Abstract
Formerly hailed as “undruggable” proteins, transcription factors (TFs) are now under investigation for targeted therapy. In cancer, this may alter, inter alia, immune evasion or replicative immortality, which are implicated in genome organization, a process that accompanies multi-step tumorigenesis and which frequently develops in a non-random manner. Still, targeting-related research on some TFs is scarce, e.g., among AP-2 proteins, which are known for their altered functionality in cancer and prognostic importance. Using public repositories, bioinformatics tools, and RNA-seq data, the present study examined the ligandability of all AP-2 members, selecting the best one, which was investigated in terms of mutations, targets, co-activators, correlated genes, and impact on genome organization. AP-2 proteins were found to have the conserved “TF_AP-2” domain, but manifested different binding characteristics and evolution. Among them, AP-2δ has not only the highest number of post-translational modifications and extended strands but also contains a specific histidine-rich region and cleft that can receive a ligand. Uterine, colon, lung, and stomach tumors are most susceptible to AP-2δ mutations, which also co-depend with cancer hallmark genes and drug targets. Considering AP-2δ targets, some of them were located proximally in the spatial genome or served as co-factors of the genes regulated by AP-2δ. Correlation and functional analyses suggested that AP-2δ affects various processes, including genome organization, via its targets; this has been eventually verified in lung adenocarcinoma using expression and immunohistochemistry data of chromosomal conformation-related genes. In conclusion, AP-2δ affects chromosomal conformation and is the most appropriate target for cancer therapy focused on the AP-2 family.
Keywords:
transcription factors; targeted therapy; ligandability; cancer; genome organization; bioinformatics; AP-2; TFAP2; AP-2δ; TFAP2D 1. Introduction
The concept of Transcription Factors (TFs) targeting is becoming increasingly popular [1]. Formerly regarded as “undruggable” DNA-binding proteins, they are now investigated in studies examining their activity depending on the use of various selective modulators [2,3]. Currently available approaches include targeting nuclear hormone receptors or protein–protein interactions, modulating proteasomal TFs degradation or TF’s drivers, degrading TFs with PROteolysis TArgeting Chimeras (PROTACs), modifying TFs post-translationally, or disrupting TF-DNA binding [4,5,6,7,8,9,10,11]. In cancer, these interventions may not only increase cell death but block the immune evasion, Epithelial-to-Mesenchymal Transition (EMT), self-renewal, treatment resistance, replicative immortality or autoregulatory cancer-driving circuits [12]. Most of these were found to be implicated in genome reorganization [13,14,15,16], the process that accompanies a multi-step tumorigenesis and which frequently develops in a non-random manner [17]. In the current post-genomics era, three-dimensional genomics is a frontier field and is primarily focused on the relationship between chromatin spatial conformation and regulation of gene transcription [18]. It is estimated that one-fifth of the so far identified oncogenes are in fact TFs [19]. Still, targeting-related research on some TF families is scarce, for example, within Activating enhancer-binding Protein 2 (AP-2); this is unexpected because the members of this family are known for their altered functionality in cancer and prognostic importance in oncological patients [20,21]. Typically, AP-2 transcription factors are expressed during embryogenesis and orchestrate the cell cycle, proliferation, and apoptosis, enabling appropriate development of, inter alia, limbs, eyes, or facial features [22,23]. Apart from our previous study that paved the way for AP-2α and AP-2γ to be considered as targeted therapy candidates [24], other authors [19] have put a different family member, AP-2δ, on the list of nearly three hundred oncogenic transcription factors and regulators, which could be a useful hint for TF-based cancer treatment. However, no studies were focused on the structural properties that would justify the rationale for targeting AP-2 proteins or could describe how to perform it selectively, without targeting other AP-2 family members. Moreover, no inhibitors are currently known for AP-2 factors, complicating the research progress. In contrast to many previous experiments, which do not consider all five (α, β, γ, δ, ε) representatives of AP-2 family at once (even our previous research focused on the two best-described ones), the present study examines the relevance of each AP-2 member to be selectively recognized; it then identifies the best AP-2 protein from the point-of-view of selective ligandability and provides related data about its mutations, genetic targets, transcriptional co-factors and correlated genes in a pan-cancer spectrum. A specific stage of research was also dedicated to genes that govern chromosome conformation, in order to propose future directions in the AP-2 context since the interplay between this family and chromosomal conformation is insufficiently researched, while transcription on its own can shape the spatial genome structure [25].
2. Results and Discussion
2.1. AP-2 Proteins Have Conserved the “TF_AP-2” Domain, but the N-Terminal Amino Acid-Rich Region of AP-2δ Is Unique
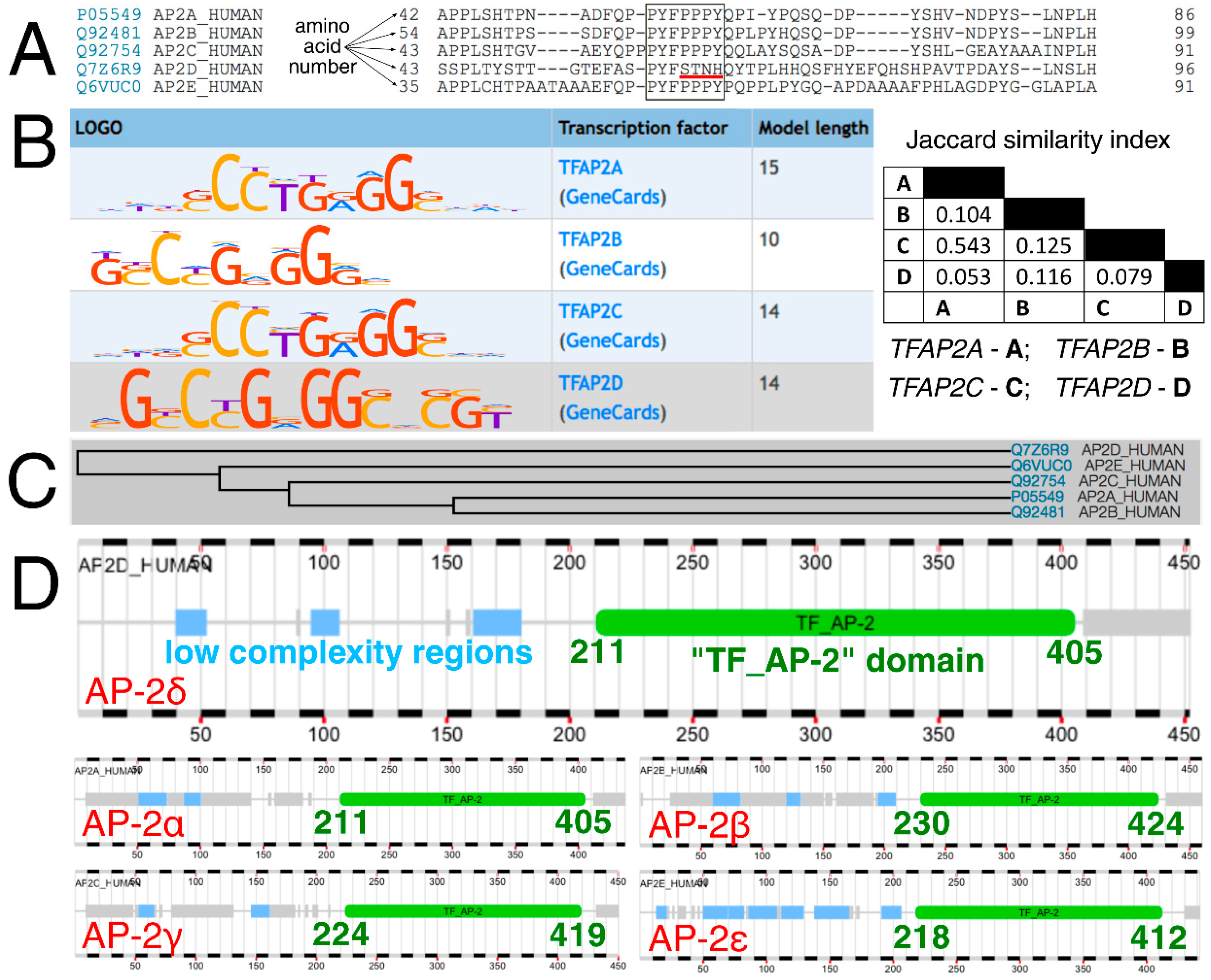
At first, AP-2 transcription factors were compared evolutionarily, functionally, and both in terms of how they bind to DNA sequences and are bound by the interacting proteins. Typically, AP-2 factors interact with other proteins with the use of a proline-rich motif (PPxY) while the cooperation within the AP-2 family is maintained via a so-called “TF_AP-2” domain that is responsible for dimerization (of homo- and hetero- dimers), as well as DNA binding (this region contains Helix-Span-Helix [HSH] motif) [26]. Clustal Omega revealed that a part of the sequence corresponding to the amino acid-rich motif has the pattern “PPPY” in AP-2α, AP-2β, AP-2γ, and AP-2ε, compared to “STNH” in AP-2δ (Figure 1A). Moreover, the DNA sequences that are recognized by AP-2 factors are also distinct (Figure 1B). While the length of the consensus logo for AP-2δ was congruent to that of AP-2α and AP-2γ, the Jaccard index suggested a slightly greater similarity of content between AP-2δ and AP-2β than between AP-2δ and AP-2α/AP-2γ. No data is available for AP-2ε based on HOmo sapiens COmprehensive MOdel COllection database (HOCOMOCO). Furthermore, the phylogenetic tree indicates that the AP-2δ evolved separately from the rest of the AP-2 family (Figure 1C). However, since the “TF_AP-2” domain is highly conserved [23] and encompasses the rest of the protein sequence in the direction of carboxyl terminus (Figure 1D), their distinction is supposedly due to differences located in the “first half” of the polypeptide chain, i.e., from amino terminus to the middle part of the amino acid sequence.
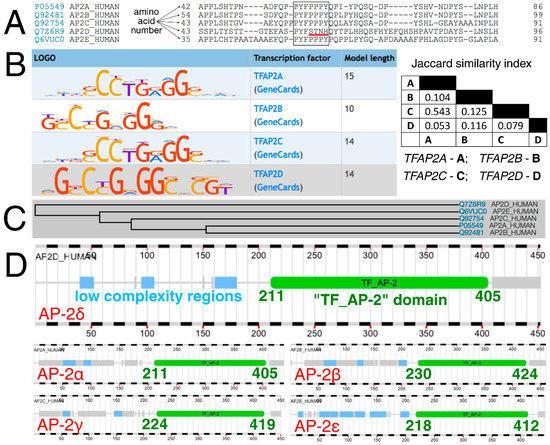
Figure 1.
Analysis of AP-2 factors’ sequence, recognizable DNA, evolution, and “TF_AP-2” domain. (A) Clustal Omega revealed that the amino acid sequence (encompassing the proline-rich motif in AP-2 members α, β, γ, ε) is different in AP-2δ (the core of the pattern is framed; the main difference in AP-2δ is underlined in red). (B) HOCOMOCO identified the consensus logo of AP-2δ to be similar in length to that of AP-2α and AP-2γ, but the sequence itself was congruent to shorter AP-2β according to MACRO-APE. (C) Phylogeny data acquired from UniProt proved the separate evolution of AP-2δ compared to other AP-2 proteins. (D) The range of the “TF_AP-2” domain among AP-2 members certifies that this part of the protein sequence is highly conserved. This indicates that the distinction of AP-2 proteins is supposedly due to differences located in the “first half” of the polypeptide chain. The “TF_AP-2” domain is marked in green, while the low complexity regions are marked in blue (acquired from FABRIC).
Although the last 20 years did not yield many findings related to the role of AP-2δ in carcinogenesis (this TF was cloned in 2001 [27], and the research focused more on embryogenesis [28,29,30,31]), some interesting observations were noted, e.g., its distinct binding affinity to conserved AP-2–binding sites [27]. This suggests that AP-2δ might regulate genes in vivo through other mechanisms than the remaining AP-2 TFs, presumably via interaction with unique coactivators. It has also been proposed that AP-2δ might function as a negative regulator, inhibiting or modulating the transactivation capability or DNA-binding affinity of the other AP-2 family members [23]. What also makes AP-2δ unique is the more restricted temporal and spatial expression pattern when compared to other AP-2 factors–among normal tissues, the expression of this TF was observed mainly in the central nervous system, retina, and developing heart [28]. AP-2δ is encoded by Transcription Factor AP-2 Delta (TFAP2D) gene, which shares the same cytological location (chromosome 6p12.3) with TFAP2B (encoding AP-2β) [26]. This explains the previous symbol of TFAP2D, originally denoted as TFAP2BL1 (“BL1” stands for “beta-like 1”) [32]. Interestingly, a human-specific Alu DNA cassette was found flanking the TFAP2D and TFAP2B genes, which may affect their regulation [33]—this leaves the investigational window open, especially since Alu elements have a role both in cancer and genomic rearrangements [34,35].
2.2. AP-2δ Has the Least Similar Protein Sequence That Contains the Highest Number of PTM Sites and Extended Strands
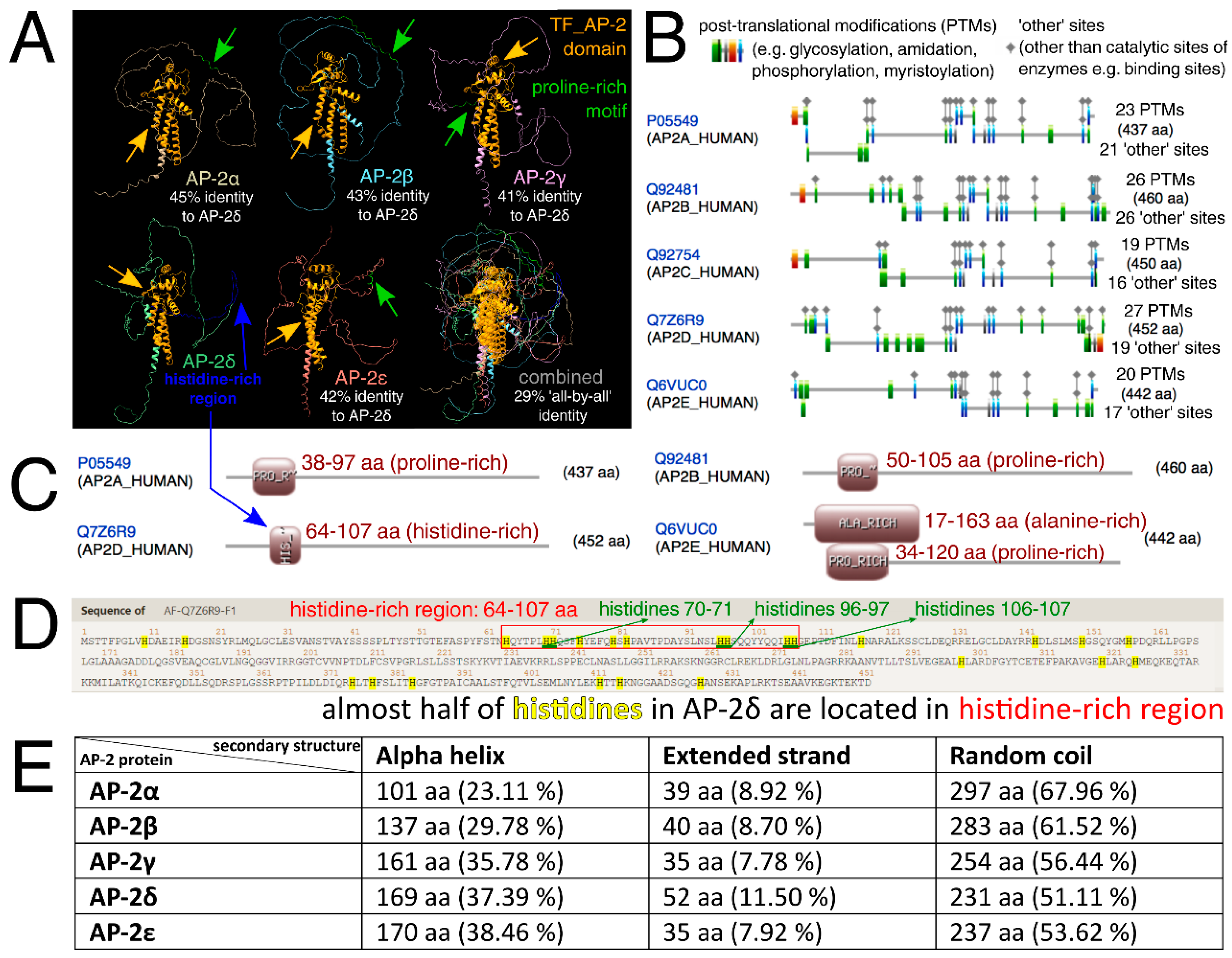
Similarities and differences in protein structures of all AP-2 factors were then investigated. Clearly, alpha-helices (that mainly correspond to the “TF_AP-2” domain and a part responsible for dimerization) are highly conserved in the entire family. However, the percentage of the shared identity of all AP-2 factors at once is about 30%, as investigated through Clustal Omega (Figure 2A). Due to the separate evolution of AP-2δ, it was also decided to consider this protein as a reference in pairwise comparisons—this revealed that AP-2δ is only almost half identical to other AP-2 factors. The percentages of identity for all comparisons are summarized in Table S1. The analysis of the amino acid sequence revealed that out of the entire family, AP-2δ possesses the highest number of Post-Translational Modifications (PTMs), which suggest vast regulation possibilities (Figure 2B). Targeting post-translation modifications is one of the TF-based therapy strategies [36,37]: more post-translational modification sites entail more therapeutic possibilities. Referring to the aforementioned “PPPY” and “STNH” patterns, the UniProt, ProSite, InterPro, and Motif Scan confirmed that AP-2α, AP-2β, AP-2γ, and AP-2ε have proline-rich motifs in this area. However, AP-2δ was the only family member lacking this motif, as it was not identified in any database (Table 1). The dissimilarity in the length of the sequence between UniProt + InterPro versus ProSite + Motif Scan is dictated by the fact that the first two databases indicate the motif, while the latter two demonstrate a wider profile of the region. Nevertheless, data from UniProt and InterPro are consistent with ProSite and Motif Scan: the amino acids of the motif exist within the wider area of the region. Subsequently, it was verified whether the lack of a proline-rich sequence in AP-2δ is compensated by another region that is rich in a specific amino acid. According to ProSite, it turned out the proline-rich region (with an additional alanine-rich region in AP-2ε) that is present in AP-2α/β/γ/ε, overlaps with the histidine-rich region of AP-2δ (Figure 2C). This region was depicted in advance so that all comparable motifs/regions and domains were present in a single graph (Figure 2A). The AP-2δ protein sequence was scanned for histidine, which revealed that almost half of histidines (10 out of 25) are located in the histidine-rich region (Figure 2D). Interestingly, this region includes three patterns where two histidines are in a row (“HH”); this would enable the histidine-rich region to be recognized selectively. For comparison (Figure S1), there is only one identical histidine pattern in AP-2β and one in AP-2γ (AP-2α and AP-2ε do not contain “HH” patterns in their sequences); the consideration of at least two such patterns would allow exclusive recognition of AP-2δ within the AP-2 family. The remaining differences investigated at this stage included the prediction of secondary structures that could be formed in the AP-2 factors. As revealed using the fourth Garnier–Osguthorpe–Robson algorithm (GOR4), out of the entire AP-2 family, AP-2δ has the largest number of extended strands but the smallest of random coils (Figure 2E). Bang et al. suggested that binding affinity could be elevated with the increase of extended strands in secondary structure [38]. Particular attention was also given to the amino acids preceding the conserved “TF_AP-2” domain; these were evaluated for their similarity, physicochemical properties, and structural characteristics (Figure 3). In brief, AP-2δ was found to be the least similar to other AP-2 TFs, which certifies the comparison of the entire protein sequences. Compared to the rest of family, the “first half” of AP-2δ polypeptide chain is more hydrophilic, contains a lower amount of conformationally special amino acids (proline, glycine) but comprises additional cysteines and threonines. Although the regions that mainly contribute to the binding affinity are hydrophobic, hydrophilicity may facilitate drug specificity [39]. The occurrence of cysteines can also reduce the off-target effect and toxicity [40]. From the structural point of view, there is an increment of amino acids having the highest strand propensity, whereas the opposite is found regarding the number of residues with a high ability to form a turn. From the drug-targeting perspective, AP-2δ was also investigated for the content of leucine (L), isoleucine (I), phenylalanine (F), and methionine (M), seeing that the recent study by Wang et al. indicated that drugs frequently bind to these amino acids [41]. Analyzing the entire protein sequences (acquired from the UniProt database), AP-2δ contains the largest sum of “LIFM” amino acids, whereas in the “first half” it is surpassed only by AP-2ε. Collectively, it appears that some properties might be of use with regard to ligand development.
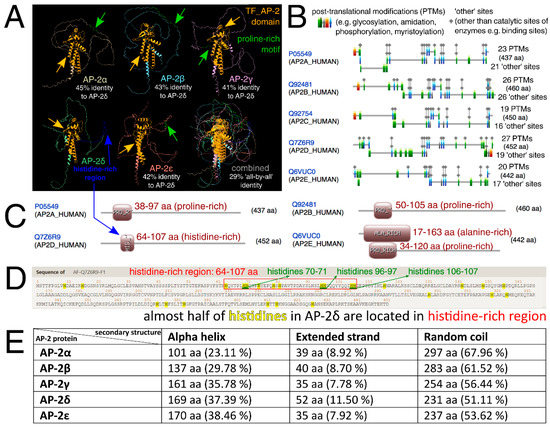
Figure 2.
Similarities and differences of AP-2 factors with regard to protein structures, frequent patterns, and amino acid-rich regions. (A) Three-dimensional protein structures of AP-2 factors, with additional overlapping, were shown via ChimeraX, while the percentages of structure identity (in relation to AP-2δ) were estimated using Clustal Omega. “TF_AP-2” domains are marked in orange, proline-rich motifs (of AP-2α/β/γ/ε) are in green, while the histidine-rich region (of AP-2δ) is in blue. (B) ScanProsite tool revealed that the highest number of PTMs can be found in AP-2δ. (C) Based on ProSite data, the histidine-rich region of AP-2δ (shown beforehand in the first subfigure) overlaps with the proline-rich region found in other AP-2 proteins. (D) Visualization of AP-2δ sequence with emphasis on histidines (highlighted in yellow) and histidine-rich region (framed in red). Two histidines in a row (“HH”) are underlined in green. (E) The GOR4 algorithm (estimating protein secondary structures) indicated that AP-2δ contains the smallest number of random coils but the highest of extended strands. Provided are both the quantity and percentage of amino acids that form these structures.

Table 1.
Occurrence of proline-rich sequence in AP-2 transcription factors according to databases.

Figure 3.
Emphasis on amino acids preceding the conserved “TF_AP-2” domain of AP-2 transcription factors. Data were acquired from the alignment of all AP-2 members using UniProt database. The quantity of amino acids presented for each protein is provided to the right of the sequence, including the far-right panel that subdivides residues based on a specific coloring scheme. Both extremes (the highest and the lowest number among AP-2 proteins) are framed in black. In the middle of the figure, the amino acid sequence encompassing the core of the proline-rich motif of AP-2α/β/γ/ε is also framed in black. “Similarity” indicates the percentage identity of each amino acid between AP-2 proteins. Physicochemical properties are grouped using the Zappo coloring scheme; serines and threonines are also presented in a separate panel. Subsequent panels present structural properties which were dichotomized. Buried index—depending on the frequency of occurrence inside a protein, the amino acid residues are colored from blue (most frequent) to light green (less frequent). Helix propensity—the ability of an amino acid to form an α-helix, colored from magenta (the highest helix propensity) to green (the lowest one). Strand propensity—the ability of an amino acid to form a β-strand, colored from yellow (the highest strand propensity) to blue (the lowest one). Turn propensity—the ability of an amino acid to form a turn, colored from red (the highest turn propensity) to cyan (the lowest one).
2.3. AP-2δ Contains the Most Relevant Ligandability-Related Cleft That Can Be Availed Using a Unique Histidine-Rich Region
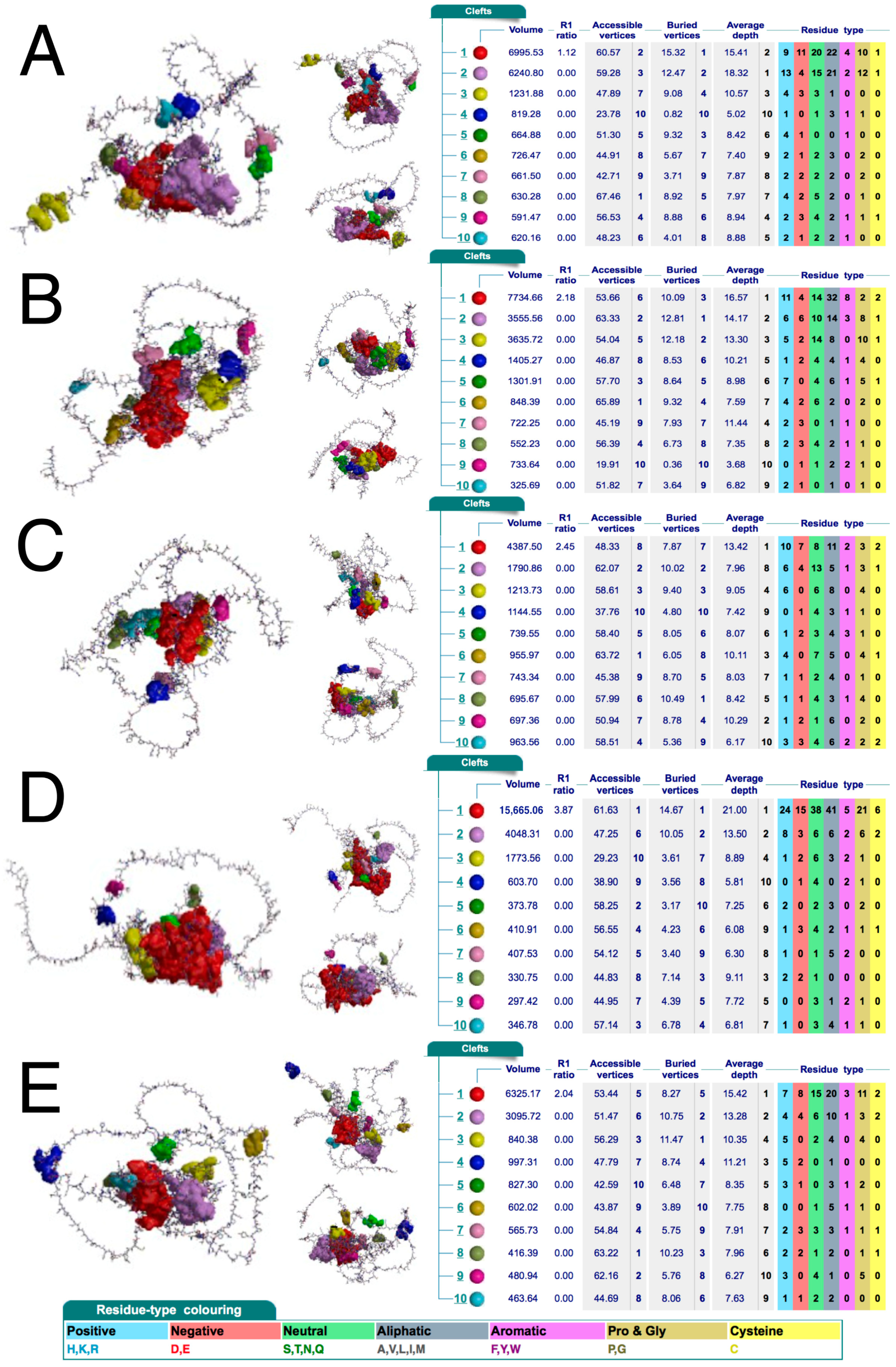
The above findings suggest that AP-2δ could be selectively recognized by a potential ligand, at least among AP-2 family members. It was then evaluated whether any relevant clefts are present in the protein structure. The cleft analysis performed using a database containing structural summaries of Protein Data Bank entries (PDBsum) indicated that AP-2δ has the largest cleft out of the entire family; this pocket is twice as large (volume: 15665 Ångströms) compared to the second largest cleft (volume: 7734 Ångströms) present in AP-2β (Figure 4). It is known that ligands preferably bind to larger clefts [42]; moreover, the so-called R1 ratio of the cleft should ideally be >2 [43]. Regarding the biggest cleft of AP-2δ, it has R1 ratio close to 4; the other AP-2 factors have their largest pockets with an R1 ratio not exceeding 2.5, except for AP-2α which has no clefts exceeding 2.
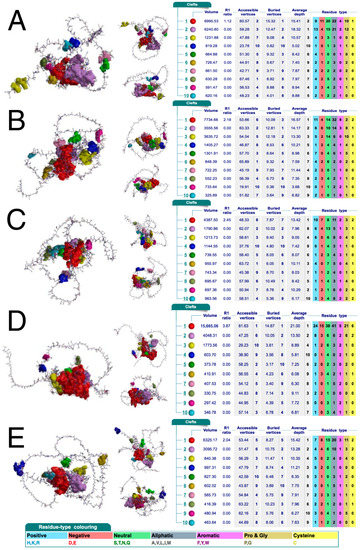
Figure 4.
Clefts in the protein structure of AP-2α (A), AP-2β (B), AP-2γ (C), AP-2δ (D), and AP-2ε (E). Three orientations of protein structure with highlighted clefts (marked in various colors that correspond to those in tables) are shown for each AP-2 transcription factor. Accompanied tables with details on the pocket’s volume, R1 ratio, depth, and residue type are visible on the right; the coloring of the latter is below the last subfigure. Data acquired from PDBsum.
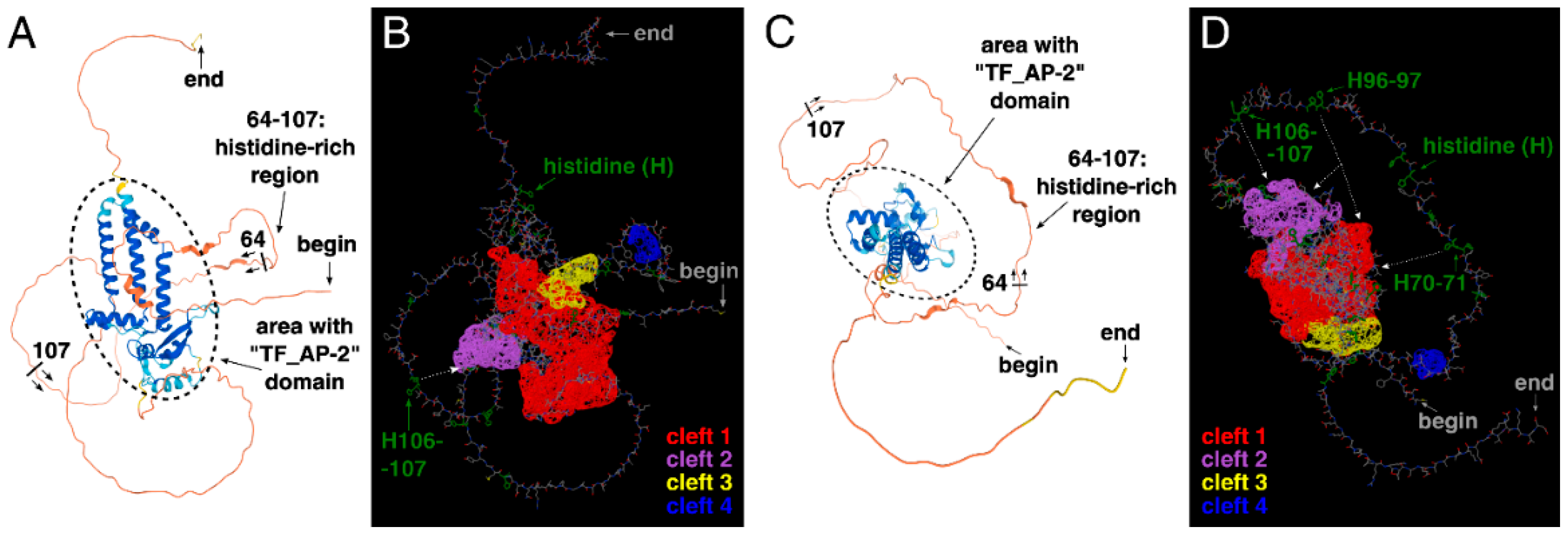
Based on these findings, the three-dimensional structure of AP-2δ was analyzed. At first glance, the presence of a histidine-rich region (in particular: three “HH” patterns) instead of a proline-rich region appears to be a good solution that allows selective identification of AP-2δ among other AP-2 proteins. It is theoretically possible to consider amino acid-rich motifs as therapeutic targets, as has been found previously [44,45]. Alternatively, these histidines could be used as a docking site so that the potential ligand could also recognize a different, nearby sequence. For example, the second-best cleft of AP-2δ is close to histidines 106–107 (H106–107) and there is a space between them (Figure 5A,B). The cleft itself is also composed of histidines (Figure 4D) but not the ones comprising a histidine-rich region; the occurrence of such amino acids in cavities can strengthen the binding [46].
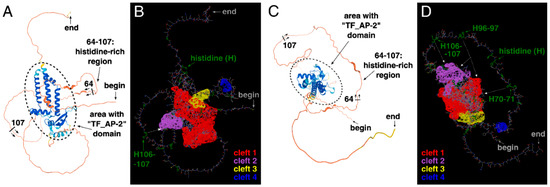
Figure 5.
The three-dimensional structure of AP-2δ visualized using AlphaFold and ChimeraX. The emphasis on the histidine-rich region is shown to spot its range, i.e., 64–107 aa; the helices mainly overlapping with the “TF_AP-2” domain are encircled (A,C). The location of “HH” patterns (two histidines in a row, marked in green) and the largest clefts (highlighted in red, purple, yellow, and blue) are shown (B,D). Both amino terminus (“begin”) and carboxyl terminus (“end”) of the protein are indicated.
However, many other proteins (outside the AP-2 family) could contain identical “HH” pattern(s); therefore, the ideal solution should be related to the simultaneous recognition of double histidines and the “TF_AP-2” domain (that is exclusive for AP-2 family representatives). Recognition of the “TF_AP-2” domain would exclude proteins that are not representatives of the AP-2 family, whereas recognition of at least two “HH” patterns would exclude other AP-2 factors than AP-2δ. In the entire AP-2δ sequence, there are three “HH” patterns, all within the histidine-rich motif, making it possible to bind to the specific location. It is also worth underlining that the best three clefts in AP-2δ mainly overlap with the “TF_AP-2” domain (Figure 4). Hence, this could be a method that would not necessarily aim at the largest best cleft only, but could act on some other cavities. Since the “TF_AP-2” domain also includes a sequence that is responsible for dimerization, the ligand binding, i.a., on the site of the “TF_AP-2” domain would act as a steric hindrance, inhibiting the function of transcription factor dimers. Hypothetically, there is a space for the ligand to recognize not only the “HH” patterns of the histidine-rich region but also some clefts (including the largest one) that overlap with the “TF_AP-2” domain (Figure 5C,D). Thus, one of the examples showing the rationality for further research in this field is presented. Unfortunately, the research methodology that needs to be performed requires the expensive verification and time-consuming description of much more detailed strategies, most probably via Computer-Aided Drug Design (CADD) and further pre-clinical molecule selection.
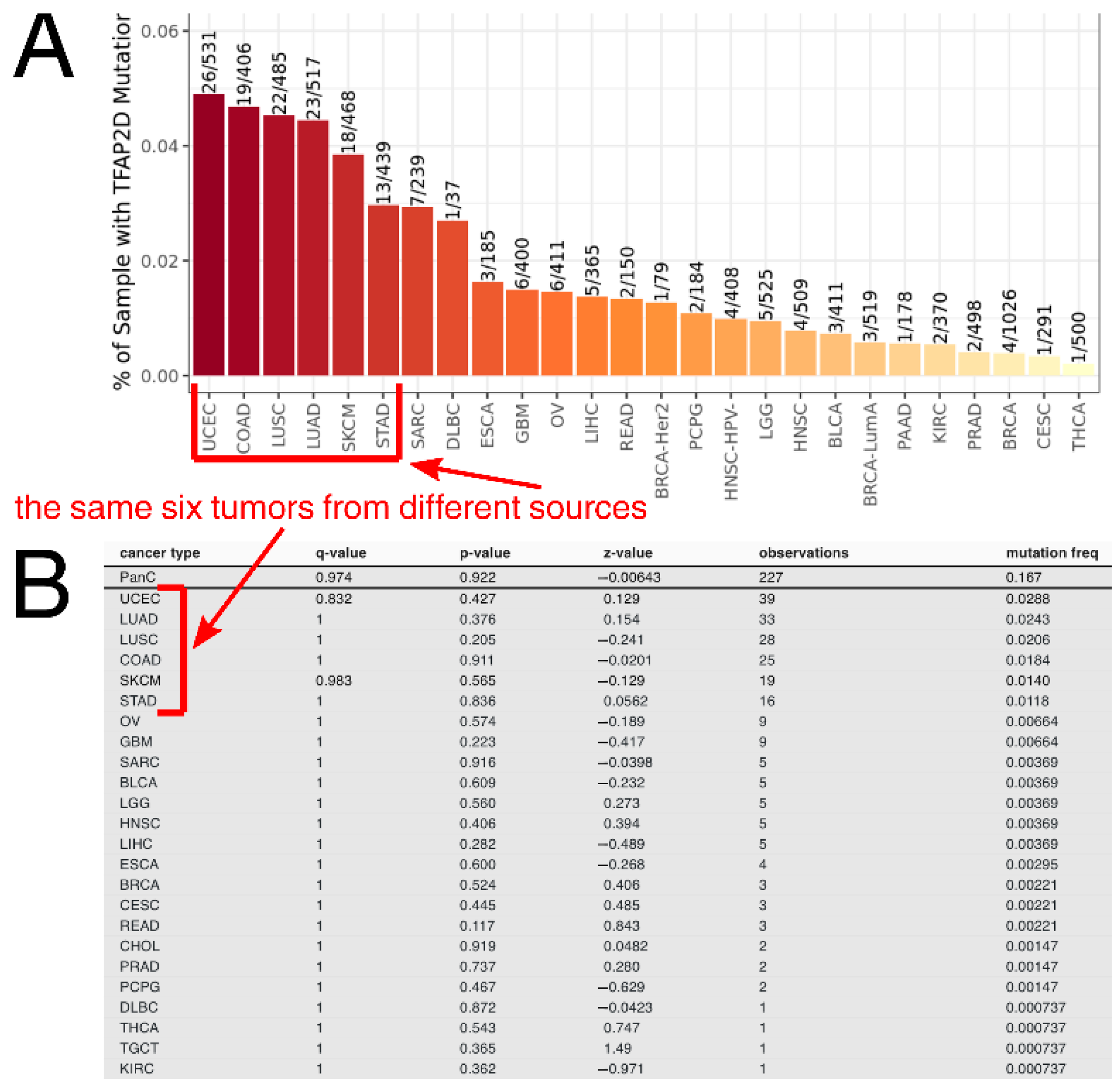
2.4. The Highest Number of Samples with TFAP2D Mutation Was Observed in UCEC, COAD, LUSC, LUAD, and STAD, the Tumors in Which Cancer-Related Genes Co-Depended with TFAP2D
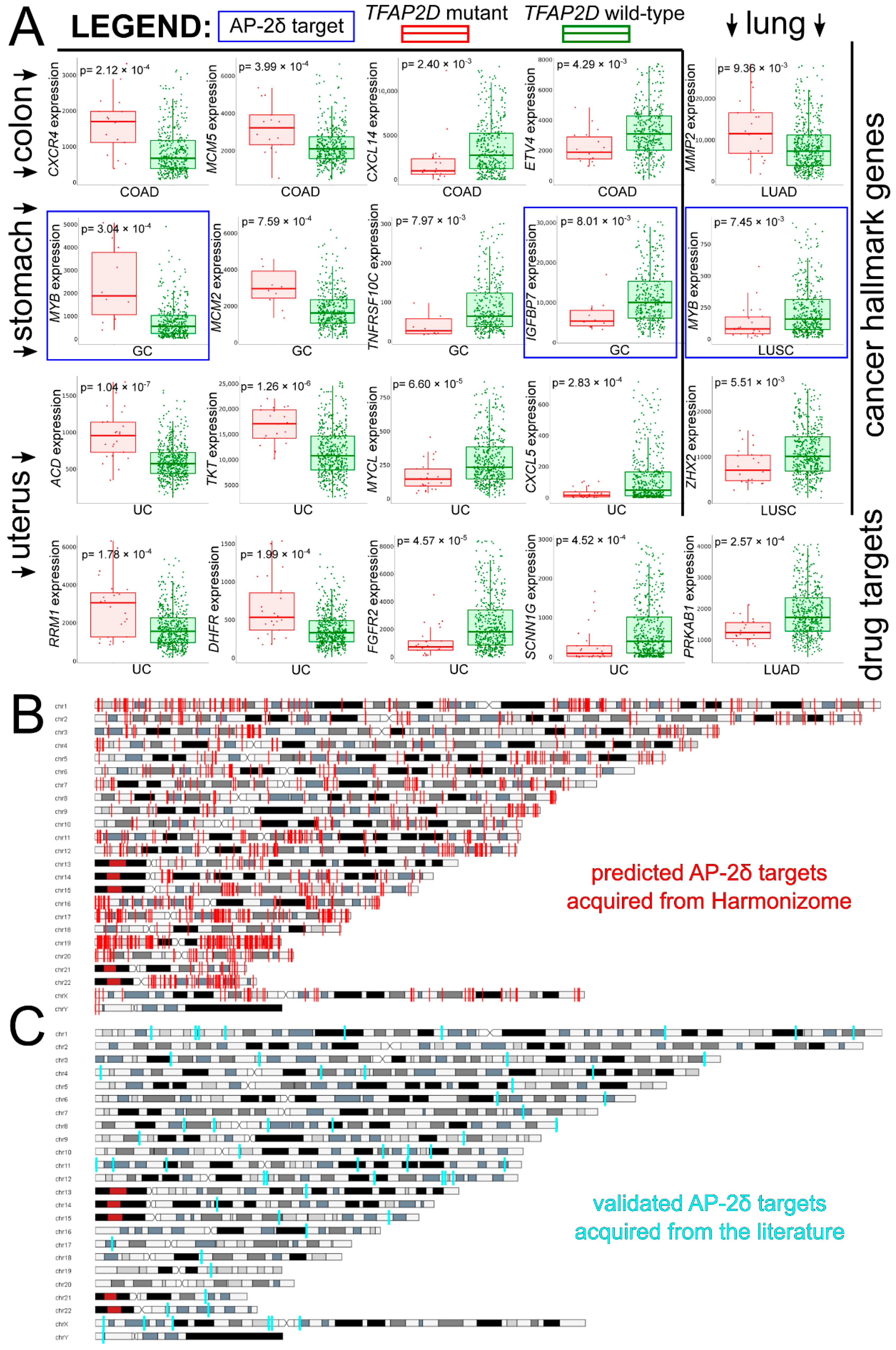
Taking a pan-cancer view of AP-2δ, the number of samples with the TFAP2D gene mutation was determined across tumor types. The Tumor Immune Estimation Resource (TIMER 2.0) indicated that the largest percentage of samples with the mutation was in uterine corpus endometrial carcinoma (Figure 6A), which is in agreement with the FABRIC Cancer Portal (Figure 6B). Collectively, the following six tumors have the highest percentages of samples with TFAP2D mutation: Uterine Corpus Endometrial Carcinoma (UCEC), Colon Adenocarcinoma (COAD), Lung Squamous Cell Carcinoma (LUSC), Lung Adenocarcinoma (LUAD), Skin Cutaneous Melanoma (SKCM), and Stomach Adenocarcinoma (STAD). However, while the mutation frequency is not statistically significant in any tumor or even in a pan-cancer view (according to FABRIC), muTarget indicates that several cancer hallmark genes and a few Food and Drug Administration (FDA)-approved drug targets demonstrate significantly different expression between TFAP2D-mutant and TFAP2D-wild samples of uterine, colon, lung, and stomach cancers (Figure 7A). Regarding cancer hallmark genes for colon, stomach and uterine cancers, only the two best up- and downregulated examples are presented in the figure; the same applies to uterine cancer in terms of FDA-approved drug targets. Supplementary File S1 summarizes the results from muTarget, which in addition to cancer hallmark genes and FDA-approved drug targets, includes data for all significant genes identified using this tool. Separately for upregulated and downregulated TFAP2D mutation-related genes, the functional analysis was performed among cohorts visible in Figure 7A. The gene ontology revealed that the “cell adhesion” and “cellular protein metabolic process” were the most frequently annotated processes, i.e., found in at least half of functional annotations. It is important to note that the tumors identified through muTarget overlap with those of TIMER/FABRIC, and that some genes from muTarget are predicted genetic targets of AP-2δ (marked with a blue frame). Regarding AP-2δ gene targets, their location in the genome was additionally visualized using Ideogram Viewer and split into predicted and validated ones (Figure 7B,C, respectively). Due to the lack of literature on AP-2δ, it is challenging to find data that can be referenced, not only in specific tumor but also in pan-cancer view. Thus, it is unknown whether some of these observations are biologically more inquisitive than the others. Especially since these genes include representatives of regulation of chemotaxis (CXCR4, CXCL14, CXCL5 [47]), DNA replication (MCM2, MCM5 [48]), Extracellular Matrix (ECM) degradation (MMP2 [49]), transcription (MYB, MYCL, ZHX2 [50,51,52]) telomere function (ACD [53]), catalysis (RRM1, DHFR [54,55]), or signaling (TNFRSF10C, IGFBP7, FGFR2, PRKAB1 [56,57,58,59]).
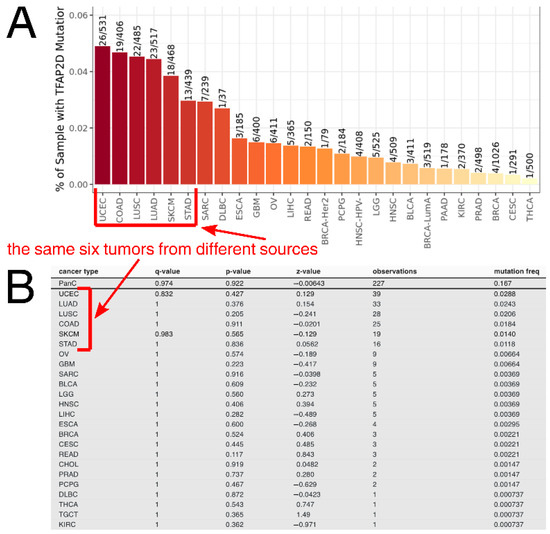
Figure 6.
TFAP2D mutation frequency across tumors according to TIMER (A) and FABRIC (B). The same six tumors having the highest mutation frequency (i.e., UCEC, COAD, LUSC, LUAD, SKCM, STAD) are marked with red frames and arrows.
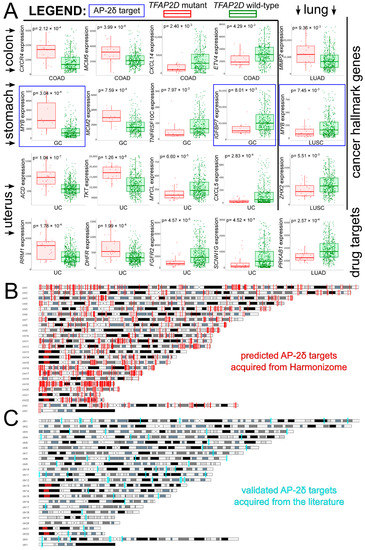
Figure 7.
Investigation of AP-2δ mutational status via muTarget and visualization of AP-2δ genetic targets through Ideogram Viewer. (A) The best cancer hallmark genes and FDA-approved drug targets of which expression co-dependence occurs with TFAP2D mutation status (complete list available in Supplementary Materials). Genes identified as AP-2δ genetic targets are framed in blue. (B) Genomic location of predicted AP-2δ targets (marked in red). (C) Genomic location of validated AP-2δ targets (marked in cyan).
2.5. Some AP-2δ Targets Might Be Located in Adjacent TADs or Act as TcoFs
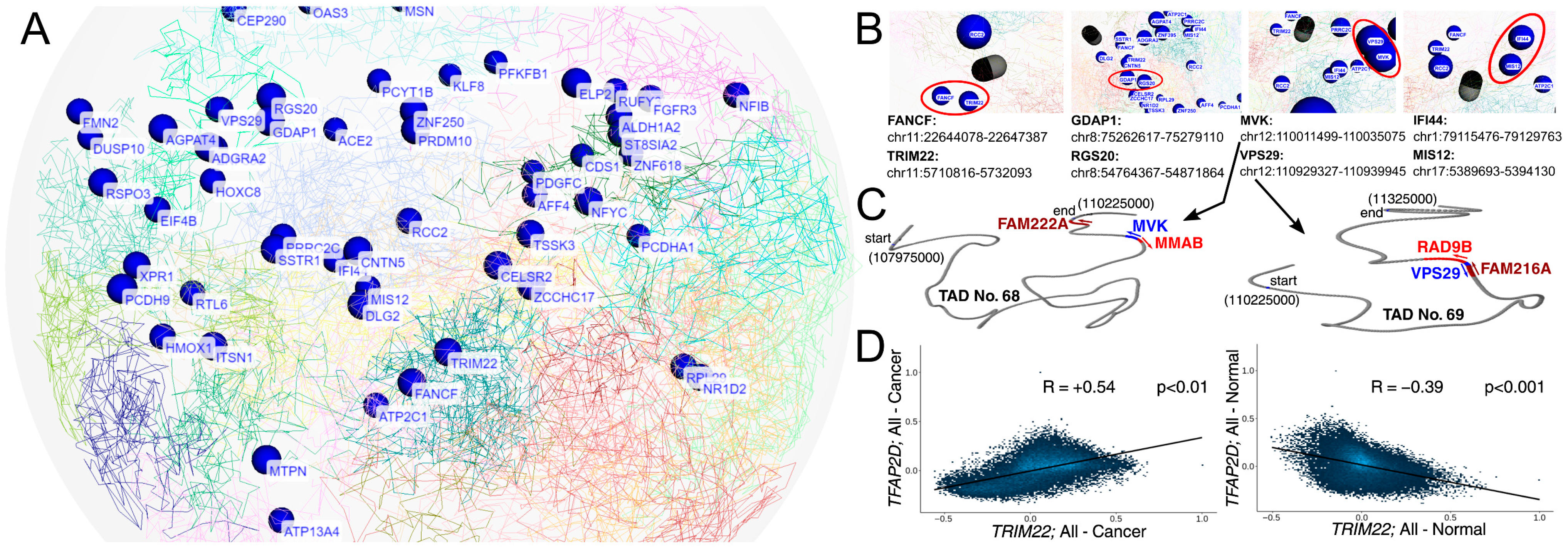
The experimentally validated AP-2δ targets were investigated for their location on chromosomes, taking into account the three-dimensional genome organization [60] (Figure 8A and Figure S2). The intention was to identify any informative insights into AP-2δ regulation, as currently there is only one Chromatin Immunoprecipitation (ChIP) method (specifically: ChIP-exo [exonuclease]) which was performed as a part of enormous and valuable research [61], but unfortunately provides a small amount of data when it comes to AP-2δ. Our WashU Browser-based investigation (Figure 8B) presented that AP-2δ targets might not only be located in close proximity on a single chromosome (e.g., TRIM22 and FANCD on chromosome 11) but also on different chromosomes (IFI44 and MIS12 on chromosomes 1 and 17, respectively). For the chromosomal location of each gene, see Supplementary File S2. Particularly interesting was the example of MVK and VPS29, in which their genomic locations were found to be the closest to each other among the presented genes. Such close proximity suggested that they may constitute a single Topologically Associating Domain (TAD) [62]; however, as investigated via TAD Knowledge Base (TADKB), they are a part of two adjacent TADs (Figure 8C and Figure S2). Although DNA sequences of single TAD interact more frequently than those from neighboring domains [63], adjacent TADs were also found to partially overlap [64], which provides a snippet of information on how AP-2δ targets might be organized in the genome.
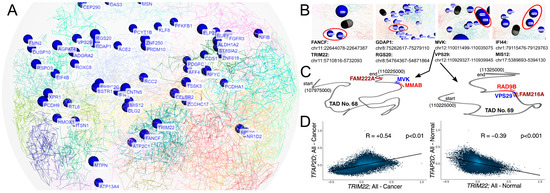
Figure 8.
Insights into the three-dimensional genomic location of validated AP-2δ targets (A,B), TAD domains of MVK and VPS29 (C), and correlation of TFAP2D with TRIM22 as the representative of TcoFs (D). In subfigure A, the emphasis is initially put on presenting all genes’ names at once. In subfigure B, the most interesting genomic locations were magnified and marked in red. In subfigure C, the locations of MVK and VPS29 (as well as the closest genes) are presented in TADs via TADKB. In subfigure (D), the CorrelationAnalyzeR was employed to correlate two genes in all available tissue types, separately for tumor and normal sample types.
In addition, the experimentally validated AP-2δ targets were investigated for their common Transcription co-Factors (TcoFs) [65] in order to identify the co-regulators that might be responsible for mutual genome control orchestrated by AP-2δ. It is worth noting that typically, TFs recruit interactive TcoFs and form physical contact loops [66]. Identification of repeating TcoFs from the TcoFBase server revealed that except for LINC02126 (which was not included in the database), only 12 TcoFs regulate the rest of the 65 validated AP-2δ targets (see Supplementary File S3 for the complete list of TcoFs for each target). These co-factors were: TLE3, TRIM22, TRIM24, TRIM25, TRIM28, TRRAP, USP7, WDR5, XRN2, YAP1, ZMIZ1, and ZMYND8. Interestingly, TRIM22 is not only present in the list of currently known AP-2δ targets but also has been experimentally validated [67]. Since the next study stages took a pan-cancer view, CorrelationAnalyzeR was used to correlate TRIM22 and TFAP2D expression in all cancer and normal specimens. Unexpectedly, it appears that depending on the sample type, TFAP2D correlates positively (cancer) or negatively (normal) with TRIM22 (Figure 8D). Together with the fact that TRIM22 is an AP-2δ target, this suggests a change in the regulation of important TcoFs and thus target genes that are commonly regulated. Although the function might depend on tissue context [68], TRIM22 was found to manifest oncogenic properties in chronic myeloid leukemia and non-small cell lung cancer [69,70].
2.6. TFAP2D-Correlated Genes Regulate Various Processes, including Genome Organization, Whereas AP-2δ Targets Regulate Transcription, as Does Their Superior TF
Subsequently, pan-cancer analysis of AP-2δ was directed to identify genes that correlate with TFAP2D in various tumors, and to indicate the most relevant ones both within a specific cancer type (correlating gene is identified for the same cancer in more than one database), and between tumors (correlating gene repeats in various cancer tissues). We concluded that such an approach is more appropriate given the scarcity of cancer research on AP-2δ, and because it might reveal genes that are in fact AP-2δ targets but are not currently considered as such. Moreover, the majority of AP-2δ targets are predicted via Harmonizome and thus not assigned to any specific tissue.
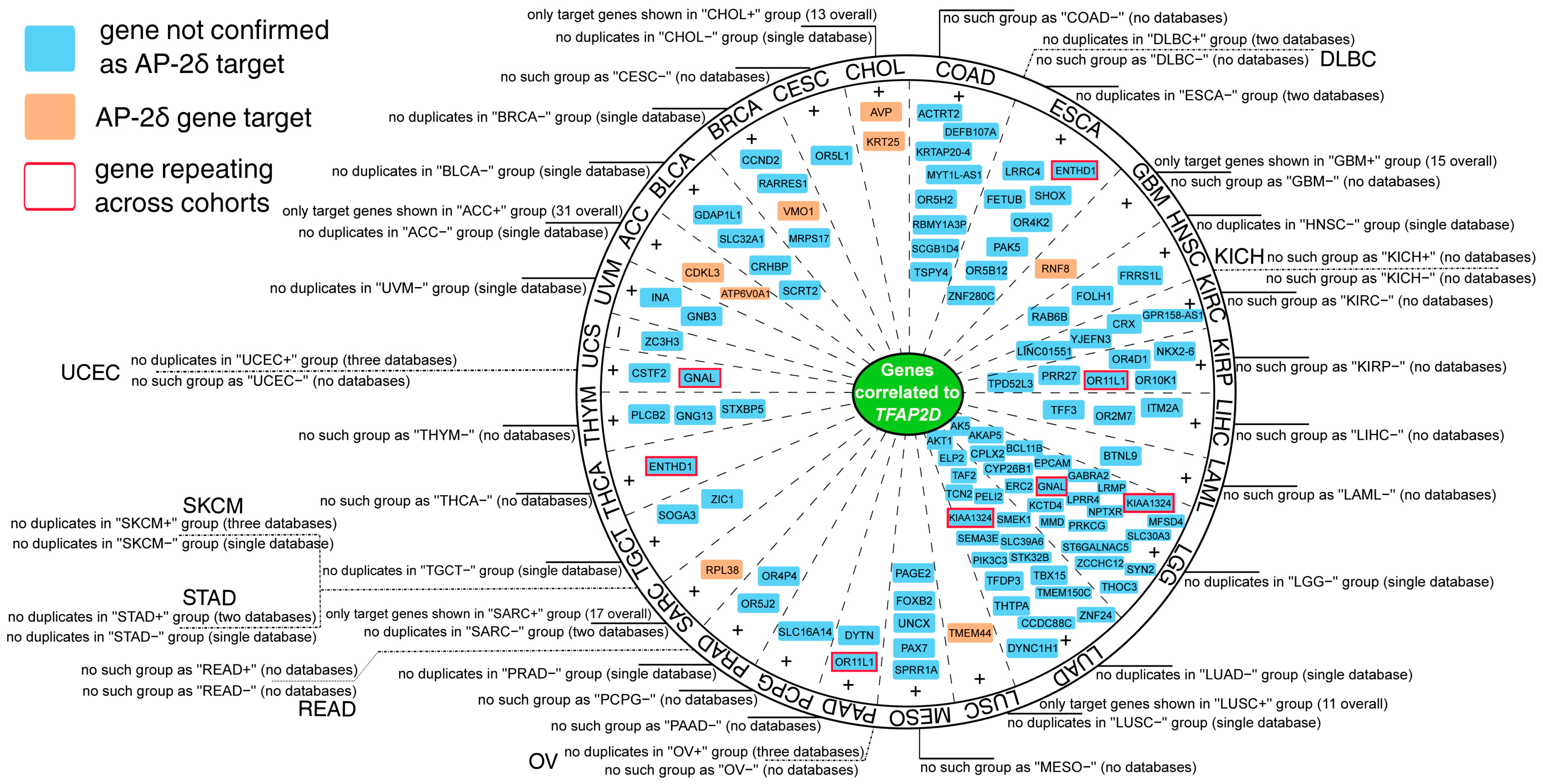
Regarding the within-cancer part, most genes that repeated in the cBioPortal, Gene Expression Profiling Interactive Analysis (GEPIA2), CorrelationAnalyzeR and University of Alabama at Birmingham Cancer (UALCAN) databases were identified as positively correlated (see Supplementary File S4 for full report). However, it was also possible to spot negatively correlated genes, as proved by the Uterine Carcinosarcoma (UCS) cohort (Figure 9). Moreover, some genes were found in the list of AP-2δ targets, namely CDKL3 and ATP6V0A1 in Adrenocortical Carcinoma (ACC), VMO1 in Breast invasive Carcinoma (BRCA), AVP and KRT25 in Cholangiocarcinoma (CHOL), RNF8 in Glioblastoma Multiforme (GBM), TMEM44 in Lung Squamous Cell Carcinoma (LUSC) and RPL38 in Sarcoma (SARC). For some cohorts, no TFAP2D positively or negatively correlated genes were identified in any database (Supplementary File S4). This applies to Diffuse Large B-cell Lymphoma (DLBC), Kidney Chromophobe (KICH), Ovarian serous Cystadenocarcinoma (OV), Rectum Adenocarcinoma (READ), Stomach Adenocarcinoma (STAD), Skin Cutaneous Melanoma (SKCM), and Uterine Corpus Endometrial Carcinoma (UCEC). In each cohort, separately for positively and negatively correlated genes, the gene ontology was performed using Database for Annotation Visualization and Integrated Discovery (DAVID), revealing a plethora of biological processes and signaling pathways (Supplementary File S5). For instance, functional annotations indicating any genome organization-related processes were identified in four cohorts: Bladder urothelial Carcinoma (BLCA), Liver Hepatocellular Carcinoma (LIHC), CHOL, and LUAD.
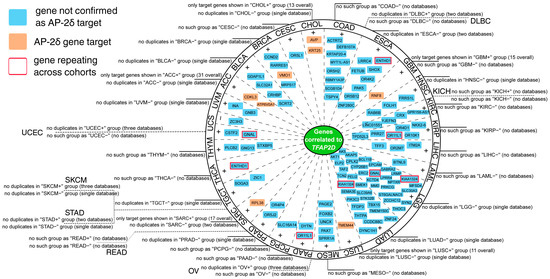
Figure 9.
Summary of the most valuable data from within-cancer correlation analysis performed via cBioPortal, GEPIA2, CorrelationAnalyzeR, and UALCAN. Some cohorts revealed no qualifying genes, the cause could be a lack of duplicates across databases (e.g., SKCM), or no results were obtained from any database (e.g., UCEC). Some genes were denoted as AP-2δ targets (orange) or the ones that repeat across cohorts (red frame). Details on data availability are provided around the circle and in Supplementary File S4.
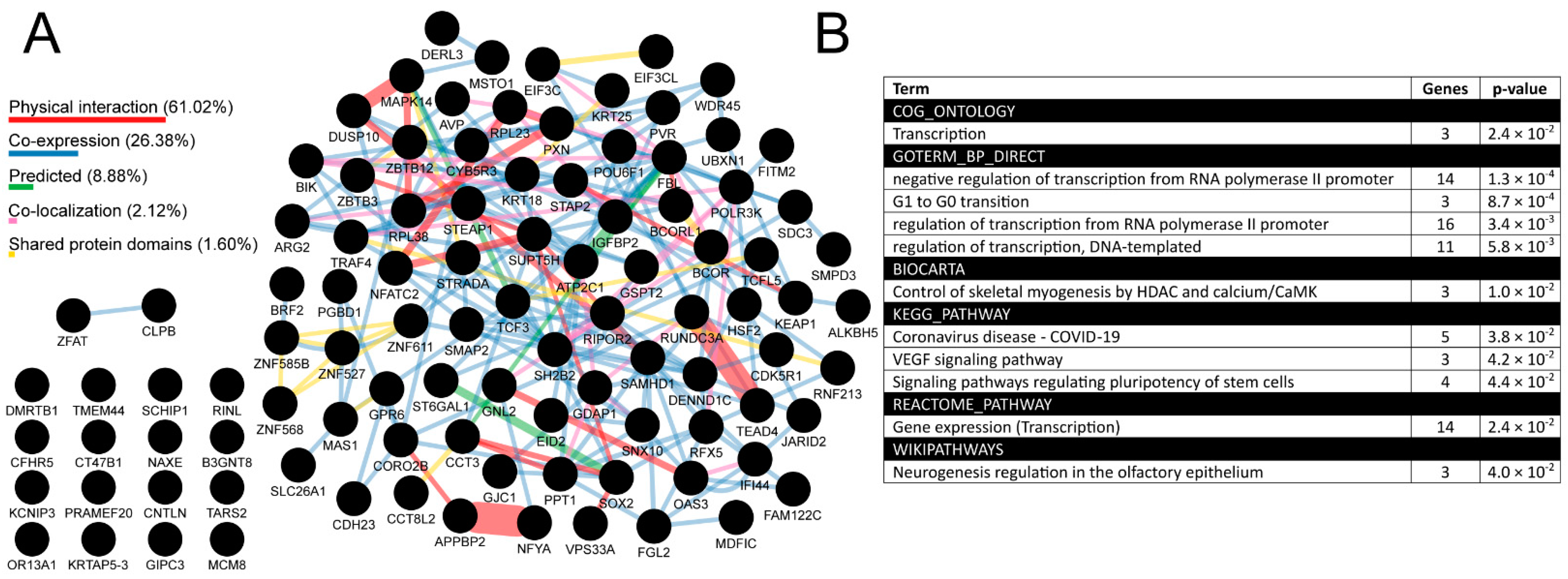
In the between-tumors analysis, 1716 TFAP2D-correlated genes were identified (Supplementary File S6), including 82 AP-2δ targets that presented both high interconnectivity based on GeneMania (Figure 10A; Supplementary File S7) and implication in, e.g., transcription, cell cycle, pluripotency, neurogenesis, and Vascular Endothelial Growth Factor (VEGF) signaling, based on DAVID (Figure 10B). Nearly 90% of connectivity (considering the network weighting) stems from physical interaction (61.02%) and co-expression (26.38%). The most numerous groups consist of more than 10 annotated genes and are all related to transcription, which allows us to view the subject from a wider perspective. Namely, it is known that spatial genome organization influences the gene expression regulation, but recently it has been also noted that at a finer scale, the transcripts or transcription seem to play a role in sub-compartmentalization, sub-TAD connections, as well as in the stabilization of enhancer–promoter interactions [25]. Knowing that AP-2δ is a protein that regulates transcription of its targets and that these genes further influence transcriptional processes, we hypothesized that AP-2δ might directly or indirectly affect the nuclear organization. In order to verify it, we narrowed the breadth of the study and focused on a suitable tumor for which such investigation would seem justifiable, given the results obtained throughout this research. It turned out that the LUAD cohort is not only in the leading tumors when it comes to TFAP2D mutation frequency (Figure 6), but also some cancer hallmark genes and drug targets were found to be differently expressed depending on TFAP2D mutation status (Figure 7). Moreover, this cancer was characterized by one of the most numerous groups of TFAP2D-correlated genes (Figure 9) and was identified as one of the four tumors (next to BLCA, CHOL, and LIHC) having any functional annotation related to chromatin organization in within-cancer gene ontology (Supplementary File S5). Since the chromatin structure was generally found to change during LUAD progression [71], we certified our decision to continue with this tumor in the remaining study stages.
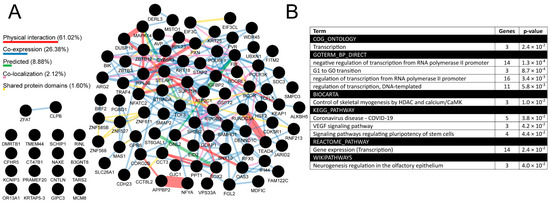
Figure 10.
Summary of the most valuable data from between-tumors correlation analysis in the form of interconnectivity (A) and gene ontology (B). Percentage contribution of a few aspects (marked in various colors) that constitute the interconnectivity of the network is provided. Analysis performed via cBioPortal, GEPIA2, CorrelationAnalyzeR, and UALCAN, visualized using GeneMania and DAVID.
2.7. Insights into LUAD Revealed the Co-Dependence of AP-2δ and Chromosomal Conformation-Related Genes That Present Various Cancer vs. Normal Tissue Staining
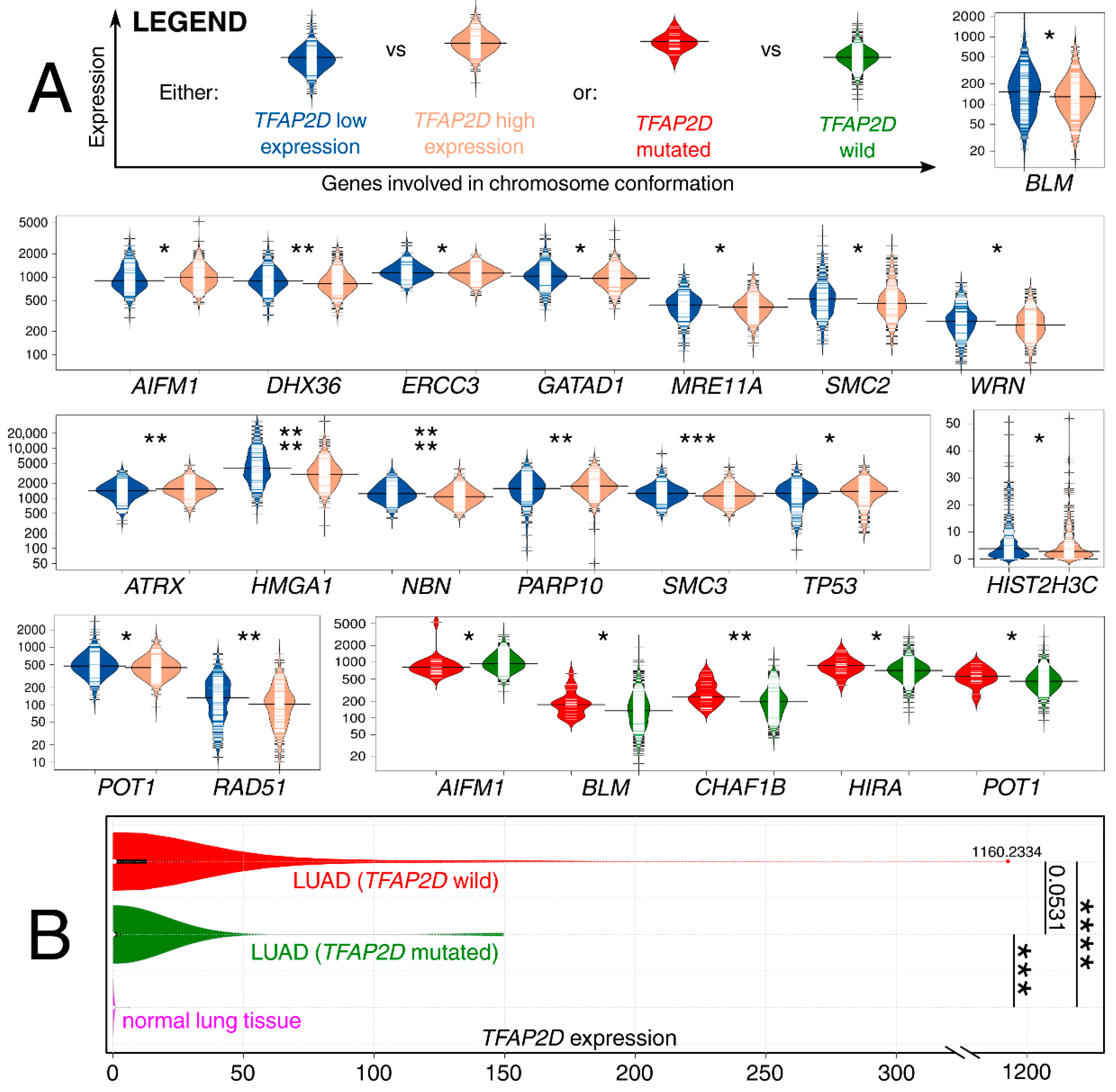
The results from the LUAD cohort revealed that both TFAP2D expression and mutation status were important for genome organization-related genes; in addition, few examples were repeated in two contexts (Figure 11A). Although these genes were collectively acquired from a single elaboration [72], in the following sentences, the dedicated references confirming the participation of genes in chromosomal conformation are included next to the symbols. Our results present that the patients with a mutation of the AP-2δ–encoding gene have lower expression of AIFM1 [73], similar to those that have lower TFAP2D expression. Contrary results were observed for both BLM [74] and POT1 [75]—lower expression of these genes was found in patients having high TFAP2D expression or lacking TFAP2D mutation. Other genes for which co-dependence with TFAP2D mutation was noted are CHAF1B [76] and HIRA [77]—both had increased expression in groups bearing TFAP2D mutation. The remaining observations concern TFAP2D expression; the genes were characterized with either lower (DHX36 [78], ERCC3 [79], GATAD1 [80], MRE11A [81], SMC2 [82], WRN [83], HMGA1 [84], NBN [81], SMC3 [85], HIST2H3C [86], RAD51 [87]) or higher (ATRX [88], PARP10 [89], TP53 [90]) expression in patients having high TFAP2D expression. Comparing the results with the AP-2δ targets list, HIRA and TP53 were identified as genes regulated by this TF. In summary, it seems that LUAD genome organization might differ in groups of different TFAP2D expression. The analysis of gene expression changes in relation to lung cancer development revealed some valuable observations. First, high expression of AIFM1 was found to drive the progression of lung cancer [91], similar to HMGA1 or RAD51 overexpression, which was connected with worse LUAD patient outcomes [92,93]. On the other hand, BLM or ATRX downregulation is associated with favorable events related to Non-Small Cell Lung Cancer (NSCLC) sensitization to radiotherapy or immunotherapy, respectively [94,95]. Finally, decreased expression of either DHX36 or ERCC3 seems to be unfavorable due to the elevated NSCLC migration and growth [96] or increased DNA adduct levels [97], even if the latter one was mainly due to ERCC5 and ERCC6 (in study by Cheng et al., the mean ERCC3 expression was lower in oncological patients but the results were not statistically significant). Additionally, the expression of TFAP2D in both the wild-type and mutated LUAD samples was compared to normal lung samples (Figure 11B).
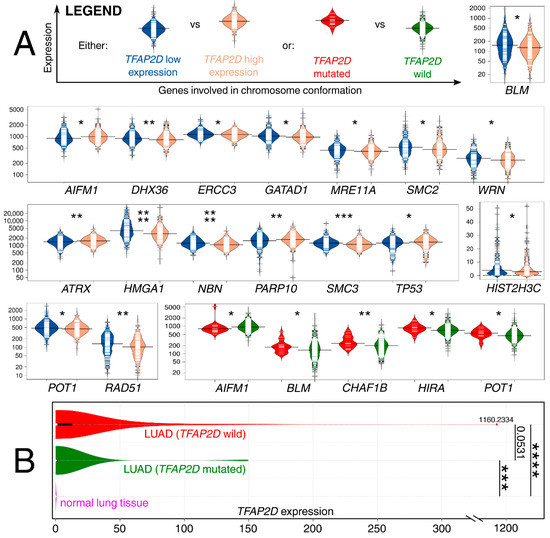
Figure 11.
Co-dependence of genes involved in chromosome conformation and either TFAP2D mutation or expression. (A) Some genes are on separate scales due to large differences in expression levels. (B) TFAP2D expression was also compared between LUAD (separately for samples with TFAP2D wild-type or mutated) and normal lung samples. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****). Data acquired from The Cancer Genome Atlas and Genomic Data Commons Portal.
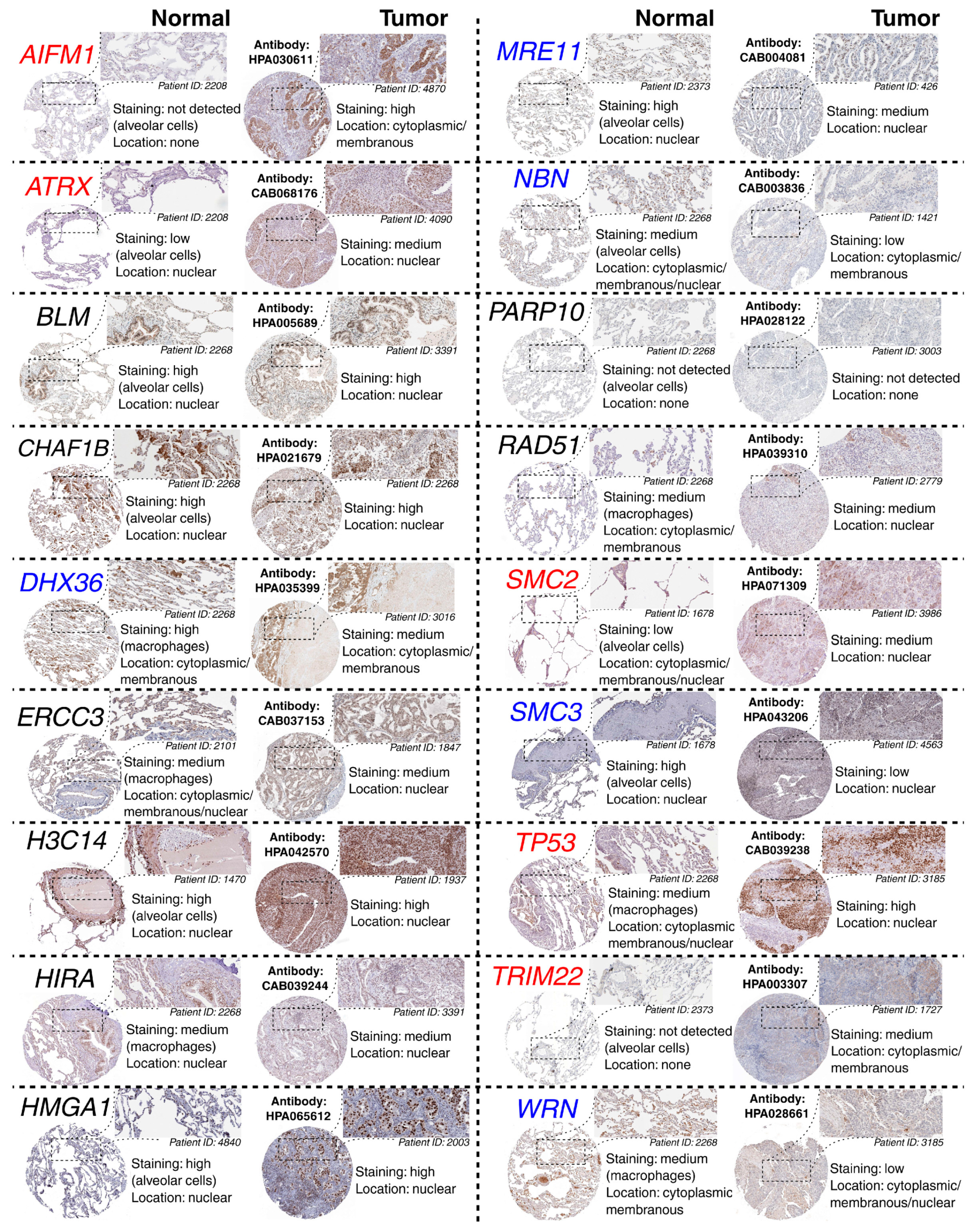
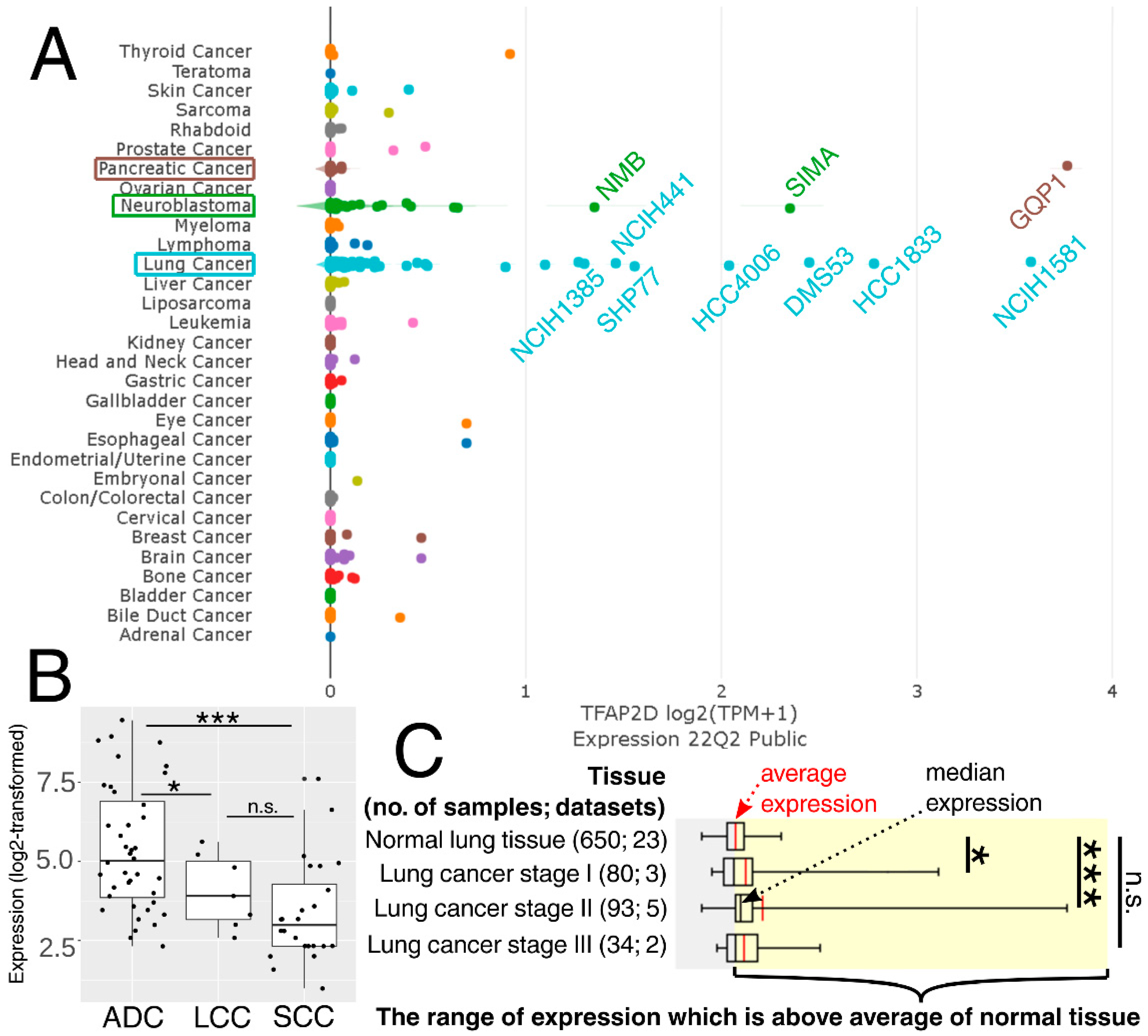
The above observations demonstrate the co-dependence of TFAP2D expression and genes that might be useful in lung cancer research. Therefore, in the last step, these genes were subjected to further evaluation of their immunohistochemistry (IHC) data (Figure 12). Out of 19 genes, no comparison was possible for GATAD1 and POT1 due to a lack of data in both the normal lung tissue and lung adenocarcinoma. For the remaining genes, IHC revealed staining differences for nine genes (AIFM1, ATRX, DHX36, MRE11, NBN, SMC2, SMC3, TP53, WRN) and no differences or undetected staining for eight genes (BLM, CHAF1B, ERCC3, HIST2H3C (also known as H3C14), HIRA, HMGA1, PARP10, RAD51). The immunohistochemical data for TRIM22 was additionally included in this step to complement the results of TcoFs analysis—the gene was found to be expressed more abundantly in LUAD than in normal lungs, which could confirm its oncogenic properties in lung cancer [70]. The other interesting genes are AIFM1, ATRX, and DHX36, which have not only various IHC data, but also the staining complements literature data on LUAD [91,95,96]. In addition, higher cancer-promoting expression is observed in patients with high AP-2δ expression, making TFAP2D a potential oncogene of lung adenocarcinoma. Further research is needed in this area, although it is already evident that the lung is the proper direction that can yield valuable data due to the fact that the highest expression of TFAP2D is mainly observed in lung carcinoma (Figure 13A; out of ten cancer cell lines with the highest expression of TFAP2D, seven stem from lung cancer). Moreover, various AP-2δ expressions can be observed in different lung tumor histological types (Figure 13B; the highest expression in adenocarcinoma), as well as cancer stages (Figure 13C; higher average TFAP2D expression in stage I and stage II tumors compared to the normal lung). Thus, when conceptualizing the experimental research, one should consider these important aspects to select cellular models with relevant AP-2δ levels.
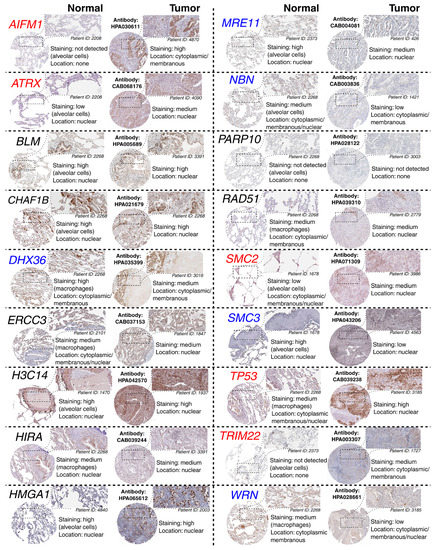
Figure 12.
Representative HPA staining data for chromosomal conformation-related genes from normal lung and adenocarcinoma. The genes with various staining in normal vs. tumor comparison are marked in red (if staining is stronger in the tumor) or blue (if staining is weaker in the tumor). One region per specimen is magnified. Antibody catalog number and staining results are provided.
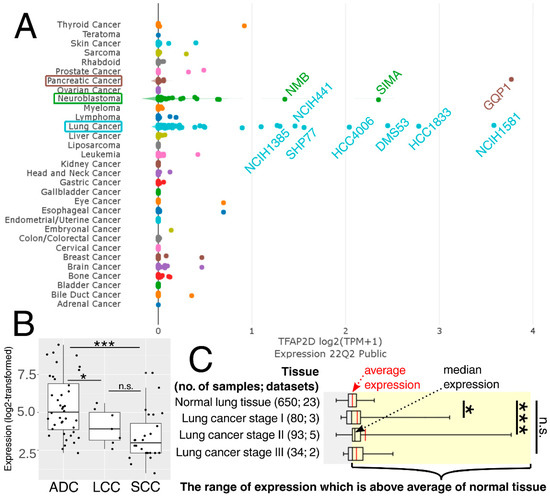
Figure 13.
Expression of TFAP2D in pan-cancer cell lines (A), as well as lung carcinoma histological types (B) and stages (C). The names of ten cancer cell lines having the highest expression are included. ADC–lung adenocarcinoma. LCC—lung large cell carcinoma. SCC—lung squamous cell carcinoma. p > 0.05 (n.s.), p < 0.05 (*), p < 0.001 (***). Data acquired from DepMap, GENT2, and Oncopression.
3. Materials and Methods
3.1. Data Acquisition of AP-2 Sequences, Recognized DNA, Evolution, Structures, and Clefts
Sequences of AP-2 proteins were acquired from Universal Protein Resource (UniProt) and aligned on the same server using Clustal Omega v1.2 (default parameters), which also visualized a phylogenetic tree and estimated percentage identity. The consensus logos representing sequences that are recognized by AP-2 factors were obtained from HOCOMOCO v11, except for AP-2ε which is not included in the database. The repository was searched using a “TFAP2” query among human binding models (“full” collection). For all AP-2 factors except for AP-2ε, the positional count matrices (PCMs) were downloaded in order to compare them using the Jaccard similarity index that was estimated through the MAtrix CompaRisOn by Approximate p-value Estimation (MACRO-APE) tool, employed with default parameters. The FABRIC database was employed to present the “TF_AP-2” domains and their range. Data on the occurrence of proline-rich sequences in all AP-2 factors were collectively acquired from UniProt, ProSite, InterPro, and Motif Scan.
The three-dimensional structure models in PDB format were acquired from the AlphaFold database and loaded into the ChimeraX v1.4 to visualize similarities and differences in protein structures of all AP-2 factors. PTMs and motifs/regions were identified via the ScanProsite tool with option “1” (submit protein(s); scan motifs). Accession identifiers for all AP-2 members were acquired from the aforementioned UniProt, and the analysis was submitted with an additional option to run the scan at high sensitivity. Histidines in AP-2 protein sequences were highlighted manually on screenshots from AlphaFold. Secondary structures that are formed in AP-2 transcription factors were estimated using the GOR4 algorithm with default parameters. UniProt database was also employed to provide the details about amino acids preceding the conserved “TF_AP-2” domain; the built-in “Highlight properties” option was used to visualize the similarity and various physicochemical or structural properties.
The presence of clefts among AP-2 members was analyzed via PDBsum–UniProt ID provided in the “Alpha Fold model” search option, and data from the “Clefts” tab was then summarized. Moreover, a three-dimensional AP-2δ structure with presentation of the binding site(s) was additionally downloaded from PDBsum in RasMol format in order to present clefts, side by side with AlphaFold models which were intended to visualize the range of the histidine-rich region.
3.2. Collection of Data Related to Mutational Status, Genetic Targets, and Transcriptional Co-Factors
Mutation frequency data were collected from FABRIC and The Tumor Immune Estimation Resource (TIMER 2.0). The connection of TFAP2D mutation status to gene expression changes was performed via muTarget. Analysis was started with the “Genotype” option; all mutation types and tumor types were included with default threshold (p < 0.01 and fold-change ≥ 1.44). Tables and boxplots were generated separately for cancer hallmark genes and FDA-approved drug targets. In addition, the summary of muTarget reports (Supplementary File S1) includes all significant genes which were further subjected to the gene ontology analysis (biological processes) using the built-in option. AP-2δ gene targets were collected from all available sources: databases (Harmonizome which contains, e.g., TRANScription FACtor database [TRANSFAC]); ChIP-exo experiment [61] from Gene Expression Omnibus (GSE151287: currently, to the best of our knowledge, there is only one such experiment); and literature data [67,98,99,100,101]. Collectively, 1318 target genes of AP-2δ were identified (Supplementary File S8).
The genomic location of currently known AP-2δ targets was visualized through Ideogram Viewer v1.0.8, according to official guidelines (i.e., genes symbols were included in the Universal Resource Locator [URL] under the “genelist=” query parameter). For clarity purposes (and to increase the findings relevance), only experimentally validated AP-2δ targets were presented via WashU Epigenome Browser. The hg19 reference genome was selected due to the fact that it allows a three-dimensional visualization of the genome from Public Data Hubs to be loaded under the “Tracks” tab. The 3dg data from the GM12878 cell line [60] were converted by the repository to g3d format using g3dtools. An additional black-gray indicator was drawn to facilitate results interpretation in various orientations (Figure S2). The most interesting observations were manually zoomed and details of genes’ location were retrieved from the left panel of WashU Browser. For MVK and VPS29, insights into TADs were visualized through TADKB and manually annotated with the help of University of California Santa Cruz Genome Browser (UCSC). For consistency, TADKB was browsed using the same reference genome and cell line (GM12878) as WashU Browser, with 50 kb resolution and the calling TAD method set as “Gaussian Mixture model And Proportion test” (GMAP).
Experimentally-validated AP-2δ targets were also investigated for their common TcoFs using the TcoFBase server. The “search by target gene” option was selected and all possible methods of TcoF identification were employed for Human species. The acquired lists were scanned for duplicates using Kutools v19 to provide TcoFs that regulate all query genes. Only one TcoF was identified as an AP-2δ target, TRIM22; it was further correlated with TFAP2D using CorrelationAnalyzeR in all available tissue types at once, but separately for tumor and normal sample types.
3.3. Correlation Analysis, Gene Ontology, and Evaluation of AP-2δ Impact on Genome Organization
TFAP2D-correlated genes were obtained from cBioPortal, GEPIA2, CorrelationAnalyzeR and UALCAN; separate lists were prepared for positively and negatively correlated genes. A correlation coefficient of at least |0.3| was considered acceptable. After merging, duplicates were first identified using Kutools v19 in data for a specific tumor. After completing the within-cancer part (summarized in circular plot using Cytoscape v3.7 with additional gene ontology via DAVID), duplicates were temporarily removed from data for specific tumors and then merged into a single list to identify duplicates between tumors. These genes were reduced only to AP-2δ targets, which were subsequently subjected to analysis of interconnectivity via GeneMania and gene ontology via DAVID. GeneMania was customized to show zero “resultant” genes (i.e., additional genes that are added to artificially increase the connectivity of query genes) while DAVID was employed under default parameters with the selected identifier set as “OFFICIAL_GENE_SYMBOL”. Only functional annotations that contained at least 3 query genes and presented statistical significance (p < 0.05) were collected from DAVID. Only interconnected nodes were subjected to functional annotation.
The assumptions made during the within-cancer and between-tumors parts were evaluated using RNA-seq expression data (level 3 RNA-seqV2, RSEM normalized) of the LUAD cohort from The Cancer Genome Atlas (TCGA). In order to split patients not only based on expression, TFAP2D-mutated samples were identified via Genomic Data Commons (GDC) Data Portal. The list of genes implicated in the nuclear organization was retrieved from Labome’s elaboration [72]. Results were visualized using BoxPlotR webtool and manually edited using Inkscape v1.2. Expression differences between groups were evaluated using GraphPad Prism v8; p < 0.05 in the Mann–Whitney test was considered statistically significant. Additionally, the representative IHC data were obtained from the publicly-available Human Protein Atlas; the same antibody for both normal and tumor lung specimens was selected using the “Tissue” and “Pathology” atlas, respectively. Selected antibodies had at least an “Approved” validation score, but some of them presented higher scores, e.g., “Supported” or “Enhanced” (Table 2). Expression of TFAP2D across cancer cell lines was determined using DepMap (Expression 22Q2 Public source); the portal was searched by gene name, and data were acquired from the “Characterization” tab and Data Explorer tool (groups were colored by the primary disease option). AP-2δ expression in various lung cancer histological types and stages was obtained from Gene Expression patterns across Normal and Tumor tissues (GENT2) and Oncopression, respectively. A “Subtype profile” workflow was selected in GENT2 with the following parameters: “Tissues” set as “Lung”, “Subtypes” as “Histology”, and “Gene Symbol” as “TFAP2D”. Oncopression was queried with the gene symbol, and lung cancer was accessed for further details on staging.

Table 2.
Validation scores of antibodies selected from HPA.
4. Conclusions
To conclude, cancer treatment-related research on the AP-2 family of transcription factors is encouraged, especially regarding AP-2δ, given its high ligandability potential, or evolutionary distinction that entails exclusive structural and functional properties. Unequivocal recognition of AP-2δ would require simultaneous identification of the histidine-rich region and “TF_AP-2” domain, in order to exclude other molecules from inside and outside the AP-2 family. The presence of the highest number of PTMs and extended strands confirms the relevance of AP-2δ among family representatives. Moreover, AP-2δ might be a more relevant therapeutic target owing to its presumed interaction with unique coactivators or functioning as a modulator of other AP-2 proteins. The second most interesting AP-2 member could be AP-2γ, for which much literature data confirm its oncogenic properties, and for which our study adds valuable information regarding available clefts.
Various steps of AP-2δ pan-cancer analysis identified different tumors that are relevant to further research. TFAP2D mutation may co-depend with changes in expression of both cancer hallmark genes and FDA-approved drug targets. Investigation of experimentally validated AP-2δ targets revealed that their close location in the genome could be due to inter-chromosomal proximity or TADs lying together on a single chromosome. Among TcoFs, the most inquisitive appears to be TRIM22, which regulates all currently validated AP-2δ and its correlation with TFAP2D changes depending on tissue context (cancer vs. normal). Based on the literature data, TRIM22 could manifest oncogenic properties in leukemia or lung cancer. Pan-cancer correlation analysis revealed a plethora of biological processes and signaling pathways that are worth investigating in the future. The between-tumors part of the investigation indicated nearly a hundred AP-2δ targets that in addition to being highly interconnected, also regulate transcription, as does their superior transcription factor. This allowed us to view the subject from a wider perspective, i.e., viewing transcription as a process that shapes the spatial organization of the genome. Assumptions were certified using a representative cohort, LUAD, in which a co-dependence was observed between AP-2δ and the expression of chromosome conformation-related genes; the latter were found to influence lung cancer development and presented various staining in cancer vs. normal comparison. Lung cancer cell lines generally present the highest expression of TFAP2D, suggesting that research in this context should be pursued. If genome organization were to be orchestrated by AP-2δ, and if cancer-driving phenomena were found to be implicated in genome organization, targeting this TF may bring tremendous benefits.
Evidently, the results from this study and the scarcity of cancer-related data on AP-2δ strongly emphasize the need for subsequent research aimed at filling both the literature and therapeutic gaps via Cleavage Under Targets and Tagmentation (CUT&Tag) or CUT&RUN (Release Using Nuclease), chromosome conformation capture and CADD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11244124/s1, Table S1. Pairwise comparisons between AP-2 proteins with an additional all-by-all percentage of identity. Figure S1. Presence of histidines in protein sequences of AP-2 transcription factors. Figure S2. Additional data for three-dimensional genome organization and TADs. Supplementary File S1. Summary of mutational status-related analysis performed using muTarget (Supplementary_File_S1.xlsx). Supplementary File S2. Chromosomal location of experimentally validated AP-2δ targets included in WashU Epigenome Browser (Supplementary_File_S2.xlsx). Supplementary File S3. Summary of TcoFs-related analysis performed using TcoFBase (Supplementary_File_S3.xlsx). Supplementary File S4. Summary of within-cancer correlation analysis performed using cBioPortal, GEPIA2, CorrelationAnalyzeR, and UALCAN (Supplementary_File_S4.xlsx). Supplementary File S5. Gene ontology based on within-cancer analyses performed using DAVID (Supplementary_File_S5.xlsx). Supplementary File S6. Summary of between-tumors correlation analysis performed using cBioPortal, GEPIA2, CorrelationAnalyzeR, and UALCAN (Supplementary_File_S6.xlsx). Supplementary File S7. An interactive file accompanying the network from between-tumors analysis (Supplementary_File_S7.cys). Supplementary File S8. The list of currently known AP-2δ targets collected from Harmonizome and literature data (Supplementary_File_S8.xlsx).
Author Contributions
D.K. conceptualized the article; D.K. and Ż.K.-K. established the methodology; D.K. and M.K. were responsible for software; D.K. and Ż.K.-K. supervised the article; D.K. and L.-Y.Z. visualized the results; D.K. wrote the original draft; D.K., L.-Y.Z., M.K., E.P. and Ż.K.-K. reviewed and edited article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article (and its additional files).
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henley, M.J.; Koehler, A.N. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat. Rev. Drug Discov. 2021, 20, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.N. A complex task? Direct modulation of transcription factors with small molecules. Curr. Opin. Chem. Biol. 2010, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Arndt, H.D. Small molecule modulators of transcription. Angew. Chem. Int. Ed. Engl. 2006, 45, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Burris, T.P.; Solt, L.A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffett, K.; Lundasen, T.; Hughes, T.; Kojetin, D.J. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 2013, 65, 710–778. [Google Scholar] [CrossRef] [PubMed]
- Arkin, M.R.; Tang, Y.; Wells, J.A. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem. Biol. 2014, 21, 1102–1114. [Google Scholar] [CrossRef]
- Geng, F.; Wenzel, S.; Tansey, W.P. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 2012, 81, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.; Toure, M.; Hellerschmied, D.; Salami, J.; Jaime-Figueroa, S.; Ko, E.; Hines, J.; Crews, C.M. Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL. Angew. Chem. Int. Ed. Engl. 2016, 55, 807–810. [Google Scholar] [CrossRef]
- Bondeson, D.P.; Mares, A.; Smith, I.E.; Ko, E.; Campos, S.; Miah, A.H.; Mulholland, K.E.; Routly, N.; Buckley, D.L.; Gustafson, J.L.; et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 2015, 11, 611–617. [Google Scholar] [CrossRef]
- Kwiatkowski, N.; Zhang, T.; Rahl, P.B.; Abraham, B.J.; Reddy, J.; Ficarro, S.B.; Dastur, A.; Amzallag, A.; Ramaswamy, S.; Tesar, B.; et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 2014, 511, 616–620. [Google Scholar] [CrossRef]
- Liu, N.; Ling, R.; Tang, X.; Yu, Y.; Zhou, Y.; Chen, D. Post-Translational Modifications of BRD4: Therapeutic Targets for Tumor. Front. Oncol. 2022, 12, 847701. [Google Scholar] [CrossRef]
- Leung, C.H.; Chan, D.S.; Ma, V.P.; Ma, D.L. DNA-binding small molecules as inhibitors of transcription factors. Med. Res. Rev. 2013, 33, 823–846. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting transcription factors in cancer—From undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Deng, S.; Feng, Y.; Pauklin, S. 3D chromatin architecture and transcription regulation in cancer. J. Hematol. Oncol. 2022, 15, 49. [Google Scholar] [CrossRef]
- Su, J.; Zhu, D.; Huo, Z.; Gingold, J.A.; Ang, Y.S.; Tu, J.; Zhou, R.; Lin, Y.; Luo, H.; Yang, H.; et al. Genomic Integrity Safeguards Self-Renewal in Embryonic Stem Cells. Cell Rep. 2019, 28, 1400–1409.e4. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.Y.; Tan, T.Z.; Sundararajan, V.; Chiu, Y.C.; Chee, E.Y.W.; Chung, V.Y.; Choolani, M.A.; Huang, R.Y. 3D genome organization in the epithelial-mesenchymal transition spectrum. Genome Biol. 2022, 23, 121. [Google Scholar] [CrossRef]
- Zhou, Y.; Petrovic, J.; Zhao, J.; Zhang, W.; Bigdeli, A.; Zhang, Z.; Berger, S.L.; Pear, W.S.; Faryabi, R.B. EBF1 nuclear repositioning instructs chromatin refolding to promote therapy resistance in T leukemic cells. Mol. Cell 2022, 82, 1003–1020.e15. [Google Scholar] [CrossRef] [PubMed]
- Standfuss, C.; Parczyk, J.; Ruhnau, J.; Klein, A. Genome reorganization in different cancer types: Detection of cancer specific breakpoint regions. Mol. Cytogenet. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Peng, W.; Wang, R.; Zhao, H.; Yu, X.; Sun, Y. Regulation of 3D Organization and Its Role in Cancer Biology. Front. Cell Dev. Biol. 2022, 10, 879465. [Google Scholar] [CrossRef]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.H. Targeting Transcription Factors for Cancer Treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef]
- Wu, H.R.; Zhang, J. AP-2alpha expression in papillary thyroid carcinoma predicts tumor progression and poor prognosis. Cancer Manag. Res. 2018, 10, 2615–2625. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Tai, J.; Chen, S.; Yu, M.; Ren, D.; Wang, L. TFAP2C promotes stemness and chemotherapeutic resistance in colorectal cancer via inactivating hippo signaling pathway. J. Exp. Clin. Cancer Res. 2018, 37, 27. [Google Scholar] [CrossRef] [PubMed]
- Hilger-Eversheim, K.; Moser, M.; Schorle, H.; Buettner, R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 2000, 260, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.; Buhl, S.; Weber, S.; Jager, R.; Schorle, H. The AP-2 family of transcription factors. Genome Biol. 2005, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Kolat, D.; Kaluzinska, Z.; Bednarek, A.K.; Pluciennik, E. Prognostic significance of AP-2alpha/gamma targets as cancer therapeutics. Sci. Rep. 2022, 12, 5497. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, B.; Furlong, E.E.M. The role of transcription in shaping the spatial organization of the genome. Nat. Rev. Mol. Cell Biol. 2019, 20, 327–337. [Google Scholar] [CrossRef]
- Kolat, D.; Kaluzinska, Z.; Bednarek, A.K.; Pluciennik, E. The biological characteristics of transcription factors AP-2alpha and AP-2gamma and their importance in various types of cancers. Biosci. Rep. 2019, 39, BSR20181928. [Google Scholar] [CrossRef]
- Zhao, F.; Satoda, M.; Licht, J.D.; Hayashizaki, Y.; Gelb, B.D. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J. Biol. Chem. 2001, 276, 40755–40760. [Google Scholar] [CrossRef]
- Zhao, F.; Lufkin, T.; Gelb, B.D. Expression of Tfap2d, the gene encoding the transcription factor Ap-2 delta, during mouse embryogenesis. Gene Expr. Patterns 2003, 3, 213–217. [Google Scholar] [CrossRef]
- Kettler, L.; Sid, H.; Schaub, C.; Lischka, K.; Klinger, R.; Moser, M.; Schusser, B.; Luksch, H. AP-2delta Expression Kinetics in Multimodal Networks in the Developing Chicken Midbrain. Front. Neural Circuits 2021, 15, 756184. [Google Scholar] [CrossRef]
- Hesse, K.; Vaupel, K.; Kurt, S.; Buettner, R.; Kirfel, J.; Moser, M. AP-2delta is a crucial transcriptional regulator of the posterior midbrain. PLoS ONE 2011, 6, e23483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Monckton, E.A.; Godbout, R. Ectopic expression of transcription factor AP-2delta in developing retina: Effect on PSA-NCAM and axon routing. J. Neurochem. 2014, 129, 72–84. [Google Scholar] [CrossRef]
- Cheng, C.; Ying, K.; Xu, M.; Zhao, W.; Zhou, Z.; Huang, Y.; Wang, W.; Xu, J.; Zeng, L.; Xie, Y.; et al. Cloning and characterization of a novel human transcription factor AP-2 beta like gene (TFAP2BL1). Int. J. Biochem. Cell Biol. 2002, 34, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.K.; Reddy, S.; Laz, N.; Eltaher, R.; Kandell, Z.; Mahmud, T.; Alenazi, L.; Haroun, B.; Hassan, M.; Ragavendra, R. A human specific Alu DNA cassette is found flanking the genes of transcription factor AP2. BMC Res. Notes 2019, 12, 222. [Google Scholar] [CrossRef]
- Zhang, W.; Edwards, A.; Fan, W.; Deininger, P.; Zhang, K. Alu distribution and mutation types of cancer genes. BMC Genom. 2011, 12, 157. [Google Scholar] [CrossRef]
- Kim, S.; Cho, C.S.; Han, K.; Lee, J. Structural Variation of Alu Element and Human Disease. Genom. Inform. 2016, 14, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Yan, F.; Yuan, T.; Yang, B.; He, Q.; Zhu, H. Targeting post-translational modification of transcription factors as cancer therapy. Drug Discov. Today 2020, 25, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liang, Z.; Zhao, K.; Luo, C. Drug design targeting active posttranslational modification protein isoforms. Med. Res. Rev. 2021, 41, 1701–1750. [Google Scholar] [CrossRef]
- Bang, J.; Park, H.; Yoo, J.; Lee, D.; Choi, W.I.; Lee, J.H.; Lee, Y.R.; Kim, C.; Koo, H.; Kim, S. Selection and identification of a novel bone-targeting peptide for biomedical imaging of bone. Sci. Rep. 2020, 10, 10576. [Google Scholar] [CrossRef]
- Guo, Z.; Li, B.; Cheng, L.T.; Zhou, S.; McCammon, J.A.; Che, J. Identification of protein-ligand binding sites by the level-set variational implicit-solvent approach. J. Chem. Theory Comput. 2015, 11, 753–765. [Google Scholar] [CrossRef]
- Du, H.; Jiang, D.; Gao, J.; Zhang, X.; Jiang, L.; Zeng, Y.; Wu, Z.; Shen, C.; Xu, L.; Cao, D.; et al. Proteome-Wide Profiling of the Covalent-Druggable Cysteines with a Structure-Based Deep Graph Learning Network. Research 2022, 2022, 9873564. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, D.; Zhang, H.; Wang, X.; Zhou, Y. Prediction of DNA-Binding Protein-Drug-Binding Sites Using Residue Interaction Networks and Sequence Feature. Front. Bioeng. Biotechnol. 2022, 10, 822392. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Luscombe, N.M.; Swindells, M.B.; Thornton, J.M. Protein clefts in molecular recognition and function. Protein Sci. 1996, 5, 2438–2452. [Google Scholar] [CrossRef]
- Purkait, D.; Ahuja, A.; Bhattacharjee, U.; Singha, A.; Rhetso, K.; Dey, T.K.; Das, S.; Sanjukta, R.K.; Puro, K.; Shakuntala, I.; et al. Molecular Characterization and Computational Modelling of New Delhi Metallo-beta-Lactamase-5 from an Escherichia coli Isolate (KOEC3) of Bovine Origin. Indian J. Microbiol. 2016, 56, 182–189. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, M.; Dunker, A.K. Proline rich motifs as drug targets in immune mediated disorders. Int. J. Pept. 2012, 2012, 634769. [Google Scholar] [CrossRef] [PubMed]
- Corbi-Verge, C.; Kim, P.M. Motif mediated protein-protein interactions as drug targets. Cell Commun. Signal 2016, 14, 8. [Google Scholar] [CrossRef]
- Merski, M.; Shoichet, B.K. The impact of introducing a histidine into an apolar cavity site on docking and ligand recognition. J. Med. Chem. 2013, 56, 2874–2884. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Mougkogianni, M.; Kalogeropoulou, A.; Giakoumakis, N.N.; Lygerou, Z.; Taraviras, S. Iotan vivo imaging of DNA-bound minichromosome maintenance complex in embryonic mouse cortex. STAR Protoc. 2021, 2, 100234. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Kim, H.; Twomey, J.D.; Hsieh, A.H. MMP-2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertebral disc. Arthritis Res. Ther. 2013, 15, R57. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Carroll, P.A.; Freie, B.W.; Mathsyaraja, H.; Eisenman, R.N. The MYC transcription factor network: Balancing metabolism, proliferation and oncogenesis. Front. Med. 2018, 12, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, L.; Jiang, C.; Chen, J.; Qin, Z.; Zhong, F.; Yan, Y.; Tong, R.; Zhou, M.; Yuan, A.; et al. The transcription factor zinc fingers and homeoboxes 2 alleviates NASH by transcriptional activation of phosphatase and tensin homolog. Hepatology 2022, 75, 939–954. [Google Scholar] [CrossRef]
- Guo, Y.; Kartawinata, M.; Li, J.; Pickett, H.A.; Teo, J.; Kilo, T.; Barbaro, P.M.; Keating, B.; Chen, Y.; Tian, L.; et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood 2014, 124, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Specks, J.; Lecona, E.; Lopez-Contreras, A.J.; Fernandez-Capetillo, O. A Single Conserved Residue Mediates Binding of the Ribonucleotide Reductase Catalytic Subunit RRM1 to RRM2 and Is Essential for Mouse Development. Mol. Cell Biol. 2015, 35, 2910–2917. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behiry, E.M.; Evans, R.M.; Guo, J.; Loveridge, E.J.; Allemann, R.K. Loop interactions during catalysis by dihydrofolate reductase from Moritella profunda. Biochemistry 2014, 53, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Evdokimova, V.; Tognon, C.E.; Benatar, T.; Yang, W.; Krutikov, K.; Pollak, M.; Sorensen, P.H.; Seth, A. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci. Signal. 2012, 5, ra92. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Ke, Y.; Wang, F. Intrinsic FGFR2 and Ectopic FGFR1 Signaling in the Prostate and Prostate Cancer. Front. Genet. 2019, 10, 12. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Kucka, K.; Wajant, H. Receptor Oligomerization and Its Relevance for Signaling by Receptors of the Tumor Necrosis Factor Receptor Superfamily. Front. Cell Dev. Biol. 2020, 8, 615141. [Google Scholar] [CrossRef]
- Tan, L.; Xing, D.; Chang, C.H.; Li, H.; Xie, X.S. Three-dimensional genome structures of single diploid human cells. Science 2018, 361, 924–928. [Google Scholar] [CrossRef]
- Lai, W.K.M.; Mariani, L.; Rothschild, G.; Smith, E.R.; Venters, B.J.; Blanda, T.R.; Kuntala, P.K.; Bocklund, K.; Mairose, J.; Dweikat, S.N.; et al. A ChIP-exo screen of 887 Protein Capture Reagents Program transcription factor antibodies in human cells. Genome Res. 2021, 31, 1663–1679. [Google Scholar] [CrossRef]
- McArthur, E.; Capra, J.A. Topologically associating domain boundaries that are stable across diverse cell types are evolutionarily constrained and enriched for heritability. Am. J. Hum. Genet. 2021, 108, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Chen, L.; Li, S.C. Detecting TAD-like domains from RNA-associated interactions. Nucleic Acids Res. 2022, 50, e88. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, M.; Hu, M.; Wang, S. TAD-like single-cell domain structures exist on both active and inactive X chromosomes and persist under epigenetic perturbations. Genome Biol. 2021, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, U.; Schmeier, S.; Bajic, V.B. TcoF-DB: Dragon database for human transcription co-factors and transcription factor interacting proteins. Nucleic Acids Res. 2011, 39, D106–D110. [Google Scholar] [CrossRef] [PubMed]
- Zabidi, M.A.; Stark, A. Regulatory Enhancer-Core-Promoter Communication via Transcription Factors and Cofactors. Trends Genet. 2016, 32, 801–814. [Google Scholar] [CrossRef]
- Sun, L.; Huang, S.; Wu, Q.; Gu, S.; Fu, X.; Yu, K.; Lu, F.; Ji, C.; Feng, C.; Sun, R.; et al. Identification of genes differentially regulated by transcription factor, AP-2delta. Front. Biosci. 2007, 12, 1699–1706. [Google Scholar] [CrossRef]
- Saito-Kanatani, M.; Urano, T.; Hiroi, H.; Momoeda, M.; Ito, M.; Fujii, T.; Inoue, S. Identification of TRIM22 as a progesterone-responsive gene in Ishikawa endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 154, 217–225. [Google Scholar] [CrossRef]
- Li, L.; Qi, Y.; Ma, X.; Xiong, G.; Wang, L.; Bao, C. TRIM22 knockdown suppresses chronic myeloid leukemia via inhibiting PI3K/Akt/mTOR signaling pathway. Cell Biol. Int. 2018, 42, 1192–1199. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.M.; Yang, F.F.; Miao, Y.; Yin, Y.; Hu, X.J.; Hou, G.; Wang, Q.Y.; Kang, J. TRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3beta/beta-catenin signaling in non-small cell lung cancer. Oncotarget 2017, 8, 62069–62080. [Google Scholar] [CrossRef]
- Druliner, B.R.; Fincher, J.A.; Sexton, B.S.; Vera, D.L.; Roche, M.; Lyle, S.; Dennis, J.H. Chromatin patterns associated with lung adenocarcinoma progression. Cell Cycle 2013, 12, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K. Chromosome Conformation Capture. Mater. Methods 2018, 8, 2662. [Google Scholar] [CrossRef]
- Galluzzi, L.; Joza, N.; Tasdemir, E.; Maiuri, M.C.; Hengartner, M.; Abrams, J.M.; Tavernarakis, N.; Penninger, J.; Madeo, F.; Kroemer, G. No death without life: Vital functions of apoptotic effectors. Cell Death Differ. 2008, 15, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Savitsky, P.; Allerston, C.K.; Bizard, A.H.; Ozer, O.; Sarlos, K.; Liu, Y.; Pardon, E.; Steyaert, J.; Hickson, I.D.; et al. Crystal structure of the Bloom’s syndrome helicase indicates a role for the HRDC domain in conformational changes. Nucleic Acids Res. 2015, 43, 5221–5235. [Google Scholar] [CrossRef]
- Opresko, P.L.; Mason, P.A.; Podell, E.R.; Lei, M.; Hickson, I.D.; Cech, T.R.; Bohr, V.A. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J. Biol. Chem. 2005, 280, 32069–32080. [Google Scholar] [CrossRef]
- Verreault, A.; Kaufman, P.D.; Kobayashi, R.; Stillman, B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 1996, 87, 95–104. [Google Scholar] [CrossRef]
- Tagami, H.; Ray-Gallet, D.; Almouzni, G.; Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 2004, 116, 51–61. [Google Scholar] [CrossRef]
- Vaughn, J.P.; Creacy, S.D.; Routh, E.D.; Joyner-Butt, C.; Jenkins, G.S.; Pauli, S.; Nagamine, Y.; Akman, S.A. The DEXH protein product of the DHX36 gene is the major source of tetramolecular quadruplex G4-DNA resolving activity in HeLa cell lysates. J. Biol. Chem. 2005, 280, 38117–38120. [Google Scholar] [CrossRef]
- Coin, F.; Oksenych, V.; Egly, J.M. Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol. Cell 2007, 26, 245–256. [Google Scholar] [CrossRef]
- Vermeulen, M.; Eberl, H.C.; Matarese, F.; Marks, H.; Denissov, S.; Butter, F.; Lee, K.K.; Olsen, J.V.; Hyman, A.A.; Stunnenberg, H.G.; et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 2010, 142, 967–980. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005, 308, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Cuvier, O.; Hirano, T. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J. Biol. Chem. 2001, 276, 5417–5420. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.N.; Machwe, A.; Chen, L.; Bohr, V.A.; Orren, D.K. The DNA structure and sequence preferences of WRN underlie its function in telomeric recombination events. Nat. Commun. 2015, 6, 8331. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Narita, M.; Krizhanovsky, V.; Nunez, S.; Chicas, A.; Hearn, S.A.; Myers, M.P.; Lowe, S.W. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 2006, 126, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.D.P.; Feldmann, A.; Hernandez-Rodriguez, B.; Diaz, N.; Brown, J.M.; Fursova, N.A.; Blackledge, N.P.; Prathapan, P.; Dobrinic, P.; Huseyin, M.K.; et al. Cohesin Disrupts Polycomb-Dependent Chromosome Interactions in Embryonic Stem Cells. Cell Rep. 2020, 30, 820–835. [Google Scholar] [CrossRef]
- Kato, D.; Osakabe, A.; Tachiwana, H.; Tanaka, H.; Kurumizaka, H. Human tNASP promotes in vitro nucleosome assembly with histone H3.3. Biochemistry 2015, 54, 1171–1179. [Google Scholar] [CrossRef]
- Benson, F.E.; Stasiak, A.; West, S.C. Purification and characterization of the human Rad51 protein, an analogue of E. coli RecA. EMBO J. 1994, 13, 5764–5771. [Google Scholar] [CrossRef]
- Ratnakumar, K.; Duarte, L.F.; LeRoy, G.; Hasson, D.; Smeets, D.; Vardabasso, C.; Bonisch, C.; Zeng, T.; Xiang, B.; Zhang, D.Y.; et al. ATRX-mediated chromatin association of histone variant macroH2A1 regulates alpha-globin expression. Genes Dev. 2012, 26, 433–438. [Google Scholar] [CrossRef]
- Yu, M.; Schreek, S.; Cerni, C.; Schamberger, C.; Lesniewicz, K.; Poreba, E.; Vervoorts, J.; Walsemann, G.; Grotzinger, J.; Kremmer, E.; et al. PARP-10, a novel Myc-interacting protein with poly(ADP-ribose) polymerase activity, inhibits transformation. Oncogene 2005, 24, 1982–1993. [Google Scholar] [CrossRef]
- Hublitz, P.; Kunowska, N.; Mayer, U.P.; Muller, J.M.; Heyne, K.; Yin, N.; Fritzsche, C.; Poli, C.; Miguet, L.; Schupp, I.W.; et al. NIR is a novel INHAT repressor that modulates the transcriptional activity of p53. Genes Dev. 2005, 19, 2912–2924. [Google Scholar] [CrossRef]
- Rao, S.; Mondragon, L.; Pranjic, B.; Hanada, T.; Stoll, G.; Kocher, T.; Zhang, P.; Jais, A.; Lercher, A.; Bergthaler, A.; et al. AIF-regulated oxidative phosphorylation supports lung cancer development. Cell Res. 2019, 29, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Saed, L.; Jelen, A.; Mirowski, M.; Salagacka-Kubiak, A. Prognostic Significance of HMGA1 Expression in Lung Cancer Based on Bioinformatics Analysis. Int. J. Mol. Sci. 2022, 23, 6933. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Zhao, L.; Li, L.; Zuo, W.; Han, L. High expression of RAD51 promotes DNA damage repair and survival in KRAS-mutant lung cancer cells. BMB Rep. 2019, 52, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xu, C.; Sun, X.; Sun, H.; Zhao, X.; He, N.; Ji, K.; Wang, Q.; Du, L.; Wang, J.; et al. BLM helicase inhibition synergizes with PARP inhibition to improve the radiosensitivity of olaparib resistant non-small cell lung cancer cells by inhibiting homologous recombination repair. Cancer Biol. Med. 2021, 19, 1150–1171. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Jiang, S.; Wang, Y.; Xie, Y.; Zhang, H.; Feng, Y.; Ma, F.; Ma, J.; Liu, X.; Hu, C. Alpha Thalassemia/Intellectual Disability X-Linked Deficiency Sensitizes Non-Small Cell Lung Cancer to Immune Checkpoint Inhibitors. Front. Oncol. 2020, 10, 608300. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Z.; Cao, J.; Lane, J.; Birkin, E.; Dong, X.; Zhang, L.; Jiang, W.G. The G4 Resolvase DHX36 Possesses a Prognosis Significance and Exerts Tumour Suppressing Function Through Multiple Causal Regulations in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 655757. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Spitz, M.R.; Hong, W.K.; Wei, Q. Reduced expression levels of nucleotide excision repair genes in lung cancer: A case-control analysis. Carcinogenesis 2000, 21, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Walsh, M.J.; Gelb, B.D. Fgfr3 is a transcriptional target of Ap2delta and Ash2l-containing histone methyltransferase complexes. PLoS ONE 2009, 4, e8535. [Google Scholar] [CrossRef]
- Tan, C.C.; Sindhu, K.V.; Li, S.; Nishio, H.; Stoller, J.Z.; Oishi, K.; Puttreddy, S.; Lee, T.J.; Epstein, J.A.; Walsh, M.J.; et al. Transcription factor Ap2delta associates with Ash2l and ALR, a trithorax family histone methyltransferase, to activate Hoxc8 transcription. Proc. Natl. Acad. Sci. USA 2008, 105, 7472–7477. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Y.; Gu, S.; Mao, Y.; Ji, C.; Xin, X. Regulation of the HMOX1 gene by the transcription factor AP-2delta with unique DNA binding site. Mol. Med. Rep. 2014, 10, 423–428. [Google Scholar] [CrossRef]
- Li, X.; Persad, A.R.; Monckton, E.A.; Godbout, R. Transcription factor AP-2delta regulates the expression of polysialyltransferase ST8SIA2 in chick retina. FEBS Lett. 2014, 588, 770–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).