Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery
Abstract
:1. Introduction
2. Methods
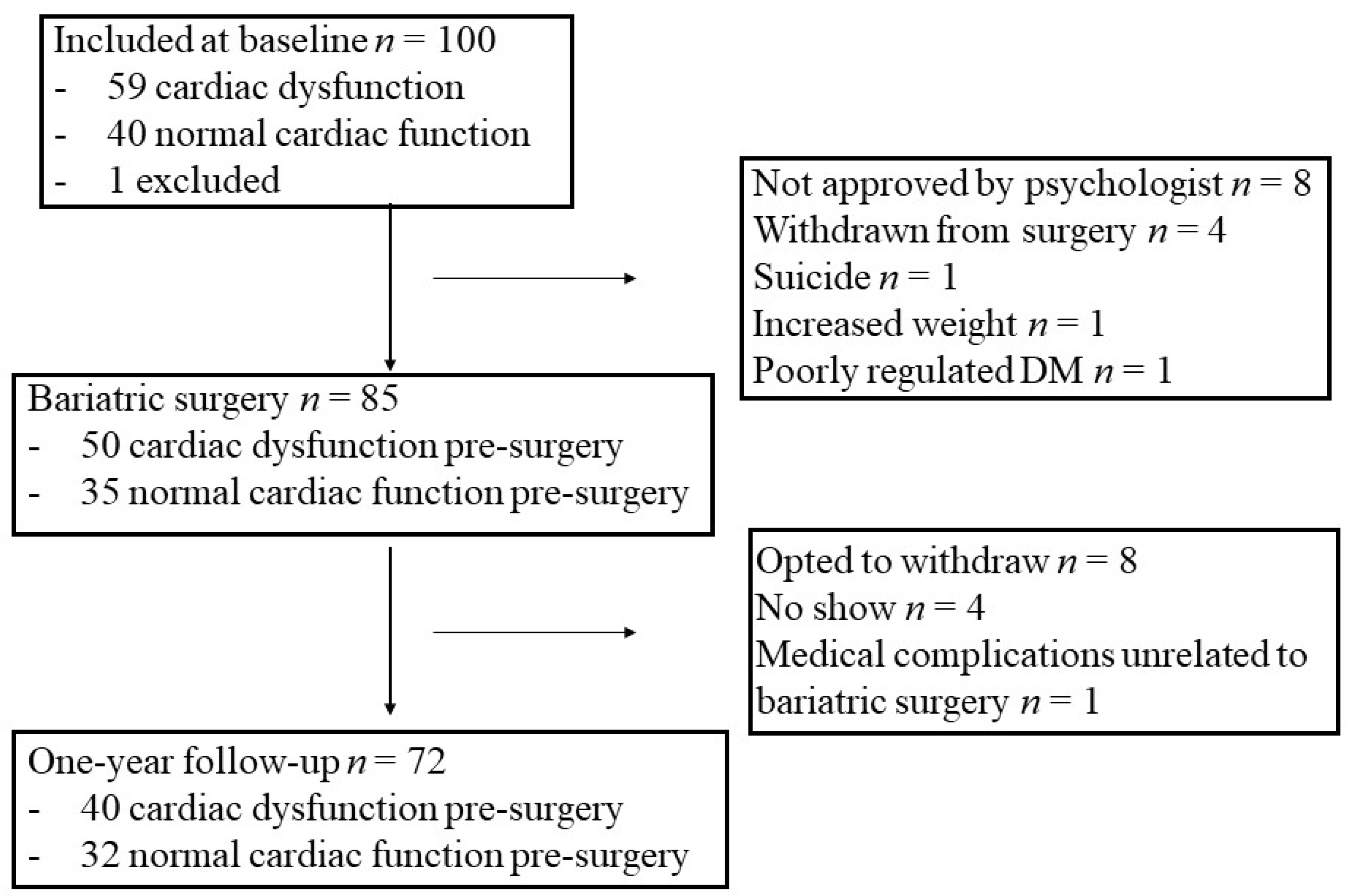
2.1. Study Design and Study Group
2.2. Laboratory Procedures
2.3. Transthoracic Echocardiography
2.4. Statistical Analysis
3. Results
3.1. Changes in Clinical Characteristics from before to One Year after Bariatric Surgery
3.2. Changes in Cardiovascular Biomarker Levels from before to One Year after Bariatric Surgery
3.3. Comparison of Baseline Values of Biomarkers in Patients with versus without Normalization of Cardiac Function after Bariatric Surgery
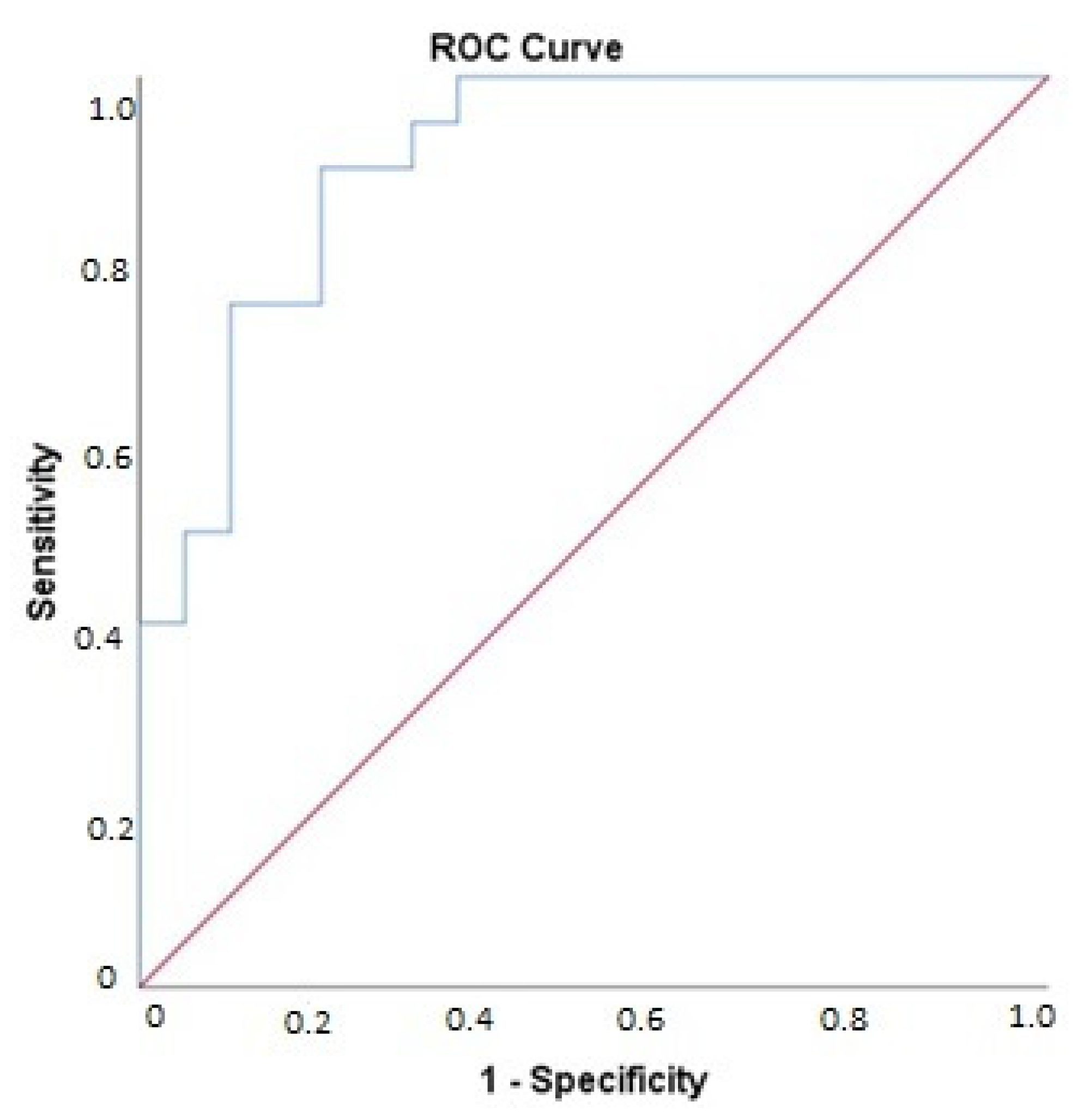
3.4. Association of Changes in Biomarker Levels with Presence of Cardiac Dysfunction Post-Bariatric Surgery
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, S.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Snelder, S.M.; de Groot-de Laat, L.E.; Biter, L.U.; Castro Cabezas, M.; Pouw, N.; Birnie, E.; Boxma-de Klerk, B.M.; Klaassen, R.A.; Zijlstra, F.; van Dalen, B.M. Subclinical cardiac dysfunction in obesity patients is linked to autonomic dysfunction: Findings from the CARDIOBESE study. ESC Heart Fail. 2020, 7, 3726–3737. [Google Scholar] [CrossRef]
- DeMaria, E.J. Bariatric surgery for morbid obesity. N. Engl. J. Med. 2007, 356, 2176–2183. [Google Scholar] [CrossRef] [Green Version]
- Sjostrom, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjostrom, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Vest, A.R.; Heneghan, H.M.; Agarwal, S.; Schauer, P.R.; Young, J.B. Bariatric surgery and cardiovascular outcomes: A systematic review. Heart 2012, 98, 1763–1777. [Google Scholar] [CrossRef]
- Snelder, S.M.; Aga, Y.; de Groot-de Laat, L.E.; Biter, L.U.; Castro Cabezas, M.; Pouw, N.; Boxma-de Klerk, B.M.; Klaassen, R.A.; Zijlstra, F.; van Dalen, B.M. Cardiac Function Normalizes 1 Year After Bariatric Surgery in Half of the Obesity Patients with Subclinical Cardiac Dysfunction. Obes. Surg. 2021, 31, 4206–4209. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Garcia-Banuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Santema, B.T.; Kloosterman, M.; Van Gelder, I.C.; Mordi, I.; Lang, C.C.; Lam, C.S.P.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; et al. Comparing biomarker profiles of patients with heart failure: Atrial fibrillation vs. sinus rhythm and reduced vs. preserved ejection fraction. Eur. Heart J. 2018, 39, 3867–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snelder, S.M.; de Groot-de Laat, L.E.; Biter, L.U.; Castro Cabezas, M.; van de Geijn, G.J.; Birnie, E.; Boxma-de Klerk, B.; Klaassen, R.A.; Zijlstra, F.; van Dalen, B.M. Cross-sectional and prospective follow-up study to detect early signs of cardiac dysfunction in obesity: Protocol of the CARDIOBESE study. BMJ Open 2018, 8, e025585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Bjorkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw 2010, 33, 1. [Google Scholar] [CrossRef] [Green Version]
- Heald, A.H.; Cruickshank, J.K.; Riste, L.K.; Cade, J.E.; Anderson, S.; Greenhalgh, A.; Sampayo, J.; Taylor, W.; Fraser, W.; White, A.; et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia 2001, 44, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Yau, S.W.; Harcourt, B.E.; Kao, K.T.; Alexander, E.J.; Russo, V.C.; Werther, G.A.; Sabin, M.A. Serum IGFBP-2 levels are associated with reduced insulin sensitivity in obese children. Clin. Obes. 2018, 8, 184–190. [Google Scholar] [CrossRef]
- Fagerholm, S.C.; Guenther, C.; Llort Asens, M.; Savinko, T.; Uotila, L.M. Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease. Front. Immunol. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschop, M.H.; et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig. 2007, 117, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Zeyda, M.; Gollinger, K.; Pfau, B.; Neuhofer, A.; Weichhart, T.; Saemann, M.D.; Geyeregger, R.; Schlederer, M.; Kenner, L.; et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010, 59, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Riedl, M.; Vila, G.; Maier, C.; Handisurya, A.; Shakeri-Manesch, S.; Prager, G.; Wagner, O.; Kautzky-Willer, A.; Ludvik, B.; Clodi, M.; et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J. Clin. Endocrinol. Metab. 2008, 93, 2307–2312. [Google Scholar] [CrossRef]
- Schaller, G.; Aso, Y.; Schernthaner, G.H.; Kopp, H.P.; Inukai, T.; Kriwanek, S.; Schernthaner, G. Increase of osteopontin plasma concentrations after bariatric surgery independent from inflammation and insulin resistance. Obes. Surg. 2009, 19, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uber, P.A.; Park, M.H.; Scott, R.L.; Ventura, H.O.; Harris, B.C.; Frohlich, E.D. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [Green Version]
- Tokat, B.; Kurt, O.; Bugra, Z.; Ozturk, O.; Yilmaz-Aydogan, H. Investigation of the monocyte diapedesis-related LFA-1 and JAM-A gene variants in Turkish coronary heart disease patients. Meta Gene 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Caligiuri, G. Mechanotransduction, immunoregulation, and metabolic functions of CD31 in cardiovascular pathophysiology. Cardiovasc. Res. 2019, 115, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.H.; Lee, Y.; Lee, B.B.; Kwon, B.; Song, H.; Kwon, B.S.; Park, J.E. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arter. Thromb. Vasc. Biol 2001, 21, 2004–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, J.W.; McVicar, D.W. TREM and TREM-like receptors in inflammation and disease. Curr. Opin. Immunol 2009, 21, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Er, L.K.; Wu, S.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Pleiotropic Associations of RARRES2 Gene Variants and Circulating Chemerin Levels: Potential Roles of Chemerin Involved in the Metabolic and Inflammation-Related Diseases. Mediat. Inflamm. 2018, 2018, 4670521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, M.J.; Tardif, J.C. Inflammation and beyond: New directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 2017, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, B.; Langer, H.; Geisler, T.; Lindemann, S.; Gawaz, M. Platelet glycoprotein VI: A novel marker for acute coronary syndrome. Semin. Thromb. Hemost. 2007, 33, 179–184. [Google Scholar] [CrossRef]
- Ricci, C.; Ferri, N. Naturally occurring PDGF receptor inhibitors with potential anti-atherosclerotic properties. Vascul. Pharmacol. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- Kim, M.; Lorinsky, M.K.; Gold, C.A.; Lahey, S.J.; Fusco, D.S.; Rosinski, D.J.; Pawlak, D.; Liang, B.T. Usefulness of Circulating Caspase-3 p17 and Caspase-1 p20 Peptides and Cardiac Troponin 1 during Cardioplegia to Gauge Myocardial Preservation. Am. J. Cardiol. 2019, 123, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Crnovcic, I.; Gan, F.; Yang, D.; Dong, L.B.; Schultz, P.G.; Shen, B. Activities of recombinant human bleomycin hydrolase on bleomycins and engineered analogues revealing new opportunities to overcome bleomycin-induced pulmonary toxicity. Bioorg Med. Chem. Lett. 2018, 28, 2670–2674. [Google Scholar] [CrossRef]
- Riise, R.; Odqvist, L.; Mattsson, J.; Monkley, S.; Abdillahi, S.M.; Tyrchan, C.; Muthas, D.; Yrlid, L.F. Bleomycin hydrolase regulates the release of chemokines important for inflammation and wound healing by keratinocytes. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Edginton, S.; Hitchon, C.; Froese, W.; El-Gabalawy, H. Effects of Rituximab and Infliximab Treatment on Carboxypeptidase B and Its Substrates in RA Synovium. J. Rheumatol. 2016, 43, 846–854. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Kobori, H.; Ritprajak, P.; Kamimura, Y.; Kozono, H.; Azuma, M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc. Natl. Acad. Sci. USA 2008, 105, 10495–10500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawecki, C.; Lenting, P.J.; Denis, C.V. von Willebrand factor and inflammation. J. Thromb. Haemost. 2017, 15, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Do most patients with obesity or type 2 diabetes, and atrial fibrillation, also have undiagnosed heart failure? A critical conceptual framework for understanding mechanisms and improving diagnosis and treatment. Eur. J. Heart Fail. 2020, 22, 214–227. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Lovren, F.; Teoh, H.; Verma, S. Obesity and atherosclerosis: Mechanistic insights. Can. J. Cardiol. 2015, 31, 177–183. [Google Scholar] [CrossRef]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Bohm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Campello, E.; Zabeo, E.; Radu, C.M.; Spiezia, L.; Gavasso, S.; Fadin, M.; Woodhams, B.; Vettor, R.; Simioni, P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb. Haemost. 2015, 113, 85–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Shah, P.; Tantry, U.S.; Gurbel, P.A. Coagulation Abnormalities in Heart Failure: Pathophysiology and Therapeutic Implications. Curr. Heart Fail. Rep. 2016, 13, 319–328. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]

| Pre-Surgery (n = 72) | 1 yr Post-Surgery (n = 72) | p-Value | |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 48 (43–54) | ||
| Female (%) | 54 (75%) | ||
| Physical examination | |||
| Weight (kg) | 122 (113–133) | 83 (74–91) | <0.001 |
| BMI (kg/m2) | 41 (39–46) | 28 (25–31) | <0.001 |
| Systolic BP (mmHg) | 146 ± 21 | 133 ± 20 | 0.003 |
| Diastolic BP (mmHg) | 79 (73–88) | 80 (75–86) | 0.18 |
| Heart rate (bpm) | 80 (73–86) | 65 (57–71) | <0.001 |
| Comorbidity | |||
| Diabetes Mellitus (%) | 16 (22%) | 6 (8%) | 0.002 |
| Hypertension (%) | 24 (33%) | 12 (17%) | 0.035 |
| Hypercholesterolemia (%) | 15 (21%) | 8 (11%) | 0.09 |
| Current smoking (%) | 11 (15%) | 3 (6%) | 0.18 |
| COPD (%) | 4 (6%) | 0 | 0.13 |
| OSAS (%) | 8 (11%) | 0 | 0.008 |
| Medication | |||
| Beta blockers (%) | 5 (7%) | 3 (4%) | 0.63 |
| ACE inhibitors/ARBs (%) | 11 (15%) | 8 (11%) | 0.012 |
| Calcium channel blockers (%) | 6 (8%) | 5 (7%) | 0.66 |
| Statins (%) | 16 (22%) | 9 (13%) | 0.039 |
| Diuretics (%) | 13 (18%) | 8 (11%) | 0.18 |
| Insulin (%) | 5 (7%) | 4 (6%) | 0.56 |
| Oral antidiabetics (%) | 10 (14%) | 4 (6%) | 0.031 |
| Abbreviation | Post-Surgery Normal Cardiac Function (n = 20) | Post-Surgery Cardiac Dysfunction (n = 20) | p-Value |
|---|---|---|---|
| AP-N | 37.6 (34.2–41.5) | 36.5 (32.6–40.1) | 0.81 |
| AZU | 7.8 (6.4–10.4) | 6.4 (5.3–7.8) | 0.61 |
| BLM hydrolase | 9.9 (8.2–10.8) | 7.4 (6.3–8.6) | 0.004 * |
| CCL15 | 126 (108–178) | 126 (110–183) | 0.37 |
| CCL16 | 155 (114–163) | 150 (104–172) | 0.97 |
| CCL24 | 41 (28–68) | 43 (23–60) | 0.90 |
| CXCL16 | 51 (40–59) | 51 (48–61) | 0.15 |
| CDH5 | 26 (23–28) | 26 (19–32) | 0.95 |
| CPA1 | 67 (52–99) | 70 (49–98) | 0.30 |
| CPB1 | 61 (46–85) | 67 (44–95) | 0.31 |
| CASP-3 | 750 (564–903) | 295 (155–551) | <0.001 * |
| CTSD | 8.3 (6.4–11.6) | 7.9 (5.9–10.5) | 0.67 |
| CTSZ | 59.5 (54.5–68.5) | 60.1 (43.2–70.6) | 0.67 |
| ALCAM | 232 (209–284) | 222 (197–244) | 0.91 |
| CHI3L1 | 21.1 (17.6–30.0) | 18.9 (13.5–27.2) | 0.96 |
| CHIT1 | 26.2 (20.0–38.1) | 36.2 (14.9–48.5) | 0.84 |
| COL1A1 | 8.2 (7.3–9.7) | 9.1 (8.0–10.8) | 0.42 |
| CD93 | 2200 (2002–2592) | 2572 (2058–2955) | 0.22 |
| CNTN1 | 29.1 (24.3–33.5) | 27.0 (24.7–32.0) | 0.95 |
| CSTB | 26.8 (19.9–33.3) | 21.5 (17.5–26.5) | 0.012 |
| SELE | 7543 (5486–9772) | 7098 (5587–10,785) | 0.38 |
| PI3 | 5.7 (5.0–7.8) | 5.8 (5.0–7.5) | 0.41 |
| EPHB4 | 49.6 (44.8–58.5) | 51.4 (46.3–60.4) | 0.57 |
| EGFR | 11.9 (11.1–13.3) | 11.1 (10.1–12.7) | 0.22 |
| Ep-CAM | 49.6 (33.5–75.1) | 51.8 (26.3–126.9) | 0.91 |
| FABP4 | 109.2 (88.9–169.3) | 104.2 (85.1–142.8) | 0.59 |
| Gal-3 | 11.5 (10.6–13.1) | 11.0 (10.4–12.4) | 0.96 |
| Gal-4 | 19.7 (13.7–23.8) | 18.0 (14.8–21.5) | 0.72 |
| GRN | 60.1 (46.6–75.5) | 59.8 (53.6–70.0) | 0.42 |
| GDF-15 | 72.0 (46.6–96.8) | 54.4 (48.1–59.4) | 0.85 |
| IGFBP-1 | 10.6 (6.4–18.8) | 9.5 (6.2–13.3) | 0.63 |
| IGFBP-2 | 159 (127–200) | 170 (133–226) | 0.22 |
| IGFBP-7 | 296 (243–323) | 275 (247–322) | 0.22 |
| ITGB2 | 58.4 (49.1–66.6) | 54.6 (46.3–65.3) | 0.37 |
| ICAM-2 | 57.3 (48.3–66.9) | 53.1 (44.0–69.8) | 0.96 |
| IL-1RT1 | 91.3 (78.1–104.4) | 81.9 (74.8–101.4) | 0.64 |
| IL-1RT2 | 57.2 (45.5–62.8) | 50.9 (40.9–54.3) | 0.91 |
| IL-17RA | 24.2 (19.3–33.6) | 20.4 (15.8–26.1) | 0.06 |
| IL-18BP | 72.2 (65.5–80.9) | 68.0 (64.6–86.3) | 0.65 |
| IL2-RA | 15.3 (14.1–17.8) | 12.4 (10.2–17.8) | 0.033 |
| IL-6RA | 5523 (4150–6345) | 4812 (3881–6379) | 0.96 |
| JAM-A | 160 (116–205) | 64 (29–103) | <0.001 * |
| KLK6 | 5.8 (5.1–7.3) | 5.4 (4.6–6.0) | 0.26 |
| LDL receptor | 27.4 (19.9–40.3) | 25.2 (21.6–31.2) | 0.82 |
| LTBR | 17.9 (15.5–19.4) | 17.3 (14.5–19.0) | 0.31 |
| MEPE | 74.8 (64.0–89.3) | 65.7 (62.4–80.6) | 0.58 |
| MMP-2 | 16.6 (14.8–19.6) | 18.8 (15.4–20.2) | 0.014 |
| MMP-3 | 183 (137–241) | 210 (168–266) | 0.018 |
| MMP-9 | 68.7 (50.8–123.1) | 60.5 (36.9–86.8) | 0.06 |
| TIMP4 | 12.3 (11.4–14.7) | 14.2 (11.8–15.5) | 0.62 |
| MCP-1 | 20.2 (17.8–24.6) | 17.5 (13.9–19.9) | 0.33 |
| PRTN3 | 19.6 (15.7–25.4) | 17.4 (14.2–24.9) | 0.92 |
| MPO | 12.0 (9.8–14.9) | 12.0 (9.3–15.0) | 0.57 |
| MB | 205 (170–267) | 226 (193–286) | 0.014 |
| NT-proBNP | 11.6 (7.2–15.2) | 7.7 (4.4–16.6) | 0.41 |
| Notch 3 | 50.2 (41.6–54.4) | 53.3 (42.0–63.4) | 0.018 |
| OPN | 224 (196–271) | 218 (165–275) | 0.71 |
| OPG | 18.0 (14.4–22.6) | 16.7 (14.0–19.7) | 0.66 |
| SELP | 3845 (2941–4907) | 2152 (1093–2368) | <0.001 * |
| PON3 | 70.7 (61.2–105.7) | 91.4 (75.1–106.8) | 0.10 |
| PGLYRP1 | 205 (175–255) | 177 (159–211) | 0.23 |
| PLC | 308 (284–336) | 315 (290–348) | 0.40 |
| PAI | 95 (74–202) | 73 (52–97) | 0.05 |
| PECAM-1 | 89 (77–116) | 56 (31–63) | <0.001 * |
| GP6 | 23 (18–27) | 12 (7–16) | <0.001 * |
| PDGF subunit A | 36 (26–48) | 19 (13–28) | <0.001 * |
| PCSK9 | 9.1 (7.5–11.8) | 9.5 (7.7–10.4) | 0.57 |
| DLK-1 | 79 (60–105) | 94 (70–105) | 0.65 |
| PSP-D | 6.4 (5.4–9.9) | 8.6 (5.6–10.8) | 0.52 |
| RETN | 87 (76–108) | 81 (71–98) | 0.73 |
| RARRES2 | 4849 (4614–5629) | 4256 (4044–4862) | 0.003 * |
| CD163 | 381 (305–455) | 367 (251–407) | 0.45 |
| SCGB3A2 | 5.1 (4.3–6.5) | 3.8(3.0–5.2) | 0.08 |
| SPON1 | 4.9 (4.4–5.3) | 4.4 (4.0–5.9) | 0.73 |
| ST2 | 33.3 (22.3–40.1) | 23.3 (15.3–27.8) | 0.047 |
| TR-AP | 15.8 (14.4–22.0) | 15.8 (13.5–18.3) | 0.93 |
| TFPI | 792 (688–850) | 742 (646–877) | 0.50 |
| t-PA | 134 (97–167) | 144 (96–170) | 0.58 |
| TR | 65 (39–87) | 62 (42–75) | 0.70 |
| TFF3 | 39 (33–46) | 38 (28–45) | 0.73 |
| TLT-2 | 73 (64–88) | 51 (42–62) | <0.001 * |
| TNFSF13B | 170 (152–184) | 174 (143–205) | 0.54 |
| TNF-R1 | 139 (128–155) | 141 (128–174) | 0.37 |
| TNF-R2 | 68 (62–78) | 65 (61–79) | 0.67 |
| TNFRSF10C | 192 (132–253) | 161 (98–199) | 0.45 |
| TNFRSF14 | 40 (37–46) | 33 (29–36) | 0.004 * |
| FAS | 79 (73–94) | 83 (75–89) | 0.17 |
| AXL | 650 (547–763) | 623 (544–809) | 0.29 |
| SHPS-1 | 16.3 (13.6–20.4) | 13.6 (11.5–17.5) | 0.62 |
| U-PAR | 54.4 (45.6–63.7) | 48.8 (39.5–60.7) | 0.53 |
| uPA | 31.0 (25.9–38.3) | 30.7 (25.0–34.3) | 0.36 |
| vWF | 211 (151–313) | 176 (108–239) | 0.85 |
| Biomarker | Odds Ratio |
|---|---|
| CPB1 | 0.94 |
| CASP3 | 1.06 |
| SELP | 1.01 |
| GP6 | 1.12 |
| PDGFsubunitA | 1.03 |
| TLT-2 | 1.22 |
| vWF | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snelder, S.M.; Pouw, N.; Aga, Y.; Castro Cabezas, M.; Biter, L.U.; Zijlstra, F.; Kardys, I.; van Dalen, B.M. Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery. Cells 2022, 11, 422. https://doi.org/10.3390/cells11030422
Snelder SM, Pouw N, Aga Y, Castro Cabezas M, Biter LU, Zijlstra F, Kardys I, van Dalen BM. Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery. Cells. 2022; 11(3):422. https://doi.org/10.3390/cells11030422
Chicago/Turabian StyleSnelder, Sanne M., Nadine Pouw, Yaar Aga, Manuel Castro Cabezas, L. Ulas Biter, Felix Zijlstra, Isabella Kardys, and Bas M. van Dalen. 2022. "Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery" Cells 11, no. 3: 422. https://doi.org/10.3390/cells11030422
APA StyleSnelder, S. M., Pouw, N., Aga, Y., Castro Cabezas, M., Biter, L. U., Zijlstra, F., Kardys, I., & van Dalen, B. M. (2022). Cardiovascular Biomarker Profiles in Obesity and Relation to Normalization of Subclinical Cardiac Dysfunction after Bariatric Surgery. Cells, 11(3), 422. https://doi.org/10.3390/cells11030422





