Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications
Abstract
:1. Introduction
2. Materials and Methods
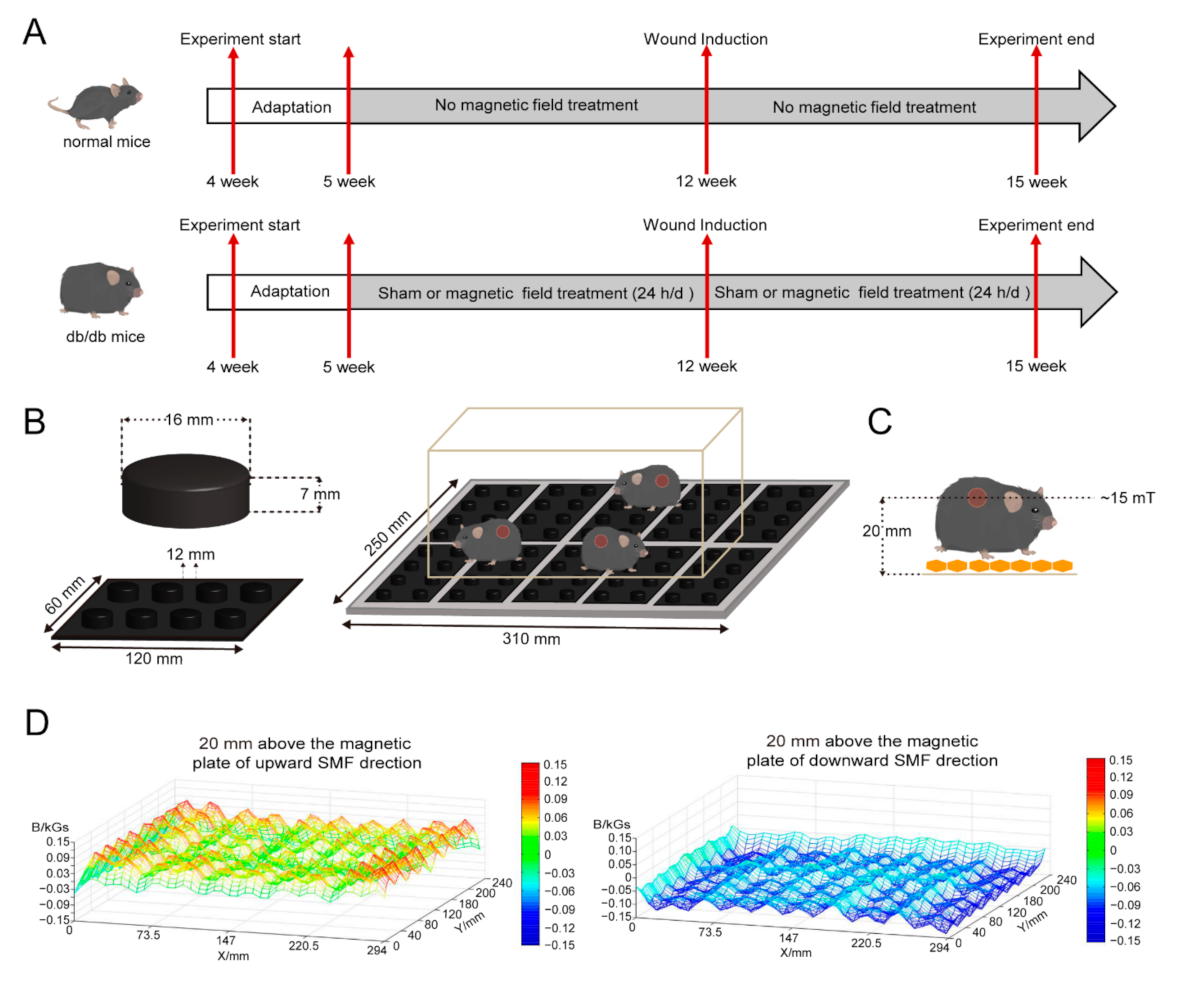
2.1. Static Magnetic Field Exposure
2.2. Animals
2.3. Wound Healing Experiments
2.4. Tissue Examinations
2.5. Serum Biochemistry
2.6. Cell Culture and CCK-8 Cytotoxicity Assays
2.7. Intracellular ROS Detection
2.8. Immunofluorescence Staining
2.9. Cell Scratch and Migration Assay
2.10. Nuclear and Cytoplasmic Protein Extraction
2.11. Western Blot Analysis
2.12. Cell-Titer Glo Test
2.13. Calcein Acetoxymethyl Ester and Propidium Iodide (Calcein-AM/PI) Staining
2.14. EdU Flow Cytometry Test
2.15. Statistical Analysis
3. Results
3.1. SMFs Alleviate Multiple Diabetic Complications
3.2. SMFs Decrease NRF2 and Increase Ki-67 to Accelerate Wound Healing in Diabetic Mice
3.3. SMFs Reduce High Glucose-Induced Cellular Oxidative Stress and Improve Cell Vitality, Proliferation, and Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowling, F.L.; Rashid, S.T.; Boulton, A.J. Preventing and treating foot complications associated with diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, B.R.; Pill, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G. Infectious Diseases Society of America clinical practice guidelines for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 29, 45–80. [Google Scholar] [CrossRef] [Green Version]
- Lavery, L.A.; Hunt, N.A.; Ndip, A.; Lavery, D.C.; Van Houtum, W.; Boulton, A.J.M. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010, 33, 2365–2369. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.J.; Song, J.B.; Nie, L.M.; Chen, X.Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxic. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Hardman, M.J.; Malone, M.; Embil, J.M.; Attinger, C.E.; Lipsky, B.A.; Aragón-Sánchez, J.; Li, H.K.; Schultz, G.; et al. Diagnosis and Management of diabetic Foot Infections; American Diabetes Association: Arlington, VA, USA, 2020. [Google Scholar]
- Lan, C.C.E.; Wu, C.S.; Huang, S.M.; Wu, I.H.; Chen, G.S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: New insights into impaired diabetic wound healing. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Thusu, K.; Cook, S.; Snyder, B.; Makowski, J.; Armstrong, D.; Nicotera, T. Oxidative damage to DNA in diabetes mellitus. Lancet 1996, 347, 444–445. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kunkemoeller, B.; Kyriakides, T.R. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef]
- Takahashi, A.; Aoshiba, K.; Nagai, A. Apoptosis of wound fibroblasts induced by oxidative stress. Exp. Lung Res. 2002, 28, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Li, F.Y.; Shao, W.; Gao, J.Q.; Ling, D.S. Promoting angiogenesis in oxidative diabetic wound microenvironment using a nanozyme-reinforced self-protecting hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Niu, H.; Liu, Z.T.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J.J. Sustained oxygenation accelerates diabetic wound healing by simultaneously promoting epithelialization and angiogenesis, and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.J.; Zhou, H.; Wang, H.R.; Xu, Y.Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Wu, H.B.; Li, F.Y.; Wang, S.F.; Lu, J.X.; Li, J.Q.; Du, Y.; Sun, X.L.; Chen, X.Y.; Gao, J.Q.; Ling, D.S. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Ikeya, N.; Woodward, J.R. Cellular autofluorescence is magnetic field sensitive. Proc. Natl. Acad. Sci. USA 2021, 118, e2018043118. [Google Scholar] [CrossRef]
- Lewis, A.M.; Fay, T.P.; Manolopoulos, D.E.; Kerpal, C.; Richert, S.; Timmel, C.R. On the low magnetic field effect in radical pair reactions. J. Chem. Phys. 2018, 149, 034103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yarema, K.; Xu, A. Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Wang, H.Z.; Zhang, X. Magnetic fields and reactive oxygen species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.F.; Wang, D.M.; Zha, M.; Yang, X.X.; Ji, X.M.; Zhang, L.; Zhang, X. Magnetic field direction differentially impacts the growth of different cell types. Electromagn. Biol. Med. 2018, 37, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Guo, W.; Hu, X.P.; Liu, M.M.; Xu, X.; Hu, F.H.; Lan, Y.H.; Lv, C.K.; Fang, Y.W.; Liu, M.Y.; et al. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, W.L.; Chen, G.L.; Li, Y.X.; Zhuo, Y.J.; Wang, Y.H.; Fang, Z.C.; Yu, Y.; Ren, H.W. Static magnetic field accelerates diabetic wound healing by facilitating resolution of inflammation. J. Diabetes Res. 2019, 2019, 5641271. [Google Scholar] [CrossRef]
- Song, B.K.; Hong, H.; Jung, Y.J.; Lee, J.H.; Kim, B.S.; Lee, H.B. Combination therapy comprising a static magnetic field with contractility improves skin wounds. Tissue Eng. Part A 2018, 24, 1354–1363. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.G.; Deng, K.Q.; Yun, P.; Gong, T. Therapeutic effects of static magnetic field on wound healing in diabetic rats. J. Diabetes Res. 2017, 2017, 6305370. [Google Scholar] [CrossRef]
- Choi, H.M.C.; Cheing, A.K.K.; Ng, G.Y.F.; Cheing, G.L.Y. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS ONE 2018, 13, e0191074. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.H.; Liu, J.Y.; Zhen, C.X.; Wang, Y.J.; Wei, Y.P.; Ren, W.H.; Shang, P. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif. 2021, 54, e12982. [Google Scholar] [CrossRef]
- Carter, C.S.; Huang, S.C.; Searby, C.C.; Cassaidy, B.; Miller, M.J.; Grzesik, W.J.; Piorczynski, T.B.; Pak, T.K.; Walsh, S.A.; Acevedo, M. Exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab. 2020, 32, 561–574. [Google Scholar] [CrossRef]
- Csillag, A.; Kumar, B.V.; Szabó, K.; Szilasi, M.; Papp, Z.; Szilasi, M.E.; Pázmándi, K.; Boldogh, I.; Rajnavölgyi, É.; Bácsi, A.; et al. Exposure to inhomogeneous static magnetic field beneficially affects allergic inflammation in a murine model. J. R. Soc. Interface 2014, 11, 20140097. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.J.; Cheng, J.; Zhang, L.; Song, C.; Tian, X.F.; Fan, Y.X.; Lv, Y.; Zhang, X. A Static Magnetic Field Improves Iron Metabolism and Prevents High-Fat-Diet/Streptozocin-Induced Diabetes. Innovation 2021, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.H.; Huang, B.B.; Hai, S.T.; Chi, L.S.; Yuan, M.D.; Wang, X.; Zhong, X.Z.; Wan, H.C.; Yu, T.Z.; Wei, T.M. High-Glucose Inhibits Human Fibroblast Cell Migration in Wound Healing via Repression of bFGF-Regulating JNK Phosphorylation. PLoS ONE 2014, 9, e108182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seok, S.E.; Qiong, H.; Zafer, G.; Sorenson, C.M.; Nader, S.; Ram, N. High Glucose Alters Retinal Astrocytes Phenotype through Increased Production of Inflammatory Cytokines and Oxidative Stress. PLoS ONE 2014, 9, e103148. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Kanao, T.; Sawada, T.; Kobayashi, Y.; Moriwaki, Y.; Ishida, Y.; Takeda, K.; Ichijo, H.; Lu, B.W.; Takahashi, R. The loss of PGAM5 suppresses the mitochondrial degeneration caused by inactivation of PINK1 in Drosophila. PLoS Genet. 2010, 6, e1001229. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Elferchichi, M.; Mercier, J.; Bourret, A.; Gross, R.; Lajoix, A.D.; Belguith, H.; Abdelmelek, H.; Sakly, M.; Lambert, K. Is static magnetic field exposure a new model of metabolic alteration? Comparison with Zucker rats. Int. J. Radiat. Biol. 2011, 87, 483–490. [Google Scholar] [CrossRef]
- Gorczynska, E.; Wegrzynowicz, R. Glucose homeostasis in rats exposed to magnetic fields. Investig. Radiol. 1991, 26, 1095–1099. [Google Scholar] [CrossRef]
- Abbasi, M.; Nakhjavani, M.; Hamidi, S.; Tarafdari, A.M.; Esteghamati, A. Constant magnetic field of 50 mT does not affect weight gain and blood glucose level in BALB/c mice. Med. Sci. Monit. 2007, 13, 151–154. [Google Scholar]
- Wrobel, M.P.; Szymborska-Kajanek, A.; Wystrychowski, G.; Biniszkiewicz, T.; Sieroń-Stołtny, K.; Sieroń, A.; Pierzchała, K.; Grzeszczak, W.; Strojek, K. Impact of low frequency pulsed magnetic fields on pain intensity, quality of life and sleep disturbances in patients with painful diabetic polyneuropathy. Diabetes Metab. 2008, 34, 349–354. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, L.; Zhu, X.Q.; Wang, J.; Han, L.C.; Cheng, P.; Jing, D.; Zhang, X.D.; Shan, Q.S. Moderate-intensity 4 mT static magnetic fields prevent bone architectural deterioration and strength reduction by stimulating bone formation in streptozotocin-treated diabetic rats. Bone 2018, 107, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.J.; Zheng, X.F.S. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.F.; Cederbaum, A.I. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology 2006, 43, 144–153. [Google Scholar] [CrossRef]
- Ayers, D.; Baron, B.; Hunter, T. miRNA Influences in NRF2 Pathway Interactions within Cancer Models. J. Nucleic Acids 2015, 2015, 143636. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Z.; Zhang, X. ROS Reduction Does Not Decrease the Anticancer Efficacy of X-Ray in Two Breast Cancer Cell Lines. Oxidative Med. Cell. Longev. 2019, 2019, 3782074. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, V.B.; Abdolmaleki, P.; Behmanesh, M. The Static Magnetic Field Remotely Boosts the Efficiency of Doxorubicin through Modulating ROS Behaviors. Sci. Rep. 2018, 8, 990. [Google Scholar] [CrossRef]
- Poniedzialek, B.; Rzymski, P.; Karczewski, J.; Jaroszyk, F.; Wiktorowicz, K. Reactive oxygen species (ROS) production in human peripheral blood neutrophils exposed in vitro to static magnetic field. Electromagn. Biol. Med. 2013, 32, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, Z.Y.; Polyakov, T.; Zablotskii, V.; Zhang, X. Effect of static magnetic field on DNA synthesis: The interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv. 2020, 2, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Song, C.; Zhang, L.; Wang, J.J.; Yu, X.; Yu, B.; Zablotskii, V.; Zhang, X. An upward 9.4 T static magnetic field inhibits DNA synthesis and increases ROS-P53 to suppress lung cancer growth. Transl. Oncol. 2021, 14, 101103. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Yu, B.; Song, C.; Wang, J.; Zhang, L.; Ji, X.; Wang, Y.; Fang, Y.; Liao, Z.; Wei, M.; et al. Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications. Cells 2022, 11, 443. https://doi.org/10.3390/cells11030443
Feng C, Yu B, Song C, Wang J, Zhang L, Ji X, Wang Y, Fang Y, Liao Z, Wei M, et al. Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications. Cells. 2022; 11(3):443. https://doi.org/10.3390/cells11030443
Chicago/Turabian StyleFeng, Chuanlin, Biao Yu, Chao Song, Junjun Wang, Lei Zhang, Xinmiao Ji, Ying Wang, Yanwen Fang, Zhongcai Liao, Min Wei, and et al. 2022. "Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications" Cells 11, no. 3: 443. https://doi.org/10.3390/cells11030443
APA StyleFeng, C., Yu, B., Song, C., Wang, J., Zhang, L., Ji, X., Wang, Y., Fang, Y., Liao, Z., Wei, M., & Zhang, X. (2022). Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications. Cells, 11(3), 443. https://doi.org/10.3390/cells11030443





