Alterations of Sphingolipid and Phospholipid Pathways and Ornithine Level in the Plasma as Biomarkers of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Amino Acid Analysis
2.3. Biogenic Amines Analysis with Liquid Chromatography Mass Spectrometry
2.4. Tryptophan Metabolites Analysis with Liquid Chromatography Mass Spectrometry
2.5. Acylcarnitines Analysis with Liquid Chromatography Mass Spectrometry
2.6. Phospholipid Analysis with Liquid Chromatography Mass Spectrometry
2.7. Measurement of α-Synuclein in Plasma
2.8. Statistical Analysis
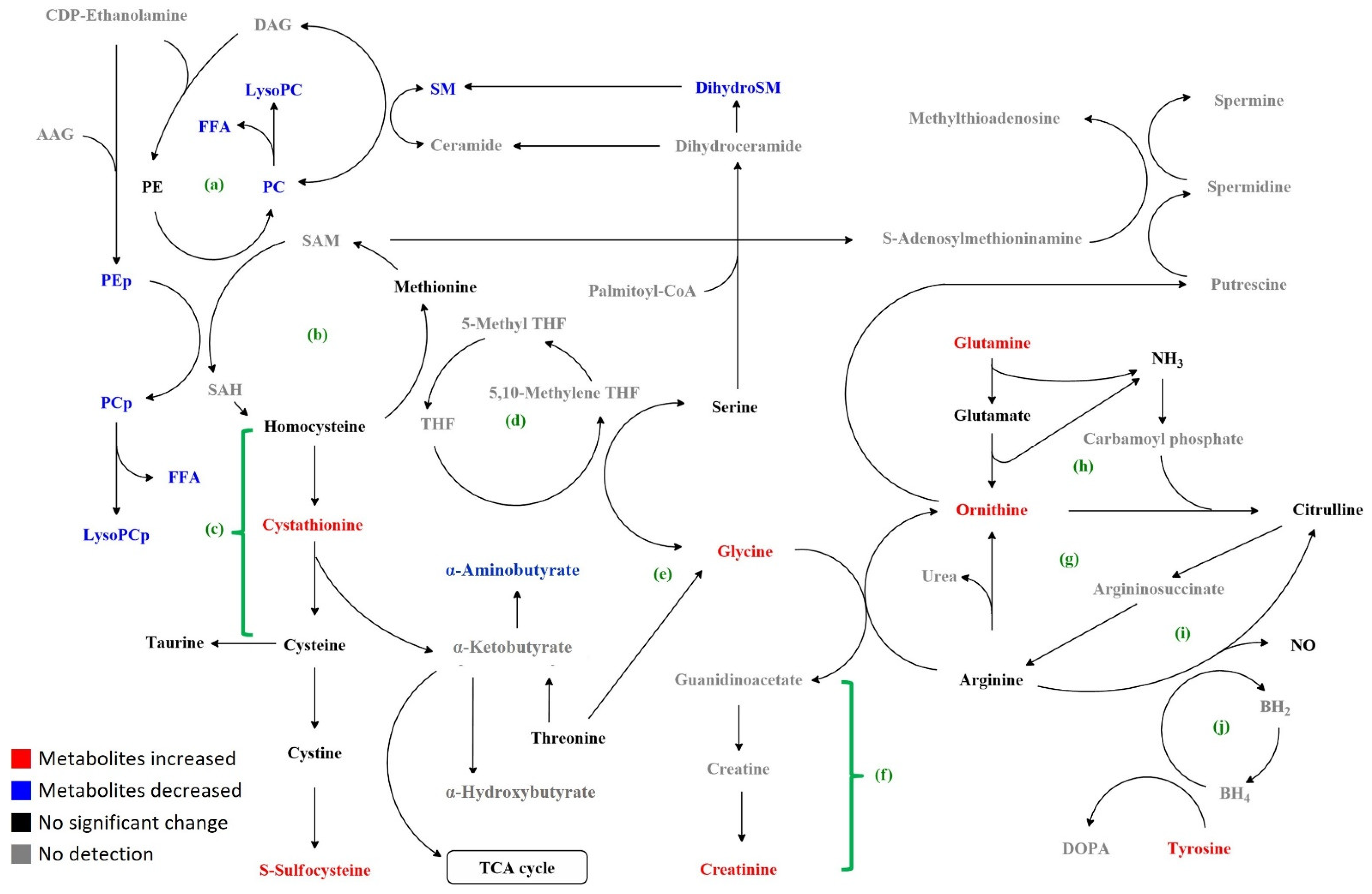
3. Results
3.1. Demography and Clinical Presentations of Subjects
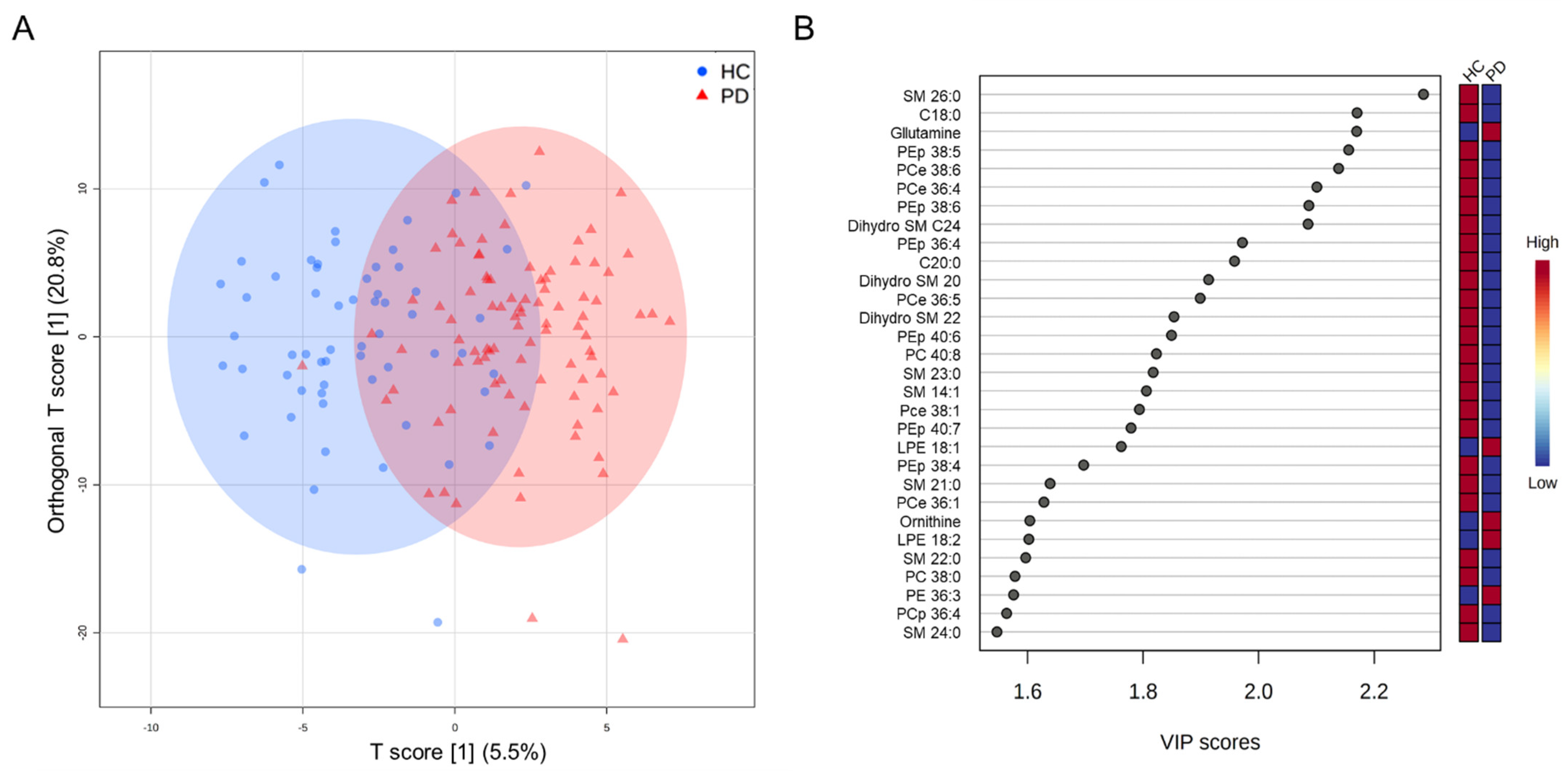
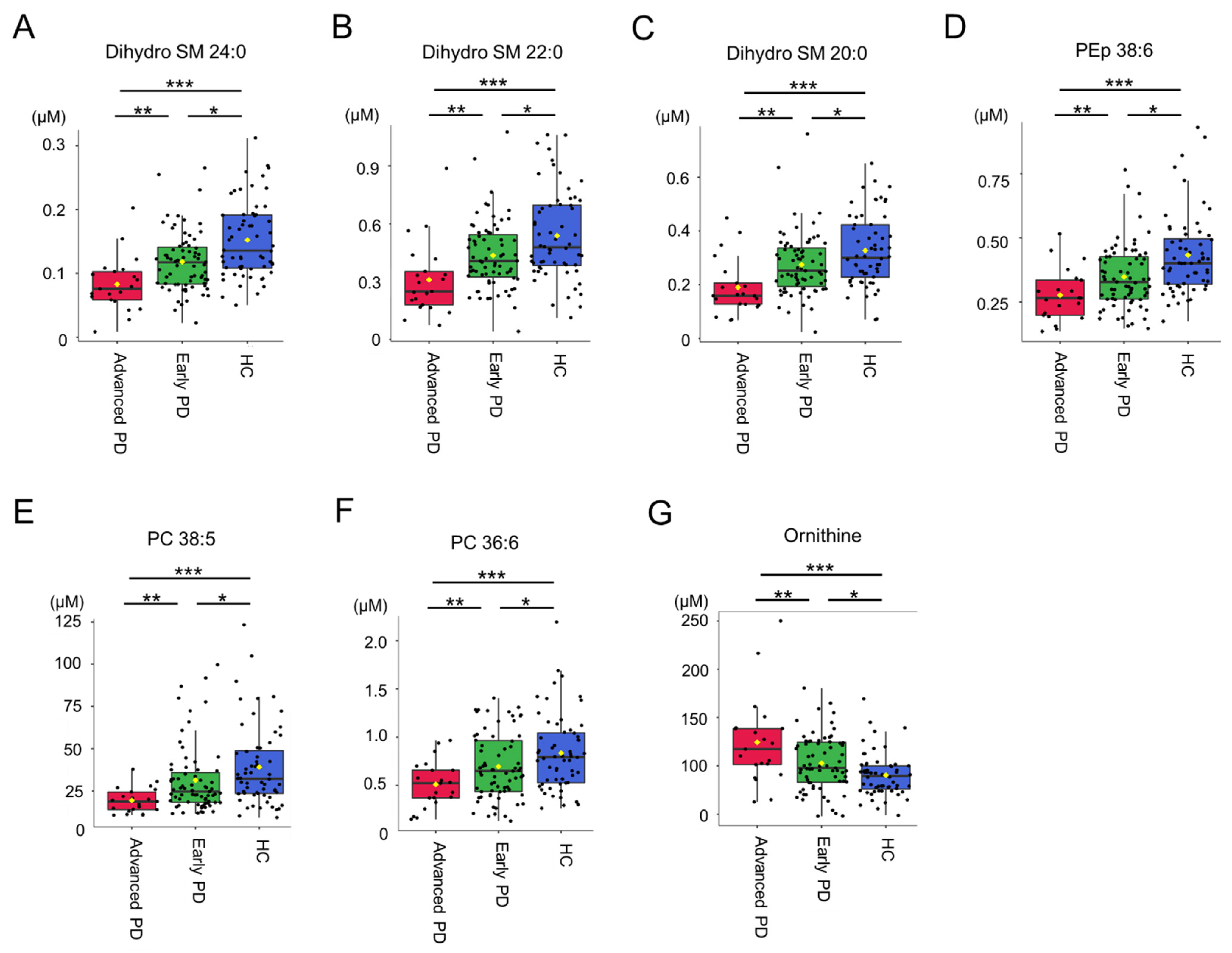
3.2. Targeted Metabolomics Analysis
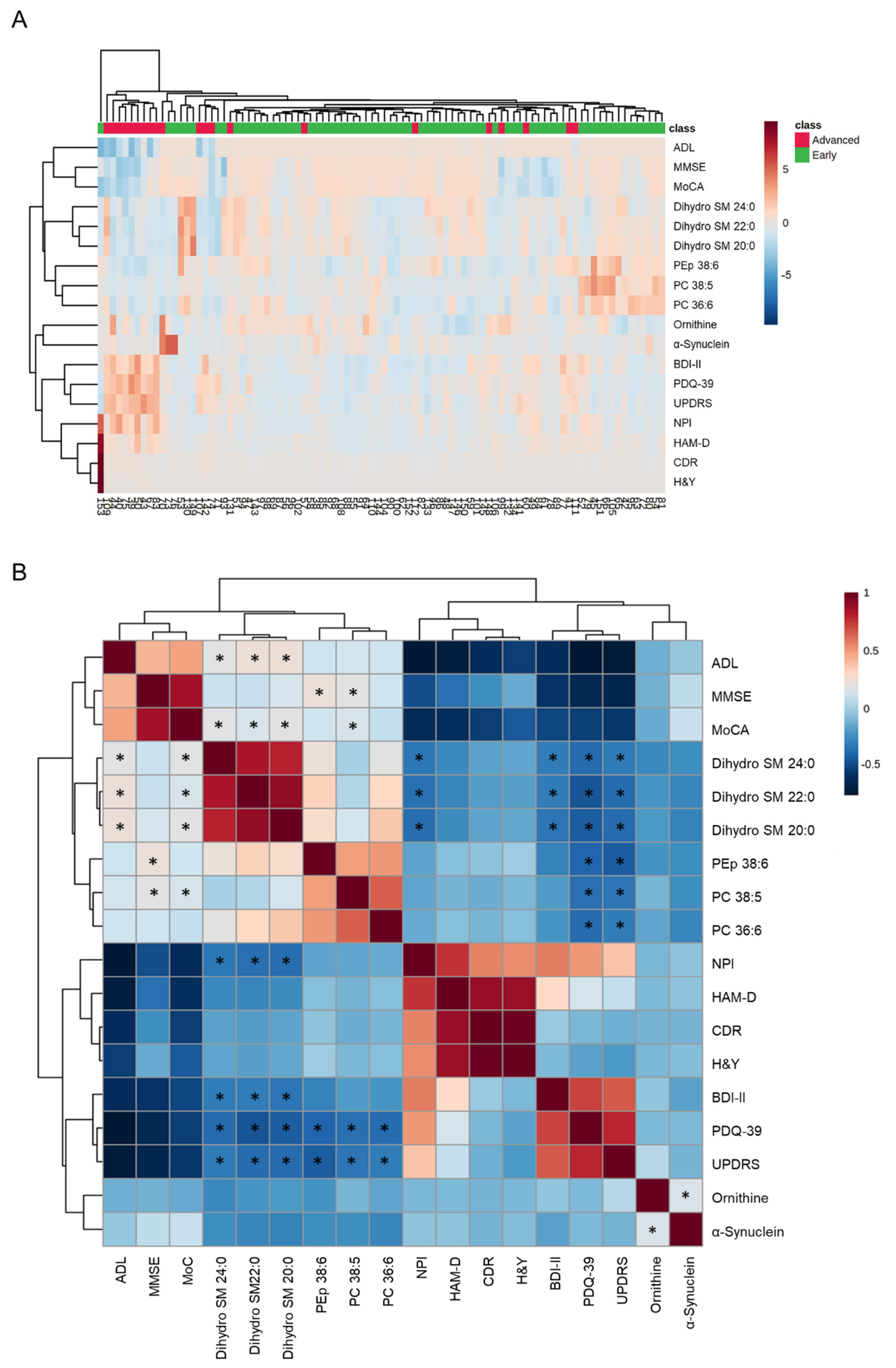
3.3. Clustering and Correlation Analysis
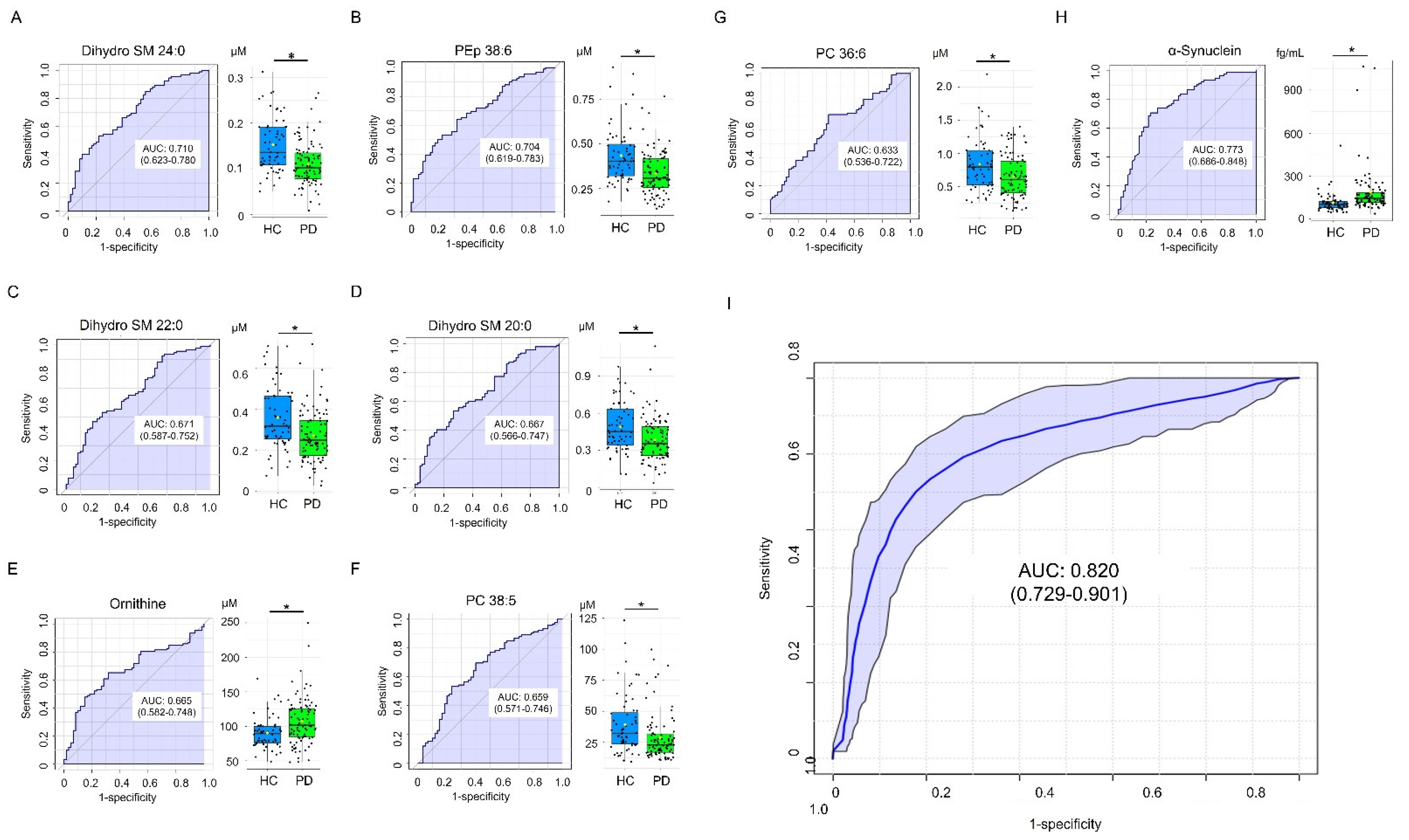
3.4. Classification Model for Differentiating PD and HC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A. Biomarkers in Parkinson’s Disease. In Neurodegenerative Diseases Biomarkers; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Springer: New York, NY, USA, 2022; pp. 155–180. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Santosh, W.; Kumar, S.; Christlet, H.T. Metabolic profiling of Parkinson’s disease: Evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 2009, 16, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, T.; Saiki, S.; Okuzumi, A.; Mohney, R.P.; Hattori, N. Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Roede, J.R.; Uppal, K.; Park, Y.; Lee, K.; Tran, V.; Walker, D.; Strobel, F.H.; Rhodes, S.L.; Ritz, B.; Jones, D.P. Serum metabolomics of slow vs. rapid motor progression Parkinson’s disease: A pilot study. PLoS ONE 2013, 8, e77629. [Google Scholar] [CrossRef] [Green Version]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Johansen, K.K.; Wang, L.; Aasly, J.O.; White, L.R.; Matson, W.R.; Henchcliffe, C.; Beal, M.F.; Bogdanov, M. Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS ONE 2009, 4, e7551. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of metabolic profile and kynurenine metabolism in the plasma of Parkinson’s disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef]
- Trupp, M.; Jonsson, P.; Ohrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J. Parkinsons Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef]
- Bogdanov, M.; Matson, W.R.; Wang, L.; Matson, T.; Saunders-Pullman, R.; Bressman, S.S.; Flint Beal, M. Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 2008, 131, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Fahn, S. Movement Disorder Society Task Force on Rating Scales for Parkinson’s, D. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Morris, J.C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory-II; Pearson: San Antonio, TX, USA, 1996. [Google Scholar]
- Hamilton, M. Rating depressive patients. J. Clin. Psychiatry 1980, 41, 21–24. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Jenkinson, C.; Peto, V.; Fitzpatrick, R.; Greenhall, R.; Hyman, N. Self-reported functioning and well-being in patients with Parkinson’s disease: Comparison of the short-form health survey (SF-36) and the Parkinson’s Disease Questionnaire (PDQ-39). Age Ageing 1995, 24, 505–509. [Google Scholar] [CrossRef]
- Cummings, J.L. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48, S10–S16. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.H.; Cheng, M.L.; Lo, C.J.; Lin, Y.H.; Lai, M.W.; Lin, W.R.; Yeh, C.T. Plasma phenylalanine and glutamine concentrations correlate with subsequent hepatocellular carcinoma occurrence in liver cirrhosis patients: An exploratory study. Sci. Rep. 2020, 10, 10926. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Fang, X.; Huang, Y.; Cai, Y.; Liang, C.; Lin, R.; Liu, L. Separation and identification of underivatized plasma acylcarnitine isomers using liquid chromatography-tandem mass spectrometry for the differential diagnosis of organic acidemias and fatty acid oxidation defects. J. Chromatogr. A 2013, 1319, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Chiu, M.J.; Lin, C.H.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H. Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J. Nanobiotechnol. 2016, 14, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoessel, D.; Schulte, C.; Teixeira Dos Santos, M.C.; Scheller, D.; Rebollo-Mesa, I.; Deuschle, C.; Walther, D.; Schauer, N.; Berg, D.; Nogueira da Costa, A.; et al. Promising metabolite profiles in the plasma and CSF of early clinical Parkinson’s disease. Front. Aging Neurosci. 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ikeda, K.; Shiojima, T.; Kinoshita, M. Increased plasma concentrations of aspartate, glutamate and glycine in Parkinson’s disease. Neurosci. Lett. 1992, 145, 175–177. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta 2014, 1841, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Li, H.; Munoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castano-Diez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Albi, E.; Cataldi, S.; Codini, M.; Mariucci, G.; Lazzarini, A.; Ceccarini, M.R.; Ferri, I.; Laurenti, M.E.; Arcuri, C.; Patria, F.; et al. Neutral sphingomyelinase increases and delocalizes in the absence of Toll-Like Receptor 4: A new insight for MPTP neurotoxicity. Prostaglandins Other Lipid Mediat. 2019, 142, 46–52. [Google Scholar] [CrossRef]
- Zhu, C.; Bilousova, T.; Focht, S.; Jun, M.; Elias, C.J.; Melnik, M.; Chandra, S.; Campagna, J.; Cohn, W.; Hatami, A.; et al. Pharmacological inhibition of nSMase2 reduces brain exosome release and alpha-synuclein pathology in a Parkinson’s disease model. Mol. Brain 2021, 14, 70. [Google Scholar] [CrossRef]
- Exton, J.H. Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1994, 1212, 26–42. [Google Scholar] [CrossRef]
- Cui, Z.; Houweling, M.; Chen, M.H.; Record, M.; Chap, H.; Vance, D.E.; Terce, F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 1996, 271, 14668–14671. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, e0191815. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.; Smith, C.A.; Hayley, S.; Smith, J. Major alterations of phosphatidylcholine and lysophosphotidylcholine lipids in the substantia nigra using an early stage model of Parkinson’s disease. Int. J. Mol. Sci. 2015, 16, 18865–18877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, E.I.; Jiang, Z.; Strub, M.P.; Lee, J.C. Effects of phosphatidylcholine membrane fluidity on the conformation and aggregation of N-terminally acetylated alpha-synuclein. J. Biol. Chem. 2018, 293, 11195–11205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.L.; Chang, K.H.; Wu, Y.R.; Chen, C.M. Metabolic disturbances in plasma as biomarkers for Huntington’s disease. J. Nutr. Biochem. 2016, 31, 38–44. [Google Scholar] [CrossRef]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; Garcia-Gomez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, S.; Liou, L.C.; Ren, Q.; Zhang, Z.; Caldwell, G.A.; Caldwell, K.A.; Witt, S.N. Phosphatidylethanolamine deficiency disrupts alpha-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, E3976–E3985. [Google Scholar] [CrossRef] [Green Version]
- Hattingen, E.; Magerkurth, J.; Pilatus, U.; Mozer, A.; Seifried, C.; Steinmetz, H.; Zanella, F.; Hilker, R. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 2009, 132, 3285–3297. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef]
- Chen, J.; Herrup, K. Glutamine as a potential neuroprotectant in Alzheimer’s disease. In Diet and Nutrition in Dementia and Cognitive Decline; Martin, C.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 761–771. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wang, Y.; Li, J.; Lu, G.; Liu, Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ. Health Prev. Med. 2019, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Paschen, W. Polyamine metabolism in different pathological states of the brain. Mol. Chem. Neuropathol. 1992, 16, 241–271. [Google Scholar] [CrossRef] [PubMed]
- Çelik, V.; Çiğdem, B.; Kapancık, S.; Kılıçgün, H.; Bolayır, E. The importance of increased serum ornithine levels in the pathogenesıs of Alzheımer and Parkınson’s dıseases. Asian J. Res. Rep. Neurol. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Wyse, A.T.S.; Grings, M.; Wajner, M.; Leipnitz, G. The role of oxidative stress and bioenergetic dysfunction in sulfite oxidase deficiency: Insights from animal models. Neurotox. Res. 2019, 35, 484–494. [Google Scholar] [CrossRef]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [Green Version]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated analyses of microbiome and longitudinal metabolome data reveal microbial-host interactions on sulfur metabolism in Parkinson’s disease. Cell Rep. 2019, 29, 1767–1777.e8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dejanovic, B.; Hetsch, F.; Semtner, M.; Fusca, D.; Arjune, S.; Santamaria-Araujo, J.A.; Winkelmann, A.; Ayton, S.; Bush, A.I.; et al. S-sulfocysteine/NMDA receptor-dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J. Clin. Investig. 2017, 127, 4365–4378. [Google Scholar] [CrossRef]
- Irino, Y.; Toh, R.; Nagao, M.; Mori, T.; Honjo, T.; Shinohara, M.; Tsuda, S.; Nakajima, H.; Satomi-Kobayashi, S.; Shinke, T.; et al. 2-Aminobutyric acid modulates glutathione homeostasis in the myocardium. Sci. Rep. 2016, 6, 36749. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Schoenknecht, P.; Kassner, S.; Hildebrandt, W.; Kinscherf, R.; Schroeder, J. Cerebrospinal fluid concentrations of functionally important amino acids and metabolic compounds in patients with mild cognitive impairment and Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 251–259. [Google Scholar] [CrossRef]
- Ullah, R.; Jo, M.H.; Riaz, M.; Alam, S.I.; Saeed, K.; Ali, W.; Rehman, I.U.; Ikram, M.; Kim, M.O. Glycine, the smallest amino acid, confers neuroprotection against D-galactose-induced neurodegeneration and memory impairment by regulating c-Jun N-terminal kinase in the mouse brain. J. Neuroinflamm. 2020, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, Y.; Castagna, C.; Mrejeru, A.; Lizardi-Ortiz, J.E.; Klein, Z.; Lindsley, C.W.; Sulzer, D. Glycine transporter-1 inhibition promotes striatal axon sprouting via NMDA receptors in dopamine neurons. J. Neurosci. 2013, 33, 16778–16789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surguchev, A.A.; Emamzadeh, F.N.; Surguchov, A. Cell Responses to Extracellular alpha-Synuclein. Molecules 2019, 24, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.W.; Yang, S.Y.; Yang, C.C.; Chang, C.W.; Wu, Y.R. Plasma and serum alpha-synuclein as a biomarker of diagnosis in patients with Parkinson’s disease. Front. Neurol. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.H.; Liu, K.C.; Lai, C.S.; Yang, S.Y.; Chen, C.M. Assessing plasma levels of alpha-synuclein and neurofilament light chain by different blood preparation methods. Front. Aging Neurosci. 2021, 13, 759182. [Google Scholar] [CrossRef]
- Lee, P.H.; Lee, G.; Park, H.J.; Bang, O.Y.; Joo, I.S.; Huh, K. The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J. Neural. Transm. 2006, 113, 1435–1439. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma alpha-synuclein predicts cognitive decline in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef]
- Mata, I.F.; Shi, M.; Agarwal, P.; Chung, K.A.; Edwards, K.L.; Factor, S.A.; Galasko, D.R.; Ginghina, C.; Griffith, A.; Higgins, D.S.; et al. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch. Neurol. 2010, 67, 1350–1356. [Google Scholar] [CrossRef]
- Park, M.J.; Cheon, S.M.; Bae, H.R.; Kim, S.H.; Kim, J.W. Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson’s disease. J. Clin. Neurol. 2011, 7, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Foulds, P.G.; Diggle, P.; Mitchell, J.D.; Parker, A.; Hasegawa, M.; Masuda-Suzukake, M.; Mann, D.M.; Allsop, D. A longitudinal study on alpha-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci. Rep. 2013, 3, 2540. [Google Scholar] [CrossRef]
- Li, Q.X.; Mok, S.S.; Laughton, K.M.; McLean, C.A.; Cappai, R.; Masters, C.L.; Culvenor, J.G.; Horne, M.K. Plasma alpha-synuclein is decreased in subjects with Parkinson’s disease. Exp. Neurol. 2007, 204, 583–588. [Google Scholar] [CrossRef] [PubMed]
| HC | PD | |||
|---|---|---|---|---|
| (n = 60) | Early Stage (n = 71) | Advanced Stage (n = 21) | Total (n = 92) | |
| Age (years) | 67.35 ± 8.08 | 66.13 ± 10.61 | 72.71 ± 9.35 | 67.63 ± 10.65 |
| Male (%) | 30 (50.00) | 37 (52.11) | 11 (52.38) | 48 (52.17) |
| Triglyceride (mg/dL) | 103.35 ± 62.96 | 112.63 ± 64.34 | 90.90 ± 37.19 | 107.67 ± 59.77 |
| Cholesterol (mg/dL) | 184.05 ± 28.95 | 176.52 ± 37.06 | 170.67 ± 28.45 | 175.18 ± 35.22 |
| Pre-prandial glucose (mg/dL) | 99.12 ± 11.02 | 104.58 ± 20.19 | 97.71 ± 18.67 | 103.01 ± 19.95 |
| BMI | 24.41 ± 3.09 | 24.63 ± 3.53 | 23.63 ± 33.56 | 24.42 ± 3.56 |
| UPDRS | 28.49 ± 15.96 | 77.61 ± 36.35 d | 40.94 ± 29.17 | |
| UPDRS-part 3 | 17.50 ± 9.17 | 42.62 ± 15.13 d | 23.30 ± 15.15 | |
| Hoehn–Yahr stage | 1.61 ± 0.48 | 3.02 ± 0.54 d | 1.93 ± 0.77 | |
| LEDD (mg) | 471.95 ± 436.44 | 1323.74 ± 668.44 d | 668.38 ± 611.51 | |
| Diabetes (%) | 3 (5.00) | 11 (15.28) b | 1 (5.00) c | 12 (13.04) a |
| CDR | 0.20 ± 0.25 | 0.34 ± 0.24 | 0.64 ± 0.39 b,c | 0.41 ± 0.30 a |
| MMSE | 29.61 ± 8.98 | 27.32 ± 3.88 | 21.81 ± 6.43 b,c | 26.07 ± 5.11 a |
| MoCA | 27.95 ± 2.38 | 24.35 ± 5.73 b | 18.00 ± 8.41 b,c | 22.90 ± 6.93 a |
| NPI | 0.53 ± 1.70 | 2.06 ± 2.96 b | 7.57 ± 7.30 b,c | 3.32 ± 4.88 a |
| BDI-II | 1.67 ± 2.90 | 6.62 ± 5.08 b | 16.10 ± 7.11 b,c | 8.70 ± 6.81 a |
| HAM-D | 1.62 ± 2.73 | 5.30 ± 3.92 b | 11.40 ± 6.76 b,c | 6.64 ± 5.30 a |
| ADL | 99.92 ± 0.65 | 99.72 ± 1.44 | 69.05 ± 28.62 b,c | 92.72 ± 18.68 a |
| PDQ-39 | 5.82 ± 8.26 | 22.42 ± 16.25 b | 67.33 ± 33.67 b,c | 32.67 ± 28.49 a |
| α-Synuclein (fg/mL) | 112.74 ± 70.25 | 182.85 ± 167.83 b | 209.25 ± 177.46 b | 188.43 ± 169.10 a |
| Compound Name | HC (n = 60) | PD (n = 92) | p Value |
|---|---|---|---|
| SM 26:0 | 0.118 ± 0.026 | 0.095 ± 0.025 | <0.001 |
| Dihydro SM 24:0 | 0.152 ± 0.059 | 0.110 ± 0.048 | 0.0014 |
| PEp 38:6 | 0.433 ± 0.158 | 0.332 ± 0.123 | 0.0014 |
| 5-Hydroxytryptophan | 0.009 ± 0.002 | 0.015 ± 0.013 | 0.0015 |
| PC 40:8 | 1.890 ± 0.530 | 1.509 ± 0.489 | 0.0015 |
| SM 14:1 | 0.523 ± 0.150 | 0.418 ± 0.151 | 0.0016 |
| FFA 18:0 | 21.333 ± 5.952 | 17.443 ± 4.427 | 0.0017 |
| Glutamine | 550.337 ± 75.570 | 602.440 ± 73.175 | 0.0018 |
| PCe 38:6 and/or PCp 38:5 | 8.123 ± 2.597 | 6.406 ± 1.951 | 0.0019 |
| PCe 36:4 | 21.490 ± 5.720 | 17.710 ± 4.745 | 0.0019 |
| PEp 38:5 | 0.360 ± 0.136 | 0.275 ± 0.119 | 0.0028 |
| FFA 20:0 | 0.387 ± 0.103 | 0.321 ± 0.100 | 0.0028 |
| SM 16:1 | 15.502 ± 3.500 | 13.099 ± 3.900 | 0.0029 |
| PC 34:0 | 5.756 ± 1.252 | 4.972 ± 1.218 | 0.0029 |
| Ornithine | 90.217 ± 22.245 | 107.580 ± 34.162 | 0.0030 |
| PCe 36:5 | 2.448 ± 1.823 | 1.426 ± 1.122 | 0.0031 |
| SM 23:0 | 12.424 ± 3.087 | 10.539 ± 2.787 | 0.0032 |
| PC 28:1 | 2.412 ± 0.613 | 2.010 ± 0.658 | 0.0032 |
| PCe 38:1 | 4.592 ± 1.166 | 3.875 ± 1.031 | 0.0032 |
| PCe 36:1 | 1.911 ± 0.429 | 1.644 ± 0.438 | 0.0039 |
| Candidate Marker | Origin | Change | Reference |
|---|---|---|---|
| Ethymalonate, myoinositol, propylene glycol, pyruvate, sorbitol | Plasma | ↑ (PD versus HC) | [3] |
| Homovanillate, 3-methoxytyrosine, 3-methytyramine sulfate, | Serum | ↑ (PD versus HC) | [4] |
| N1,N8-diacetylspermidine, N1,N12-diacetylspermine, N1-acetylspermidine, N1-acetylspermine, N8-acetylspermidine | Serum | ↑ (PD versus HC) | [6] |
| Quinolinic acid | Plasma | ↑ (PD versus HC) | [8] |
| Alanine, methionine, 2-oxoisocaproic acid, pyroglutamate, malate, serine | Plasma | ↑ (PD versus HC) | [9] |
| 8-Hydroxy-2-deoxyguanosin, glutathione | Plasma | ↑ (PD versus HC) | [10] |
| L-arginyl-L-alanine, 1,3-dimethyluracil, Lyso-platelet activating factor C16, α-N-phenylacetyl-L-glutamine, PC 44:5, PC 44:6, sarcosine, | Plasma | ↑ (PD versus HC) | [26] |
| Aspartate, glutamate and glycine | Plasma | ↑ (PD versus HC) | [27] |
| Acetate, ascorbate, citrate, ethanolamine, galactitol, glucolate, gluconate, glutarate, glycerol, isocitrate, malate, methylamine, methylmalonate, suberate, succinate, threonate, trimethylamine | Plasma | ↓ (PD versus HC) | [3] |
| Spermine | Serum | ↓ (PD versus HC) | [6] |
| Hypoxanthine | Plasma | ↓ (PD versus HC) | [7] |
| Kynurenic acid, | Plasma | ↓ (PD versus HC) | [8] |
| Hexadecenoic acid, linoleic acid | Plasma | ↓ (PD versus HC) | [9] |
| Uric acid | Plasma | ↓ (PD versus HC) | [10] |
| Ethanolamine, L-glutamyl-L-isoleucine, N-lauroylglycine, PE 34:1, PC 35:6, SM d30:1, SM d32:1, SM d39:1 | Plasma | ↓ (PD versus HC) | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-H.; Cheng, M.-L.; Tang, H.-Y.; Huang, C.-Y.; Wu, H.-C.; Chen, C.-M. Alterations of Sphingolipid and Phospholipid Pathways and Ornithine Level in the Plasma as Biomarkers of Parkinson’s Disease. Cells 2022, 11, 395. https://doi.org/10.3390/cells11030395
Chang K-H, Cheng M-L, Tang H-Y, Huang C-Y, Wu H-C, Chen C-M. Alterations of Sphingolipid and Phospholipid Pathways and Ornithine Level in the Plasma as Biomarkers of Parkinson’s Disease. Cells. 2022; 11(3):395. https://doi.org/10.3390/cells11030395
Chicago/Turabian StyleChang, Kuo-Hsuan, Mei-Ling Cheng, Hsiang-Yu Tang, Cheng-Yu Huang, Hsiu-Chuan Wu, and Chiung-Mei Chen. 2022. "Alterations of Sphingolipid and Phospholipid Pathways and Ornithine Level in the Plasma as Biomarkers of Parkinson’s Disease" Cells 11, no. 3: 395. https://doi.org/10.3390/cells11030395
APA StyleChang, K.-H., Cheng, M.-L., Tang, H.-Y., Huang, C.-Y., Wu, H.-C., & Chen, C.-M. (2022). Alterations of Sphingolipid and Phospholipid Pathways and Ornithine Level in the Plasma as Biomarkers of Parkinson’s Disease. Cells, 11(3), 395. https://doi.org/10.3390/cells11030395







