Harnessing the Potential of NK Cell-Based Immunotherapies against Multiple Myeloma
Abstract
:1. Introduction
1.1. NK Cell Function
1.2. Major Subsets of NK Cells
1.3. NK Cell Activation
2. Killing of Tumor Cells by NK Cells
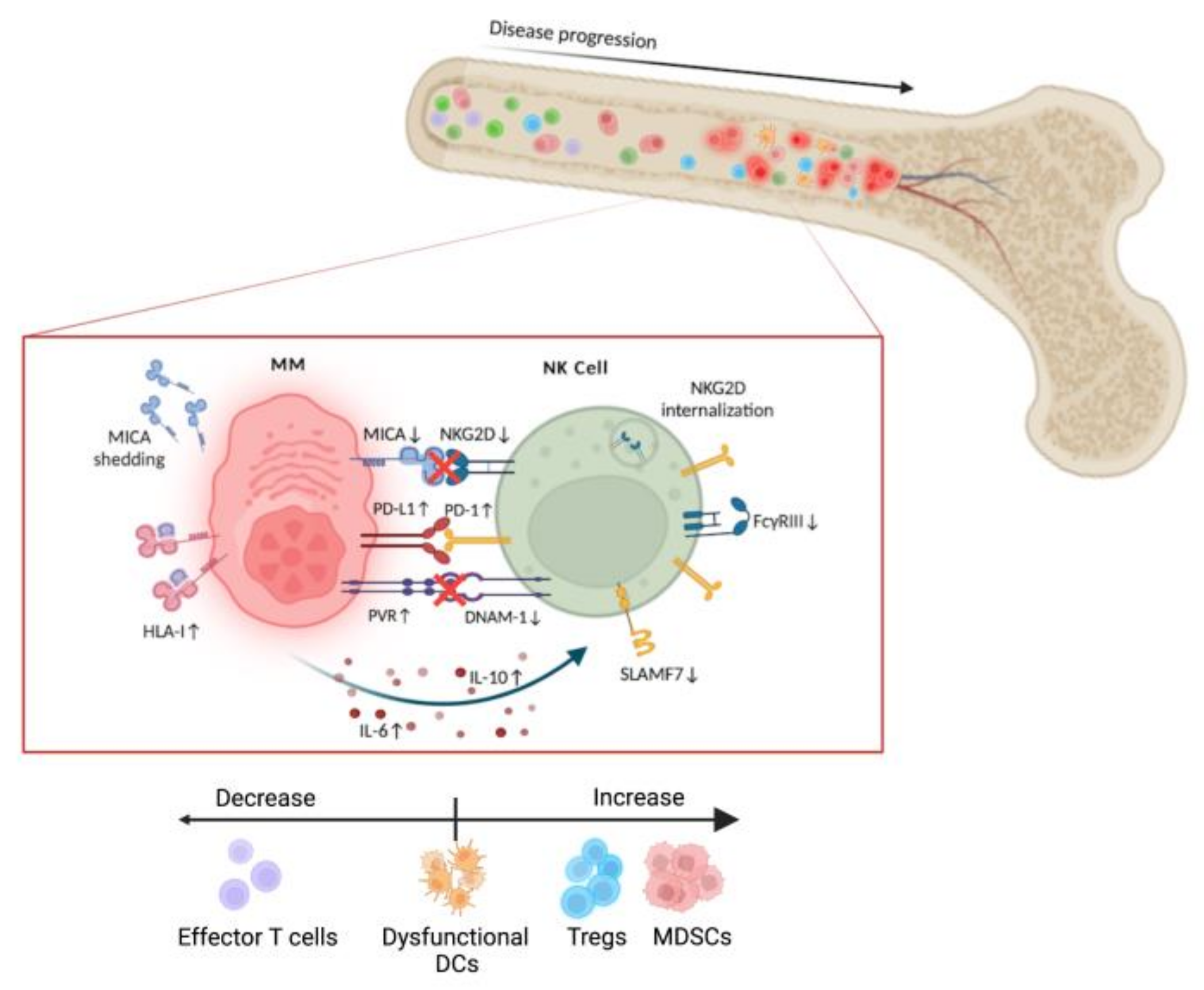
3. NK Cells in Multiple Myeloma
3.1. Current Therapies for Multiple Myeloma
3.2. Antibody-Based Therapy of Multiple Myeloma
3.3. MM Microenvironment. NK Cell Dysfunction
4. NK Cell-Based Treatment for MM
4.1. Autologous NK Cells
4.2. Allogeneic NK Cells
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nature Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Herberman, R.; Nunn, M.; Holden, H.; Lavrin, D. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Wigzell, H. ”Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975, 5, 117–121. [Google Scholar] [CrossRef]
- Herberman, R.; Ortaldo, J. Natural killer cells: Their roles in defenses against disease. Science 1981, 214, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Savoy, S.; Boudreau, J. The Evolutionary Arms Race between Virus and NK Cells: Diversity Enables Population-Level Virus Control. Viruses 2019, 11, 959. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Iizuka, K.; Aguila, H.; Weissman, I.; Yokoyama, W. In vivo natural killer cell activities revealed by natural killer cell-deficient mice Proc. Natl. Acad. Sci. USA 2000, 97, 2731–2736. [Google Scholar] [CrossRef] [Green Version]
- Muntasell, A.; Rojo, F.; Servitja, S.; Rubio-Perez, C.; Cabo, M.; Tamborero, D.; Costa-García, M.; Martínez-Garcia, M.; Menendez, S.; Vazquez, I.; et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2+ Breast Cancer Predict and Uncouple Pathological Response and Disease-free Survival. Clin. Cancer Res. 2019, 25, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Freud, A.; Mundy-Bosse, B.; Yu, J.; Caligiuri, M. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [Green Version]
- Trinchieri, G.; Valiante, N. Receptors for the Fc fragment of IgG on natural killer cells. Nat. Immunol. 1993, 12, 218–234. [Google Scholar]
- Freud, A.; Yu, J.; Caligiuri, M. Human natural killer cell development in secondary lymphoid tissues. Semin. Immunol. 2014, 26, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Collonna, M.; Smaridis, J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science 1995, 268, 405–408. [Google Scholar] [CrossRef]
- Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nature Rev. Immunol. 2005, 5, 201–214. [Google Scholar] [CrossRef]
- Symons, H.; Fuchs, E. Hematopoietic SCT from partially HLA-mismatched (HLA-haploidentical) related donors. Bone Marrow Transplant. 2008, 42, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, H.G.; Karre, K. Host resistance directed selectively gainst H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 1985, 162, 1745–1795. [Google Scholar] [CrossRef]
- Carretero, M.; Cantoni, C.; Bellón, T.; Bottino, C.; Biassoni, R.; Rodríguez, A.; Pérez-Villar, J.; Moretta, L.; Moretta, A.; López-Botet, M. The CD94 and NKG2-A C-type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur. J. Immunol. 1997, 27, 563–567. [Google Scholar] [CrossRef]
- Borrego, F.; Masilamani, M.; Kabat, J.; Sanni, T.; Coligan, J. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol. Immunol. 2005, 42, 485–488. [Google Scholar] [CrossRef]
- Baychelier, F.; Sennepin, A.; Ermonval, M.; Dorgham, K.; Debré, P.; Vieillard, V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013, 122, 2935–2942. [Google Scholar] [CrossRef] [Green Version]
- Schmiedel, D.; Mandelboim, O. NKG2D Ligands-Critical Targets for Cancer Immune Escape and Therapy. Front. Immunol. 2018, 9, 2040. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Borderías, A.; Romo, N.; Magri, G.; Gumá, M.; Angulo, A.; López-Botet, M. IL-12-Dependent Inducible Expression of the CD94/NKG2A Inhibitory Receptor Regulates CD94/NKG2C+ NK Cell Function. J. Immunol. 2009, 182, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Fionda, C.; Soriani, A.; Zingoni, A.; Santoni, A.; Cippitelli, M. NKG2D and DNAM-1 Ligands: Molecular Targets for NK Cell-Mediated Immunotherapeutic Intervention in Multiple Myeloma. BioMed Res. Int. 2015, 2015, 178698. [Google Scholar] [CrossRef]
- Anel, A.; Aguiló, J.I.; Catalán, E.; Garaude, J.; Rathore, M.G.; Pardo, J.; Villalba, M. Protein kinase C-q (PKC-q) in natural killer cell function and anti-tumor immunity. Front. Immunol. 2012, 3, 187. [Google Scholar] [CrossRef] [Green Version]
- Willemze, R.; Rodrigues, C.A.; Labopin, M.; Sanz, G.; Michel, G.; Socie, G.; Rio, B.; Sirvent, A.; Renaud, M.; Madero, L.; et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia 2009, 23, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martínez, D.; Allende-Vega, N.; Orecchioni, S.; Talarico, G.; Cornillon, A.; Vo, D.; Rene, C.; Lu, Z.; Krzywinska, E.; Anel, A.; et al. Expansion of allogeneic NK cells with efficient antibody-dependent cell cytotoxicity against multiple tumors. Theranostics 2018, 8, 3856–3869. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [Green Version]
- Van den Broek, M.F.; Kägi, D.; Zinkernagel, R.M.; Hengartner, H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur. J. Immunol. 1995, 25, 3514–3516. [Google Scholar] [CrossRef]
- Pardo, J.; Balkow, S.; Anel, A.; Simon, M.M. Granzymes are critically involved in NK-mediated control of RMA-S tumor growth in vivo. Eur. J. Immunol. 2002, 32, 2881–2886. [Google Scholar] [CrossRef]
- De Miguel, D.; Ramirez-Labrada, A.; Uranga, I.; Hidalgo, S.; Santiago, L.; Galvez, E.; Arias, M.; Pardo, J. Inflammatory cell death induced by cytotoxic lymphocytes: A dangerous but necessary liaison. FEBS J. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Screpanti, V.; Wallin, R.P.A.; Ljunggren, H.G.; Grandien, A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J. Immunol. 2001, 167, 2068–2073. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Kelly, J.M.; Baxter, A.G.; Körner, H.; Sedgwick, J.D. An Essential Role for Tumor Necrosis Factor in Natural Killer Cell–mediated Tumor Rejection in the Peritoneum. J. Exp. Med. 1998, 188, 1611–1619. [Google Scholar] [CrossRef]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [PubMed]
- Garrido, F.; Algarra, I.; García-Lora, A.M. The escape of cancer from T lymphocytes: Immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol. Immunother. 2010, 59, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.; Shukla, S.; Wu, C.; Getz, G.; Hacohen, N. Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 2015, 160, 48–61. [Google Scholar] [PubMed] [Green Version]
- Zaretsky, J.; Garcia-Diaz, A.; Shin, D.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.; Abril-Rodriguez, G.; Sandoval, S.; Ribas, A.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Eng. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Campana, D. Expansion and Activation of Natural Killer Cells for Cancer Immunotherapy. Korean J. Lab. Med. 2009, 29, 89–96. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Ruggeri, L.; Mancusi, A.; Bernardo, M.E.; de Angelis, C.; Bucher, C.; Locatelli, F.; Aversa, F.; Velardi, A. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 2008, 112, 2990–2995. [Google Scholar] [CrossRef] [Green Version]
- Calvo, T.; Reina-Ortiz, C.; Giraldos, D.; Gascón, M.; Woods, D.; Asenjo, J.; Marco-Brualla, J.; Azaceta, G.; Izquierdo, I.; Palomera, L.; et al. Expanded and activated allogeneic NK cells are cytotoxic against B-chronic lymphocytic leukemia (B-CLL) cells with sporadic cases of resistance. Sci. Rep. 2020, 10, 19398. [Google Scholar] [CrossRef]
- Curti, A.; Ruggeri, L.; Parisi, S.; Bontadini, A.; Dan, E.; Motta, M.; Rizzi, S.; Trabanelli, S.; Ocadlikova, D.; Lecciso, M.; et al. Larger Size of Donor Alloreactive NK Cell Repertoire Correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia Patients. Clin. Cancer Res. 2016, 22, 1914–1921. [Google Scholar] [CrossRef] [Green Version]
- Leivas, A.; Perez-Martinez, A.; Blanchard, M.; Martın-Clavero, E.; Fernandez, L.; Lahuerta, J.; Martinez-Lopez, J. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology 2016, 5, e1250051. [Google Scholar] [CrossRef] [Green Version]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.; Wagner, J.; Jewell, B.; Schappe, T.; Leong, J.; Abdel-Latif, S.; Schneider, S.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Szmania, S.; Lapteva, N.; Garg, T.; Greenway, A.; Lingo, J.; Nair, B.; Stone, K.; Woods, E.; Khan, J.; Stivers, J.; et al. Ex Vivo Expanded Natural Killer Cells Demonstrate Robust Proliferation In Vivo In High-Risk Relapsed Multiple Myeloma Patients. J. Immunother. 2015, 38, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Miller, K.; Fuchs, H.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Del Valle, A.; Anel, A.; Naval, J.; Marzo, I. Immunogenic Cell Death and Immunotherapy of Multiple Myeloma. Front. Cell Dev. Biol. 2019, 7, 50. [Google Scholar]
- Rajkumar, S. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [Green Version]
- Attal, M.; Harousseau, J.; Stoppa, A.; Sotto, J.; Fuzibet, J.; Rossi, J.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N. Eng. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef]
- Luptakova, K.; Rosenblatt, J.; Glotzbecker, B.; Mills, H.; Stroopinsky, D.; Kufe, T.; Vasir, B.; Arnason, J.; Tzachanis, D.; Zwicker, J.; et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol. Immunother. 2013, 62, 39–49. [Google Scholar] [CrossRef]
- Görgün, G.; Samur, M.; Cowens, K.; Paula, S.; Bianchi, G.; Anderson, J.; White, R.; Singh, A.; Ohguchi, H.; Suzuki, R.; et al. Lenalidomide Enhances Immune Checkpoint Blockade-Induced Immune Response in Multiple Myeloma. Clin. Cancer Res. 2015, 21, 4607–4618. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Hou, J.; Chen, G.; Yu, D.; Xie, Y.; Gao, L.; Xiao, W.; Kong, Y.; Shi, J. Carfilzomib combined with ex vivo-expanded patient autologous natural killer cells for myeloma immunotherapy. Neoplasma 2018, 65, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Petrucci, M.; Vozella, F. The Anti-CD38 Antibody Therapy in Multiple Myeloma. Cells 2019, 8, 1629. [Google Scholar] [CrossRef] [Green Version]
- Robak, P.; Drozdz, I.; Szemra, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar]
- Lokhorst, H.; Plesner, T.; Laubach, J.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Eng. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef]
- Nakamura, A.; Suzuki, S.; Kanasugi, J.; Ejiri, M.; Hanamura, I.; Ueda, R.; Seto, M.; Takami, A. Synergistic Effects of Venetoclax and Daratumumab on Antibody-Dependent Cell-Mediated Natural Killer Cytotoxicity in Multiple Myeloma. Int. J. Mol.Sci. 2021, 22, 10761. [Google Scholar] [CrossRef]
- Van der Veer, M.; de Weers, M.; van Kessel, B.; Bakker, J.; Wittebol, S.; Parren, P.W.; Lokhorst, H.M.; Mutis, T. Towards effective immunotherapy of myeloma: Enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica 2011, 96, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Van de Donk, N.; Richardson, P.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar]
- Wang, Y.; Zhang, Y.; Hughes, T.; Zhang, J.; Caliguri, M.; Benson, D.; Yu, J. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin. Cancer Res. 2018, 24, 4006–4017. [Google Scholar] [CrossRef] [Green Version]
- Casneuf, T.; Xu, X.; Adams, H.; Axel, A.; Chiu, C.; Khan, I.; Ahmadi, T.; Yan, X.; Lonial, S.; Plesner, T.; et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017, 1, 2105–2114. [Google Scholar] [CrossRef]
- Kararoudi, M.; Nagai, Y.; Elmas, E.; de Souza Fernandes Pereira, M.; Abbas Ali, S.; Hollingsworth Imus, P.; Wethington, D.; Marques Borrello, I.; Lee, D.; Ghiaur, G. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 2020, 136, 2416–2427. [Google Scholar] [CrossRef]
- Lejeune, M.; Duray, E.; Peipp, M.; Clémenceau, B.; Baron, F.; Beguin, Y.; Caers, J. Balancing the CD38 Expression on Effector and Target Cells in Daratumumab-Mediated NK Cell ADCC against Multiple Myeloma. Cancers 2021, 13, 3072. [Google Scholar] [CrossRef]
- Reina-Ortiz, C.; Constantinides, M.; Fayd-Herbe-de-Maudave, A.; Présumey, J.; Hernandez, J.; Cartron, G.; Giraldos, D.; Díez, R.; Izquierdo, I.; Azaceta, G.; et al. Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against tumor cells from multiple myeloma patients. Oncoimmunology 2020, 10, e1853314. [Google Scholar] [CrossRef] [PubMed]
- Mahaweni, N.; Bos, G.; Mitsiades, C.; Tilanus, M.; Wieten, L. Daratumumab augments alloreactive natural killer cell cytotoxicity towards CD38+ multiple myeloma cell lines in a biochemical context mimicking tumour microenvironment conditions. Cancer Immunol. Immunother. 2018, 67, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhael, J.; Richardson, P.; Usmani, S.; Raje, N.; Bensinger, W.; Karanes, C.; Campana, F.; Kanagavel, D.; Dubin, F.; Liu, Q.; et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood 2019, 134, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.; et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Bringhen, S.; Pour, L.; Vorobyev, V.; Vural, F.; Warzocha, K.; Benboubker, L.; Koh, Y.; Maisnar, V.; Karlin, L.; Pavic, M.; et al. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma according to prior lines of treatment and refractory status: ICARIA-MM subgroup analysis. Leuk. Res. 2021, 104, 106576. [Google Scholar] [CrossRef] [PubMed]
- Hsi, E.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008, 14, 2775–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Eng. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.; Bakan, C.; Swartzel, G.; Hofmeister, C.; Efebera, Y.; Kwon, H.; Starling, G.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavidij, O.; Haradhvala, N.; Mouhieddine, T.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- Cho, S.; Xing, L.; Anderson, K.; Tai, Y. Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers 2021, 13, 6136. [Google Scholar]
- Rawstron, A.; Davies, F.; Owen, R.; English, A.; Pratt, G.; Child, J.; Jack, A.; Morgan, G. B-lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B-cell progenitors and plasma cell precursors. Br. J. Hematol. 1998, 100, 176–183. [Google Scholar] [CrossRef]
- Joshua, D.; Suen, H.; Brown, R.; Bryant, C.; Ho, P.; Hart, D.; Gibson, J. The T Cell in Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 537–542. [Google Scholar] [CrossRef]
- Braga, W.; da Silva, B.; de Carvalho, A.; Maekawa, Y.; Bortoluzzo, A.; Rizzatti, E.; Atanackovic, D.; Colleoni, G. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4(+) T regulatory cells. Cancer Immunol. Immunother. 2014, 63, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Malek, E.; de Lima, M.; Letterio, J.; Kim, B.; Finke, J.; Driscoll, J.; Giralt, S. Myeloid-derived suppressor cells: The green light for myeloma immune escape. Blood Rev. 2016, 30, 341–348. [Google Scholar]
- Mahindra, A.; Hideshima, T.; Anderson, K. Multiple myeloma: Biology of the disease. Blood Rev. 2010, 24, S5–S11. [Google Scholar]
- Leone, P.; Berardi, S.; Frassanito, M.; Ria, R.; De Re, V.; Cicco, S.; Battaglia, S.; Ditonno, P.; Dammacco, F.; Vacca, A.; et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood 2015, 126, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.; Pope, B.; Murray, A.; Esdale, W.; Sze, D.; Gibson, J.; Ho, P.; Hart, D.; Joshua, D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood 2001, 98, 2992–2998. [Google Scholar] [CrossRef] [Green Version]
- Derenne, S.; Monia, B.; Dean, N.; Taylor, J.; Rapp, M.; Harousseau, J.; Bataille, R.; Amiot, M. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 2002, 100, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Leone, P.; Solimando, A.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune Cells in the Myeloma Niche. Front. Oncol. 2020, 10, 599098. [Google Scholar]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-gamma and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007, 110, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Yousef, S.; Marvin, J.; Steinbach, M.; Langemo, A.; Kovacsovics, T.; Binder, M.; Kröger, N.; Luetkens, T.; Atanackovic, D. Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J. 2015, 5, e285. [Google Scholar] [CrossRef] [Green Version]
- Krzywinska, E.; Allende-Vega, N.; Cornillon, A.; Vo, D.; Cayrefourcq, L.; Panabieres, C.; Vilches, C.; Déchanet-Merville, J.; Hicheri, Y.; Rossi, J.; et al. Identification of Anti-tumor Cells Carrying Natural Killer (NK) Cell Antigens in Patients with Hematological Cancers. EBioMedicine 2015, 2, 1364–1376. [Google Scholar] [PubMed] [Green Version]
- Krzywinska, E.; Cornillon, A.; Allende-Vega, N.; Vo, D.; Rene, C.; Lu, Z.; Pasero, C.; Olive, D.; Fegueux, N.; Ceballos, P.; et al. CD45 Isoform Profile Identifies Natural Killer (NK) Subsets with Differential Activity. PLoS ONE 2016, 11, e0150434. [Google Scholar]
- García-Sanz, R.; González, M.; Orfão, A.; Moro, M.; Hernández, J.; Borrego, D.; Carnero, M.; Casanova, F.; Bárez, A.; Jiménez, R.; et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br. J. Hematol. 1996, 93, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Schütt, P.; Brandhorst, D.; Stellberg, W.; Poser, M.; Ebeling, P.; Müller, S.; Buttkereit, U.; Opalka, B.; Lindemann, M.; Grosse-Wilde, H.; et al. Immune parameters in multiple myeloma patients: Influence of treatment and correlation with opportunistic infections. Leuk. Lymphoma 2006, 47, 1570–1582. [Google Scholar] [CrossRef]
- Rueff, J.; Medinger, M.; Heim, D.; Passweg, J.; Stern, M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol. Blood Marrow Transplant. 2014, 20, 896–899. [Google Scholar]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar]
- Bernal, M.; Garrido, P.; Jiménez, P.; Carretero, R.; Almagro, M.; López, P.; Navarro, P.; Garrido, F.; Ruiz-Cabello, F. Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: Implications for tumor evasion of T and NK cells. Hum. Immunol. 2009, 70, 854–857. [Google Scholar]
- Bedel, R.; Thiery-Vuillemin, A.; Grandclement, C.; Balland, J.; Remy-Martin, J.; Kantelip, B.; Pallandre, J.; Pivot, X.; Ferrand, C.; Tiberghien, P.; et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res. 2011, 71, 1615–1626. [Google Scholar]
- Jinushi, M.; Vanneman, M.; Munshi, N.; Tai, Y.; Prabhala, R.; Ritz, J.; Neuberg, D.; Anderson, K.; Carrasco, D.; Dranoff, G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl. Acad. Sci. USA 2008, 105, 1285–1290. [Google Scholar]
- Pazina, T.; MacFarlane, A.; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.; Robbins, M.; Ross, E.; Campbell, K.; et al. Alterations of NK Cell Phenotype in the Disease Course of Multiple Myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef]
- Benson, D.; Bakan, C.; Mishra, A.; Hofmeister, C.; Efebera, Y.; Becknell, B.; Baiocchi, R.; Zhang, J.; Yu, J.; Smith, M.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar]
- Costa, F.; Marchica, V.; Storti, P.; Malavasi, F.; Giuliani, N. PD-L1/PD-1 Axis in Multiple Myeloma Microenvironment and a Possible Link with CD38-Mediated Immune-Suppre. Cancers 2021, 13, 164. [Google Scholar] [CrossRef]
- Jelinek, T.; Paiva, B.; Hajek, R. Update on PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2431. [Google Scholar]
- Biran, N.; Paleoudis, E.; Feinman, R.; Vesole, D.; Zenreich, J.; Wang, S.; Ahn, J.; Bansal, M.; Rowley, S.; Donato, M.; et al. Pembrolizumab, lenalidomide and dexamethasone post autologous transplant in patients with high-risk multiple myeloma. Am. J. Hematol. 2021, 96, E430–E433. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2017, 7, 72961–72977. [Google Scholar] [CrossRef] [Green Version]
- Lad, D.; Huang, Q.; Hoeppli, R.; Garcia, R.; Xu, L.; Levings, M.; Song, K.; Broady, R. Evaluating the role of Tregs in the progression of multiple myeloma. Leuk. Lymphoma 2019, 60, 2134–2142. [Google Scholar] [CrossRef]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine profile in multiple myeloma. Cytokine 2020, 136, 155271. [Google Scholar]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of natural killer cell cytotoxicity by interleukin-6: Implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015, 67, 3037. [Google Scholar] [CrossRef]
- Fisher, D.; Appenheimer, M.; Evans, S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar]
- Konjević, G.; Vuletić, A.; Martinović, K.; Larsen, A.; Jurišić, V. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [PubMed]
- Lu, A.; Pallero, M.; Lei, W.; Hong, H.; Yang, Y.; Suto, M.; Murphy-Ullrich, J. Inhibition of Transforming Growth Factor-beta Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am. J. Pathol. 2016, 186, 678–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Jin, Y.; Sattar, H.; Liu, H.; Xie, W.; Zhou, F. Natural killer cell immunotherapy against multiple myeloma: Progress and possibilities. J. Leukoc. Biol. 2018, 103, 821–828. [Google Scholar] [PubMed]
- Rosenberg, S.; Lotze, M.; Muul, L.; Leitman, S.; Chang, A.; Ettinghausen, S.; Matory, Y.; Skibber, J.; Shiloni, E.; Vetto, J.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Eng. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Noonan, K.; Huff, C.; Davis, J.; Lemas, M.; Fiorino, S.; Bitzan, J.; Ferguson, A.; Emerling, A.; Luznik, L.; Matsui, W.; et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci. Transl. Med. 2015, 7, 288ra78. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Savani, B.; Mielke, S.; Adams, S.; Uribe, M.; Rezvani, K.; Yong, A.; Zeilah, J.; Kurlander, R.; Srinivasan, R.; Childs, R.; et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia 2007, 21, 2145–2152. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, K.; Barrett, A.; Schaffer, M.; Hägglund, H.; Ljungman, P.; Ringdén, O.; Remberger, M. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol. Blood Marrow Transplant. 2009, 15, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Tschan-Plessl, A.; Kalberer, C.; Wieboldt, R.; Stern, M.; Siegler, U.; Wodnar-Filipowicz, A.; Gerull, S.; Halter, J.; Heim, D.; Tichelli, A. Cellular immunotherapy with multiple infusions of in vitro-expanded haploidentical natural killer cells after autologous transplantation for patients with plasma cell myeloma. Cytotherapy 2020, 23, 329–338. [Google Scholar] [CrossRef]
- Shah, N.; Li, L.; McCarty, J.; Kaur, I.; Yvon, E.; Shaim, H.; Muftuoglu, M.; Liu, E.; Orlowski, R.; Cooper, L.; et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Hematol. 2017, 177, 457–466. [Google Scholar]
- Williams, B.; Law, A.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef] [Green Version]

| Trial ID. | Specific NK Cell Source | Additional Treatment | Cytokine Support | Phase | Status (# Patients) | Trial Title |
|---|---|---|---|---|---|---|
| NCT01884688 | K562-mb15-41BBL | - | IL-2 | II | Completed (1) | A Phase II Study of Autologous Expanded Natural Killer Cell Therapy for Asymptomatic Multiple Myeloma |
| NCT01313897 | K562-mb15-41BBL | Bor | IL-2 | II | Completed (10) | UARK 2010-35, A Phase II Study of Expanded Natural Killer Cell Therapy for Multiple Myeloma |
| NCT03003728 | K562-mb15-41BBL | Elo, Mel | IL-15 (ALT-803) | I | Withdrawn (10) | 2015-10: A Phase II Pilot Study of Expanded Natural Killer Cells and Elotuzumab to Eradicate High-Risk Myeloma Post Autologous Stem Cell Transplant |
| NCT04558853 | Autologous | - | - | I/II | Active, NR (6) | A Safety Study of CellProtect, an Autologous ex Vivo Expanded and Activated Natural Killer (NK) Cell Product, in Patients with Multiple Myeloma |
| NCT00720785 | Autologous | Bor | - | I | Completed (35, * MM) | Safety and the Anti- Tumor Effects of Escalating Doses of Adoptively Infused Ex Vivo Expanded Autologous Natural Killer (NK) Cells Against Metastatic Cancers or Hematological Malignancies Sensitized to NK-TRAIL Cytotoxicity with Bortezomib |
| NCT02481934 | K562-mb15-41BBL | Len, Bor | - | I | Completed (5) | Phase 1 Clinical Trial to Evaluate Security and Dose of Expanded and Activated Autologous NK Cells Infusions in Consolidation of Multiple Myeloma Patients Treatment on Second or Later Relapse |
| NCT04558931 | Autologous | Isa | - | II | Recruiting | An Open, Randomized, Controlled, Phase II Trial of CellProtect in Combination with Isatuximab Antibody Versus Isatuximab Antibody Alone as Maintenance Treatment in Patients with Multiple Myeloma Undergoing High Dose Treatment |
| Trial ID | Specific NK Cell Source | Additional Treatment | Cytokine Support | Phase | Status (# Patients) | Trial Title |
|---|---|---|---|---|---|---|
| NCT0008945 | KIR-L mismatch haploidentical | Dex, Cyc, Mel, Flu, Bor | IL-2 | I/II | Completed, NRP (10) | UARK 2003-18, A Phase II Study of KIR-Ligand Mismatched Haplo-Identical Natural Killer Cells Transfused Before Autologous Stem Cell Transplant in Relapsed Multiple Myeloma |
| NCT00569283 | Allogenic | - | - | I | Completed, NRP (18, * MM) | Donor Natural Killer Cell Infusion for the Prevention of Relapse or Graft Failure After HLA-Haploidentical Familial Donor Bone Marrow Transplantation-A Phase I Study |
| NCT00660166 | HLA Class I Haplotype Mismatched | Ben | - | I | Completed, NRP (13, * MM) | HLA Class I Haplotype Mismatched Natural Killer Cell Infusions After Autologous Stem Cell Transplant for Hematological Malignancies |
| NCT00789776 | Allogenic | - | - | I/II | Completed (41, * MM) | A Phase I/II Study Evaluating the Safety and Efficacy of Adding a Single Prophylactic Donor Lymphocyte Infusion (DLI) of Natural Killer Cells Early After Nonmyeloablative, HLA-Haploidentical Hematopoietic Cell Transplantation—A Multi-Center Trial |
| NCT00823524 | Allogenic | - | - | I/II | Completed (47, * MM) | Donor NK Cell Infusion for Progression/Recurrence of Underlying Malignant Disorders After HLA-haploidentical HCT—a Phase 1-2 Study |
| NCT00990717 | NK-92 cells | - | - | I | Completed (11) | A Dose Escalation Study of NK-92 Cell Infusions in Patients with Hematological Malignancies in Relapse After Autologous Stem Cell Transplantation |
| NCT02955550 | PNK-007 | Mel | rhIL-2 | I | Completed (15) | A Phase 1, Multicenter, Open-label, Safety Study of Human Cord Blood Derived, Culture-expanded, Natural Killer Cell (PNK-007) Infusion Following Autologous Stem Cell Transplant for Multiple Myeloma |
| NCT01040026 | Haploidentical | Mel | - | I/II | Active, NR (10) | A Phase I/II Single Center Study to Assess Tolerability and Feasibility of Infusions of Allogeneic Expanded Haploidentical Natural Killer (NK) Cells in Patients Treated with High Dose Melphalan Chemotherapy and Autologous Stem Cell Transplantation for a Multiple Myeloma |
| NCT04309084 | CYNK-001 | - | - | I | Active, NR (29, * MM) | A Phase I Study of Human Placental Hematopoietic Stem Cell Derived Natural Killer Cells (CYNK 001) in Multiple Myeloma Patients Following Autologous Stem Cell Transplant in the Front-line Setting |
| NCT01619761 | UCB | Mel, Len, Flu, Myc, Cyc, Rit | - | I | Active, NR (12) | Natural Killer Cells in Allogeneic Cord Blood Transplantation |
| NCT01729091 | UCB | Elo, Len, Mel | - | II | Active, NR (72) | Phase II Study of Umbilical Cord Blood-Derived Natural Killer Cells in Conjunction with Elotuzumab, Lenalidomide and High Dose Melphalan Followed by Autologous Stem Cell Transplant for Patients With Multiple Myeloma |
| NCT02890758 | Non-HLA matched donor | - | ALT803 | I | Active, NR (14, * MM) | Phase I Trial of Universal Donor NK Cell Therapy in Combination With ALT-803 |
| NCT02727803 | NK-92 | ATG, Flu, Cyc, Clo, Bus | - | II | Recruiting | Personalized NK Cell Therapy in Cord Blood Transplantation |
| NCT03019666 | NAM-NK | Cyc, Flu | - | I | Recruiting | A Phase I Trial Testing NAM Expanded Haploidentical or Mismatched Related Donor Natural Killer (NK) Cells Followed by a Short Course of IL-2 for the Treatment of Relapsed/Refractory Multiple Myeloma and Relapsed/Refractory CD20+ Non-Hodgkin Lymphoma |
| NCT04754100 | agent-797 | - | - | I | Recruiting | A Phase I Open-Label Study of the Safety, Tolerability and Preliminary Clinical Activity of Allogeneic Invariant Natural Killer (iNKT) Non-transduced Cells (agenT-797) in Patients with Relapsed/Refractory Multiple Myeloma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reina-Ortiz, C.; Giraldos, D.; Azaceta, G.; Palomera, L.; Marzo, I.; Naval, J.; Villalba, M.; Anel, A. Harnessing the Potential of NK Cell-Based Immunotherapies against Multiple Myeloma. Cells 2022, 11, 392. https://doi.org/10.3390/cells11030392
Reina-Ortiz C, Giraldos D, Azaceta G, Palomera L, Marzo I, Naval J, Villalba M, Anel A. Harnessing the Potential of NK Cell-Based Immunotherapies against Multiple Myeloma. Cells. 2022; 11(3):392. https://doi.org/10.3390/cells11030392
Chicago/Turabian StyleReina-Ortiz, Chantal, David Giraldos, Gemma Azaceta, Luis Palomera, Isabel Marzo, Javier Naval, Martín Villalba, and Alberto Anel. 2022. "Harnessing the Potential of NK Cell-Based Immunotherapies against Multiple Myeloma" Cells 11, no. 3: 392. https://doi.org/10.3390/cells11030392








