmetabolism in Alzheimer’s Disease: A Starring Role for Astrocytes? Antioxidants 2021, 10, 1890. [Google Scholar] [CrossRef]
- Button, E.B.; Boyce, G.K.; Wilkinson, A.; Stukas, S.; Hayat, A.; Fan, J.; Wadsworth, B.J.; Robert, J.; Martens, K.M.; Wellington, C.L. ApoA-I Deficiency Increases Cortical Amyloid Deposition, Cerebral Amyloid Angiopathy, Cortical and Hippocampal Astrogliosis, and Amyloid-Associated Astrocyte Reactivity in APP/PS1 Mice. Alzheimers. Res. Ther. 2019, 11, 44. [Google Scholar] [CrossRef]
- Ito, J.; Michikawa, M. ApoA-I/HDL Generation and Intracellular Cholesterol Transport through Cytosolic Lipid-Protein Particles in Astrocytes. J. Lipids 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Shelby, M.; Gilbile, D.; Grant, T.; Bauer, W.; Segelke, B.; He, W.; Evans, A.; Crespo, N.; Fischer, P.; Pakendorf, T.; et al. Crystallization of ApoA1 and ApoE4 Nanolipoprotein Particles and Initial XFEL-Based Structural Studies. Crystals 2020, 10, 886. [Google Scholar] [CrossRef]
- Pourmousa, M.; Pastor, R.W. Molecular Dynamics Simulations of Lipid Nanodiscs. Biochim. Biophys. Acta - Biomembr. 2018, 1860, 2094–2107. [Google Scholar] [CrossRef]
- Rawat, V.; Wang, S.; Sima, J.; Bar, R.; Liraz, O.; Gundimeda, U.; Parekh, T.; Chan, J.; Johansson, J.O.; Tang, C.; et al. ApoE4 Alters ABCA1 Membrane Trafficking in Astrocytes. J. Neurosci. 2019, 39, 9611–9622. [Google Scholar] [CrossRef]
- Barber, C.N.; Raben, D.M. Lipid Metabolism Crosstalk in the Brain: Glia and Neurons. Front. Cell. Neurosci. 2019, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Merched, A.; Xia, Y.; Visvikis, S.; Serot, J.M.; Siest, G. Decreased High-Density Lipoprotein Cholesterol and Serum Apolipoprotein AI Concentrations Are Highly Correlated with the Severity of Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Kusumo, H.; Costa, L.G.; Guizzetti, M. Cholesterol Efflux Is Differentially Regulated in Neurons and Astrocytes: Implications for Brain Cholesterol Homeostasis. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2013, 1831, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Slot, R.E.R.; Van Harten, A.C.; Kester, M.I.; Jongbloed, W.; Bouwman, F.H.; Teunissen, C.E.; Scheltens, P.; Veerhuis, R.; Van Der Flier, W.M. Apolipoprotein A1 in Cerebrospinal Fluid and Plasma and Progression to Alzheimer’s Disease in Non-Demented Elderly. J. Alzheimer’s Dis. 2017, 56, 687–697. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-Secreted ApoE. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef]
- Sierri, G.; Dal Magro, R.; Vergani, B.; Leone, B.E.; Formicola, B.; Taiarol, L.; Fagioli, S.; Kravicz, M.; Tremolizzo, L.; Calabresi, L.; et al. Reduced Levels of ABCA1 Transporter Are Responsible for the Cholesterol Efflux Impairment in Β-amyloid-induced Reactive Astrocytes: Potential Rescue from Biomimetic HDLs. Int. J. Mol. Sci. 2022, 23, 102. [Google Scholar] [CrossRef]
- Akram, A.; Schmeidler, J.; Katsel, P.; Hof, P.R.; Haroutunian, V. Increased Expression of Cholesterol Transporter ABCA1 Is Highly Correlated with Severity of Dementia in AD Hippocampus. Brain Res. 2010, 1318, 167–177. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Vijayakumar, K.K.; Murugesan, S.; Kunjiappan, S. Liposomes: An Emerging Carrier for Targeting Alzheimer’s and Parkinson’s Diseases. Heliyon 2022, 8, e09575. [Google Scholar] [CrossRef]
- Suesca, E.; Alejo, J.L.; Bolaños, N.I.; Ocampo, J.; Leidy, C.; González, J.M. Sulfocerebrosides Upregulate Liposome Uptake in Human Astrocytes without Inducing a Proinflammatory Response. Cytom. Part A 2013, 83, 627–635. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Tsao, C.W. Neuroprotection against Apoptosis of SK-N-MC Cells Using RMP-7- and Lactoferrin-Grafted Liposomes Carrying Quercetin. Int. J. Nanomedicine 2017, 12, 2857–2869. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kayama, T.; Noguchi-Shinohara, M.; Hamaguchi, T.; Yamada, M.; Abe, K.; Kobayashi, S. Rosmarinic Acid Suppresses Tau Phosphorylation and Cognitive Decline by Downregulating the JNK Signaling Pathway. NPJ Sci. Food 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Dal Prá, M.; Azambuja, J.H.; Endres, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Barschak, A.G.; Teixeira, H.F.; Braganhol, E. Glioprotective Effect of Chitosan-Coated Rosmarinic Acid Nanoemulsions Against Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Rat Astrocyte Primary Cultures. Cell. Mol. Neurobiol. 2020, 40, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Michels, L.R.; Azambuja, J.H.; Lenz, G.S.; Gelsleichter, N.E.; Endres, M.; Scholl, J.N.; Schuh, R.S.; Barschak, A.G.; Figueiró, F.; et al. Chitosan-Coated Rosmarinic Acid Nanoemulsion Nasal Administration Protects against LPS-Induced Memory Deficit, Neuroinflammation, and Oxidative Stress in Wistar Rats. Neurochem. Int. 2020, 141, 104875. [Google Scholar] [CrossRef]
- Urban, D.J.; Roth, B.L. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Roth, B.L. DREADD: A Chemogenetic GPCR Signaling Platform. Int. J. Neuropsychopharmacol. 2015, 18, pyu007. [Google Scholar] [CrossRef] [PubMed]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Jendryka, M.; Palchaudhuri, M.; Ursu, D.; van der Veen, B.; Liss, B.; Kätzel, D.; Nissen, W.; Pekcec, A. Pharmacokinetic and Pharmacodynamic Actions of Clozapine-N-Oxide, Clozapine, and Compound 21 in DREADD-Based Chemogenetics in Mice. Sci. Rep. 2019, 9, 4522. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Wang, C.; Li, X.-D.; Guo, W.-L.; Liu, G.-Y.; Zhang, H.-B.; Sun, Y.; Zhu, D.-F.; Xu, Q. Activation of Cholinergic Basal Forebrain Neurons Improved Cognitive Functions in Adult-Onset Hypothyroid Mice. Biomed. Pharmacother. 2022, 153, 113495. [Google Scholar] [CrossRef]
- Shan, Q.; Fang, Q.; Tian, Y. Evidence That GIRK Channels Mediate the DREADD-HM4Di Receptor Activation-Induced Reduction in Membrane Excitability of Striatal Medium Spiny Neurons. ACS Chem. Neurosci. 2022, 13, 2084–2091. [Google Scholar] [CrossRef]
- Hirbec, H.; Déglon, N.; Foo, L.C.; Goshen, I.; Grutzendler, J.; Hangen, E.; Kreisel, T.; Linck, N.; Muffat, J.; Regio, S.; et al. Emerging Technologies to Study Glial Cells. Glia 2020, 68, 1692–1728. [Google Scholar] [CrossRef]
- Kim, J.H.; Rahman, M.H.; Lee, W.H.; Suk, K. Chemogenetic Stimulation of the Gi Pathway in Astrocytes Suppresses Neuroinflammation. Pharmacol. Res. Perspect. 2021, 9, e00822. [Google Scholar] [CrossRef] [PubMed]
- Dusaban, S.S.; Purcell, N.H.; Rockenstein, E.; Masliah, E.; Cho, M.K.; Smrcka, A.V.; Brown, J.H. Phospholipase Cε Links G Protein-Coupled Receptor Activation to Inflammatory Astrocytic Responses. Proc. Natl. Acad. Sci. USA 2013, 110, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.H.; Han, K.S.; Lee, J.; Won, W.; Koh, W.; Bae, J.Y.; Woo, J.; Kim, J.; Kwong, E.; Choi, T.Y.; et al. Activation of Astrocytic μ-Opioid Receptor Causes Conditioned Place Preference. Cell Rep. 2019, 28, 1154–1166.e5. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Vaidyanathan, T.V.; Collard, M.; Yokoyama, S.; Reitman, M.E.; Poskanzer, K.E. Cortical Astrocytes Independently Regulate Sleep Depth and Duration via Separate GPCR Pathways. Elife 2021, 10, e63329. [Google Scholar] [CrossRef]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. G i/o Protein-coupled Receptors Inhibit Neurons but Activate Astrocytes and Stimulate Gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte–Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef]
- Van Den Herrewegen, Y.; Sanderson, T.M.; Sahu, S.; De Bundel, D.; Bortolotto, Z.A.; Smolders, I. Side-by-Side Comparison of the Effects of Gq- and Gi-DREADD-Mediated Astrocyte Modulation on Intracellular Calcium Dynamics and Synaptic Plasticity in the Hippocampal CA1. Mol. Brain 2021, 14, 144. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid Lipid Nanoparticles as a Drug Delivery System for the Treatment of Neurodegenerative Diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity. Front. Pharmacol. 2019, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Piantino, M.; Louis, F.; Shigemoto-Mogami, Y.; Kitamura, K.; Sato, K.; Yamaguchi, T.; Kawabata, K.; Yamamoto, S.; Iwasaki, S.; Hirabayashi, H.; et al. Brain Microvascular Endothelial Cells Derived from Human Induced Pluripotent Stem Cells as in Vitro Model for Assessing Blood-Brain Barrier Transferrin Receptor-Mediated Transcytosis. Mater. Today Bio 2022, 14, 100232. [Google Scholar] [CrossRef] [PubMed]
- Manfredsson, F.P.; Mandel, R.J. Development of Gene Therapy for Neurological Disorders. Available online: https://pubmed.ncbi.nlm.nih.gov/20350486/ (accessed on 30 October 2022).
- Sloane, E.; Ledeboer, A.; Seibert, W.; Coats, B.; van Strien, M.; Maier, S.F.; Johnson, K.W.; Chavez, R.; Watkins, L.R.; Leinwand, L.; et al. Anti-Inflammatory Cytokine Gene Therapy Decreases Sensory and Motor Dysfunction in Experimental Multiple Sclerosis: MOG-EAE Behavioral and Anatomical Symptom Treatment with Cytokine Gene Therapy. Brain. Behav. Immun. 2009, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Exogenous Leukemia Inhibitory Factor Stimulates Oligodendrocyte Progenitor Cell Proliferation and Enhances Hippocampal Remyelination. J. Neurosci. 2012, 32, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Kriaučiūnaitė, K.; Kaušylė, A.; Pajarskienė, J.; Tunaitis, V.; Lim, D.; Verkhratsky, A.; Pivoriūnas, A. Immortalised Hippocampal Astrocytes from 3xTG-AD Mice Fail to Support BBB Integrity In Vitro: Role of Extracellular Vesicles in Glial-Endothelial Communication. Cell. Mol. Neurobiol. 2021, 41, 551–562. [Google Scholar] [CrossRef]
- Pan, S.M.; Zhou, Y.F.; Zuo, N.; Jiao, R.Q.; Kong, L.D.; Pan, Y. Fluoxetine Increases Astrocytic Glucose Uptake and Glycolysis in Corticosterone-Induced Depression through Restricting GR-TXNIP-GLUT1 Pathway. Front. Pharmacol. 2022, 13, 872375. [Google Scholar] [CrossRef]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-Glycoprotein: New Insights into Structure, Physiological Function, Regulation and Alterations in Disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 Reductions Exacerbate Alzheimer’s Disease Vasculo-Neuronal Dysfunction and Degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef]
- Ding, Y.; Zhong, Y.; Baldeshwiler, A.; Abner, E.L.; Bauer, B.; Hartz, A.M.S. Protecting P-Glycoprotein at the Blood–Brain Barrier from Degradation in an Alzheimer’s Disease Mouse Model. Fluids Barriers CNS 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-Neuron Interactions in the Mammalian Retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Luo, Q.; Stevens, J.A.A.; Giovagnoni, C.; van Kruining, D.; Bode, G.; den Hoedt, S.; Hobo, B.; Scheithauer, A.L.; Walter, J.; et al. CERTL Reduces C16 Ceramide, Amyloid-β Levels, and Inflammation in a Model of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Ghate, V.; Nampoothiri, M.; Lewis, S. Multifunctional Role of Exosomes in Viral Diseases: From Transmission to Diagnosis and Therapy. Cell. Signal. 2022, 94, 110325. [Google Scholar] [CrossRef] [PubMed]
- Adolf, A.; Rohrbeck, A.; Münster-Wandowski, A.; Johansson, M.; Kuhn, H.-G.; Kopp, M.A.; Brommer, B.; Schwab, J.M.; Just, I.; Ahnert-Hilger, G.; et al. Release of Astroglial Vimentin by Extracellular Vesicles: Modulation of Binding and Internalization of C3 Transferase in Astrocytes and Neurons. Glia 2019, 67, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated with a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front. Cell. Neurosci. 2019, 12, 526. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes Secrete Exosomes Enriched with Proapoptotic Ceramide and Prostate Apoptosis Response 4 (PAR-4). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-Derived Extracellular Vesicles: Neuroreparative Properties and Role in the Pathogenesis of Neurodegenerative Disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Croft, C.L.; Liu, X.; Ryu, D.H.; Ceballos-Diaz, C.; Tejeda, G.; Marrero, M.; Moran, C.; Cruz, P.E.; Ladd, T.; Moore, B.D.; et al. Elucidating the Relationship between TGF-β Signaling and Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, e043570. [Google Scholar] [CrossRef]
- Patel, M.R.; Weaver, A.M. Astrocyte-Derived Small Extracellular Vesicles Promote Synapse Formation via Fibulin-2-Mediated TGF-β Signaling. Cell Rep. 2021, 34, 108829. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes from Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Xiao, O.M.; Banwait, S.; Jin, K.; Greenberg, D.A. Neuroglobin Attenuates β-Amyloid Neurotoxicity in Vitro and Transgenic Alzheimer Phenotype in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 19114–19119. [Google Scholar] [CrossRef] [PubMed]
- Bavisotto, C.C.; Scalia, F.; Gammazza, A.M.; Carlisi, D.; Bucchieri, F.; de Macario, E.C.; Macario, A.J.L.; Cappello, F.; Campanella, C. Extracellular Vesicle-Mediated Cell–Cell Communication in the Nervous System: Focus on Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 434. [Google Scholar] [CrossRef]
- Nafar, F.; Williams, J.B.; Mearow, K.M. Astrocytes Release HspB1 in Response to Amyloid-β Exposure in Vitro. J. Alzheimer’s Dis. 2015, 49, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lin, M.C.; Tsai, J.S.; He, P.L.; Luo, W.T.; Herschman, H.; Li, H.J. EP4 Antagonist-Elicited Extracellular Vesicles from Mesenchymal Stem Cells Rescue Cognition/Learning Deficiencies by Restoring Brain Cellular Functions. Stem Cells Transl. Med. 2019, 8, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of Neuronal Cell Survival by Astrocyte-Derived Exosomes under Hypoxic and Ischemic Conditions Depends on Prion Protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Bhatia, S.; Kim, W.S.; Shepherd, C.E.; Halliday, G.M. Apolipoprotein D Upregulation in Alzheimer’s Disease but Not Frontotemporal Dementia. J. Mol. Neurosci. 2019, 67, 125–132. [Google Scholar] [CrossRef]
- Dassati, S.; Waldner, A.; Schweigreiter, R. Apolipoprotein D Takes Center Stage in the Stress Response of the Aging and Degenerative Brain. Neurobiol. Aging 2014, 35, 1632–1642. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kim, H.-S.; Shin, T.-H.; Kang, I.; Lee, J.Y.; Kim, J.-J.; Kang, H.K.; Seo, Y.; Lee, S.; Yu, K.-R.; et al. PGE2 Maintains Self-Renewal of Human Adult Stem Cells via EP2-Mediated Autocrine Signaling and Its Production Is Regulated by Cell-to-Cell Contact. Sci. Rep. 2016, 6, 26298. [Google Scholar] [CrossRef]
- Kim, H.; Shin, T.; Lee, B.; Yu, K.; Seo, Y.; Lee, S.; Seo, M.; Hong, I.; Choi, S.W.; Seo, K.; et al. Human Umbilical Cord Blood Mesenchymal Stem Cells Reduce Colitis in Mice by Activating NOD2 Signaling to COX2. Gastroenterology 2013, 145, 1392–1403.e8. [Google Scholar] [CrossRef]
- Chang, J.-K.; Li, C.-J.; Wu, S.-C.; Yeh, C.-H.; Chen, C.-H.; Fu, Y.-C.; Wang, G.-J.; Ho, M.-L. Effects of Anti-Inflammatory Drugs on Proliferation, Cytotoxicity and Osteogenesis in Bone Marrow Mesenchymal Stem Cells. Biochem. Pharmacol. 2007, 74, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Dias, B.; Jovanovic, K.; Gonsalves, D.; Moodley, K.; Reusch, U.; Knackmuss, S.; Weinberg, M.S.; Little, M.; Weiss, S.F.T. The 37kDa/67kDa Laminin Receptor Acts as a Receptor for Aβ42 Internalization. Sci. Rep. 2014, 4, 5556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, B. Alzheimer’s Disease and Prion Protein. Intractable Rare Dis. Res. 2013, 2, 35. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Moniruzzaman, M.; Dastgheyb, R.M.; Yoo, S.W.; Wang, M.; Hao, H.; Liu, J.; Casaccia, P.; Nogueras-Ortiz, C.; Kapogiannis, D.; et al. Astrocytes Deliver CK1 to Neurons via Extracellular Vesicles in Response to Inflammation Promoting the Translation and Amyloidogenic Processing of APP. J. Extracell. Vesicles 2020, 10, e12035. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine Loaded PLGA PEGylated Nanoparticles for Alzheimer’s Disease: In Vitro and in Vivo Characterization. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-Coated Gold Nanoparticles Transfer across Human Brain Endothelium and Enter Astrocytes in Vitro. PLoS ONE 2013, 8, 81043. [Google Scholar] [CrossRef]
- Shui, B.; Tao, D.; Cheng, J.; Mei, Y.; Jaffrezic-Renault, N.; Guo, Z. A Novel Electrochemical Aptamer-Antibody Sandwich Assay for the Detection of Tau-381 in Human Serum. Analyst 2018, 143, 3549–3554. [Google Scholar] [CrossRef]
- Elbassal, E.A.; Morris, C.; Kent, T.W.; Lantz, R.; Ojha, B.; Wojcikiewicz, E.P.; Du, D. Gold Nanoparticles as a Probe for Amyloid-β Oligomer and Amyloid Formation. J. Phys. Chem. C 2017, 121, 20007–20015. [Google Scholar] [CrossRef]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M.; et al. Impact of Gold Nanoparticles on Amyloid β-Induced Alzheimer’s Disease in a Rat Animal Model: Involvement of STIM Proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; Silveira, G.d.; de Souza, D.L.; Brandolfi, J.d.; de Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold Nanoparticles Prevent Cognitive Deficits, Oxidative Stress and Inflammation in a Rat Model of Sporadic Dementia of Alzheimer’s Type. Mater. Sci. Eng. C 2017, 77, 476–483. [Google Scholar] [CrossRef]

| Sr.no | Astrocyte Transcription Factor | Role in Astrocytes | Reference |
|---|---|---|---|
| 1 | STAT3 | Increases reactive astrocytes, Aβ deposition, and cognition impairment. | [106,107,108] |
| 2 | PAX6 | Control neurogenesis and decrease neurodegenerative markers | [111] |
| 3 | TFEB | Clearance of Aβ plaques via lysosomal biogenesis. | [112,113] |
| 4 | TFAM | Protects mitochondria against Aβ-42 peptide via mitochondrial biogenesis | [114] |
| 5 | NFIA | Enhances learning and memory | [115] |
| 6 | CEBPβ | Upregulate pro-inflammatory cytokines | [116] |
| 7 | YY-1 | Generation of Aβ peptides | [117,118] |
| 8 | Epigenetic HDAC | Inhibits Aβ induced HDAC-2 and HDAC-5 expression | [119] |
| 9 | CREB | Enhances long-term memory | [120] |
| 10 | Nurr1 | Controls memory and learning functions | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, A.; Kodi, T.; Satarker, S.; Chowdari Gurram, P.; Upadhya, D.; SM, F.; Mudgal, J.; Nampoothiri, M. Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions. Cells 2022, 11, 4111. https://doi.org/10.3390/cells11244111
Nassar A, Kodi T, Satarker S, Chowdari Gurram P, Upadhya D, SM F, Mudgal J, Nampoothiri M. Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions. Cells. 2022; 11(24):4111. https://doi.org/10.3390/cells11244111
Chicago/Turabian StyleNassar, Ajmal, Triveni Kodi, Sairaj Satarker, Prasada Chowdari Gurram, Dinesh Upadhya, Fayaz SM, Jayesh Mudgal, and Madhavan Nampoothiri. 2022. "Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions" Cells 11, no. 24: 4111. https://doi.org/10.3390/cells11244111
APA StyleNassar, A., Kodi, T., Satarker, S., Chowdari Gurram, P., Upadhya, D., SM, F., Mudgal, J., & Nampoothiri, M. (2022). Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions. Cells, 11(24), 4111. https://doi.org/10.3390/cells11244111






