miRNAs as Biomarkers and Possible Therapeutic Strategies in Rheumatoid Arthritis
Abstract
:1. Introduction
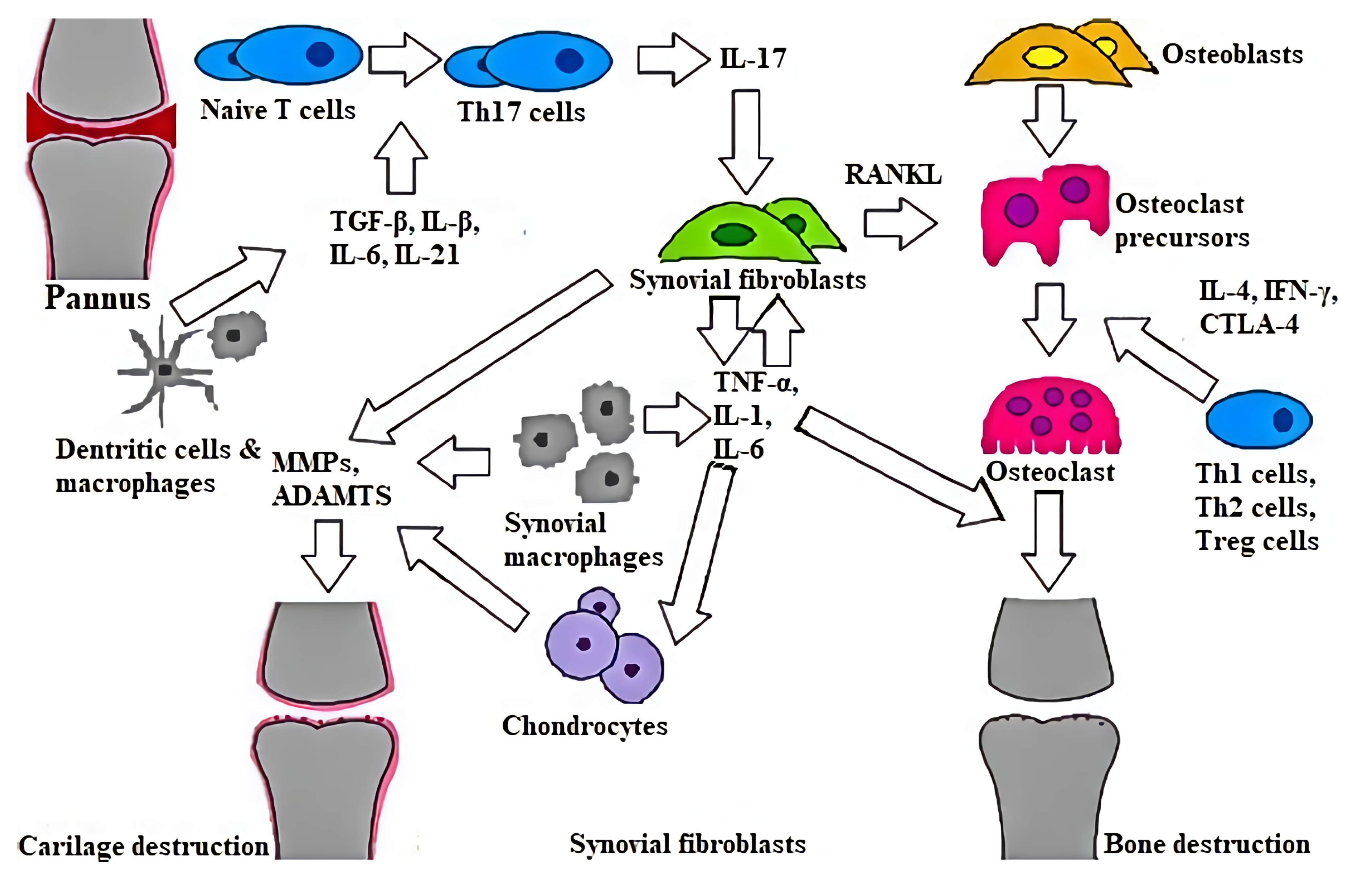
2. Characteristics and Pathology of RA
3. microRNAs and Their Role in Rheumatoid Arthritis Pathogenesis
3.1. miRNAs Affecting Rheumatoid Arthritis Synovial Fibroblasts
3.2. miRNA Affecting Signaling Pathways
3.3. Role of miRNAs in Inflammatory Responses
3.4. Role of miRNAs in Synovial Hyperplasia Responses
3.5. Role of miRNAs in Bone Destruction
4. miRNA—Diagnostic and/or Prognostic Biomarkers for RA?
5. miRNA-Based Therapy—Present or Future?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACPA | Anti-citrullinated protein antibody |
| ADA | Adalimumab |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| Bcl-2 | B cell lymphoma 2 |
| bDMARDs | Biological disease-modifying anti-rheumatic drugs |
| Btk | Bruton’s tyrosine kinase |
| CRP | C-reactive protein |
| csDMARDs | Conventional synthetic disease-modifying anti-rheumatic drugs |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CXCL 12 | C-X-C motif chemokine ligand 12 |
| DAS | Disease activity scale |
| DNMT1 | DNA (cytosine-5)-methyltransferase-1 |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| ETA | Etanercept |
| Foxp3 | Forkhead transcription factor |
| GM-CSF | Granulated macrophage colony-stimulating factor |
| GOL | Golimumab |
| hATTR | Hereditary transthyretin amyloidosis |
| HCV | Hepatitis C virus |
| HDAC | Histone deacetylase |
| HIF1α | Hypoxia-inducible factor 1α |
| IFX | Infliximab |
| ILs | Interleukins |
| JAK | Janus kinase |
| LNA | Locked nucleic acid |
| M-CSF | Macrophage colony-stimulating factor |
| miRNA | MicroRNA |
| MMPs | Matrix metalloproteinases |
| MSC | Mesenchymal stem cells |
| MTX | Methotrexate |
| NF-κB | Nuclear factor kappa-beta |
| OA | Osteoarthritis |
| PBMC | Peripheral blood mononuclear cell |
| PI3K | Phosphoinositide 3-kinase |
| PIK3R2 | Phosphatidylinositol 3-kinase regulatory subunit 2 |
| PsA | Psoriatic arthritis |
| qRT-PCR | Quantitative reverse transcription–polymerase chain reaction |
| RA | Rheumatoid arthritis |
| RANKL | Receptor activator of nuclear factor-kappa B ligand |
| RASF | Rheumatoid arthritis synovial fibroblast |
| RF | Rheumatoid factor |
| RORC2 | Retinoic acid receptor-related orphan receptor variant 2 |
| RTX | Rituximab |
| siRNA | Small interfering RNA |
| SLE | Systemic lupus erythematosus |
| SS | Sjogren’s syndrome |
| STAT3 | Signal transducers and activators of transcription |
| Syk | Spleen tyrosine kinase |
| TCZ | Tocilizumab |
| TGF-β | Transforming growth factor-β |
| Th cells | T helper cells, helper T cells |
| TIMPs | Tissue inhibitors of metalloproteinase |
| TNF | Tumor necrosis factor |
| TLR | Toll-like receptor 2 |
| Treg cells | T regulatory cells, regulatory T cells |
| tsDMARDS | Targeted synthetic disease-modifying anti-rheumatic drugs |
| Tyk2 | Tyrosine kinase 2 |
| UC | Ulcerative colitis |
| VEGF | Vascular endothelial growth factor |
| VEGFRI | VEGF receptor I |
| Wnt | Wingless/integrated |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Knedla, A.; Tennie, C.; Kampmann, A.; Wunrau, C.; Dinser, R.; Korb, A.; Schnaker, E.-M.; Tarner, I.H.; Robbins, P.D.; et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 2009, 15, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Viatte, S.; Plant, D.; Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 141–153. [Google Scholar] [CrossRef]
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar]
- Huang, R.Y.; Wu, J.Q.; Liu, Z.H.; Sun, S.L. MicroRNAs in rheumatoid arthritis: What is the latest with regards to diagnostics. Expert Rev. Mol. Diagn. 2019, 19, 363–366. [Google Scholar] [CrossRef]
- Chen, J.Q.; Papp, G.; Szodoray, P.; Zeher, M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun. Rev. 2016, 15, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Evangelatos, G.; Fragoulis, G.E.; Koulouri, V.; Lambrou, G. MicroRNAs in rheumatoid arthritis: From pathogenesis to clinical impact. Autoimmun. Rev. 2019, 18, 102391. [Google Scholar] [CrossRef]
- Jin, S.; Sun, S.; Ling, H.; Ma, J.; Zhang, X.; Xie, Z.; Zhan, N.; Zheng, W.; Li, M.; Qin, Y.; et al. Protectin DX restores Treg/Th 17 cell balance in rheumatoid arthritis by inhibiting NLRP3 inflammasome via miR-20a. Cell Death Dis. 2021, 12, 280. [Google Scholar] [CrossRef]
- Fraenke, L.; Bathon, J.M.; England, B.R.; Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology guideline for the threatment of rheumatoid arthritis. Arthritis Rheumatol. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Alten, R.; Mischkewitz, M. 2021 ACR guideline reflects changes in RA treatment. Nat. Rev. Rheumatol. 2021, 17, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Steeland, S.; Libert, C.; Vandenbroucke, R.E. A New Venue of TNF Targeting. Int. J. Mol. Sci. 2018, 19, 1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hizinga, T.W.; Fleischmann, R.M.; Jasson, M.; Radin, A.R.; van Adelsberg, J.; Fiore, S.; Huang, X.; Yancopoulos, G.D.; Stahl, N.; Genovese, M.C.; et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: Efficacy and safety results from the randomized SARIL-RA-MOBILITY Part A trial. Ann. Rheum. Dis. 2014, 73, 1626–1634. [Google Scholar] [CrossRef]
- Gabay, C.; Mishid, J.; Ziberstein, M.; Paccard, C.; Lin, Y.; Graam, N.M.H.; Boyapati, A. Identification of sarilumab pharmacodynamic and predictive markers in patients with inadequate response to TNF inhibition: A biomarker sub study of the phase 3 TARGET study. Rheum. Musculoskelet. Dis. 2018, 4, e000607. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behranin, I.; Muller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Fridman, J.S.; Sherie, P.A.; Collins, R.; Burn, T.C.; Li, Y.; Li, J.; Covington, M.B.; Thomas, B.; Collier, P.; Favata, M.F. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: Preclinical characterization of INCB028050. J. Immunol. 2010, 184, 5298–5307. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.K.; Bamert, R.S.; Patel, O.; Wang, C.; Walden, P.M.; Wilks, A.F.; Fantino, E.; Rossjohn, J.; Lucet, I.S. Dissecting specificity in the Janus Kinases: The structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J. Mol. Biol. 2009, 387, 219–232. [Google Scholar] [CrossRef]
- Keystone, E.C.; Taylor, P.C.; Drescher, E.; Schlichting, D.E.; Beattie, S.D.; Berclaz, P.Y.; Lee, C.H.; Fidelus-Gort, R.K.; Luchi, M.E.; Rooney, T.P.; et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann. Rheum. Dis. 2015, 74, 333–340. [Google Scholar] [CrossRef]
- Smolen, J.S.; Kremer, J.M.; Gaich, C.L.; DeLozier, A.M.; Schlichting, D.E.; Xie, L.; Stoykov, I.; Rooney, T.; Bird, P.; Sanchez Burson, J.M.; et al. Patient-reported outcomes from a randomized phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann. Rheum. Dis. 2017, 76, 694–700. [Google Scholar] [CrossRef]
- Dougados, M.; van der Heijde, D.; Chen, Y.C.; Greenwald, M.; Drescher, E.; Liu, J.; Beattie, S.; Witt, S.; de la Torre, I.; Gaich, C.; et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from RA-BUILD study. Ann. Rheum. Dis. 2017, 76, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lin, G.J.; Chen, J.W. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, J.S.; Landewe, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Vashaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef]
- Wielinska, J.; Bogunia-Kubik, K. miRNAs as potential biomarkers of treatment outcome in rheumatoid arthritis and ankylosing spondylitis. Pharmacogenomics 2021, 22, 291–301. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 2009, 5, 667–676. [Google Scholar] [CrossRef]
- Villarion, A.V.; Gadina, M.; O’Shea, J.J.; Kanno, Y. SnapShot: Jak-STAT Signaling II. Cell 2020, 181, 1696. [Google Scholar] [CrossRef]
- Yang, X.P.; Ghoreschi, K.; Steward-Tharp, S.M.; Rodrigues-Canales, J.; Zhu, J.; Grainger, J.R.; Hirahara, K.; Sun, H.-W.; Wei, L.; Vahedi, G.; et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011, 12, 247–254. [Google Scholar] [CrossRef] [Green Version]
- McInnes, I.B.; Shett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Blingham, C.O.; Birnbaum, N.S.; Runmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Tateiwa, D.; Yoshikawa, H.; Kaito, T. Cartilage and bone destruction in arthritis: Pathogenesis and treatment strategy: A literature review. Cells 2019, 8, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrijevic, M.; Arsenovic-Ranin, N.; Kosec, D.; Bufan, B.; Nacka-Aleksic, M.; Pilipovic, I. Sexual dimorphism in Th17/Treg axix in lymph nodes draining inflamed joints in rats with collagen-induced arthritis. Brain Behav. Immun. 2019, 76, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Manning, C.; Tsay, A.; Naito, A.; Pan, C.; Amento, E.; Goldring, S.R. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000, 43, 250–258. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 1, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotopoulos, V.C.; Tzinia, A.; Tzurbakis, M.; Kalfakakou, V.; Stefanou, S.L.; Georgoulis, A. Expression levels of matrix metalloproteinase {MMP}-9 and its specific inhibitor TIMP-1, in septic and aseptic arthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1159–1167. [Google Scholar] [CrossRef]
- Ahrens, D.; Koch, A.E.; Pope, R.M.; Stein-Picarella, M.; Niedbala, M.J. Expression of matrix metalloproteinase 9 {96-kd gelatinase B} in human rheumatoid arthritis. Arthritis Rheum. 1996, 39, 1576–1587. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [Green Version]
- Samson, M.; Audia, S.; Janikashvili, N.; Ciudad, M.; Trad, M.; Franszczak, J.; Ornetti, P.; Maillefert, J.-F.; Miossec, P.; Bonnette, B. Brief report: Inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheumatol. 2012, 64, 2499–2503. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Sun, X.; Zhang, J.; Zhu, H.; Li, C.; Gao, N.; Jia, Y.; Xu, D.; Huang, F.-P.; Li, N.; et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann. Rheum. Dis. 2015, 74, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodoma, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cells subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Li, N.; Zhao, X.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. Low-dose Interleukin-2: Biology and therapeutic prospects in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102645. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Manetti, M.; Caterbi, S.; Ibba-Manneschi, L.; Bistoni, O.; Bartoloni, E.; Valentini, V.; Terenzi, R.; Gerli, R. Altered immunoregulation in rheumatoid arthritis: The role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediat. Imflamm. 2015, 2015, 751793. [Google Scholar] [CrossRef] [PubMed]
- Podshivalova, K.; Salomon, D.R. MicroRNA regulation of T-lymphocyte immunity: Modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit. Rev. Immunol. 2013, 33, 435–476. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.F.; Boldin, M.P.; Chaudgry, A.; Lin, L.L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef] [Green Version]
- Jeker, L.T.; Bluestone, J.A. MicroRNA regulation of T-cell differentiation and function. Imuunol. Rev. 2013, 253, 65–81. [Google Scholar] [CrossRef] [Green Version]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical significance of MiRNAs in autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Sondag, G.R.; Haqqi, T.M. The role of MicroRNAs and their targets in osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Baxter, D.; McInnes, I.B.; Kurowska-Stolarska, M. Novel regulatory mechanism in inflammatory arthritis: A role of microRNA. Immunol. Cell Biol. 2012, 90, 288–292. [Google Scholar] [CrossRef]
- Chen, X.M.; Huang, Q.C.; Tang, S.L. Role of Micro RNAs in the pathogenesis of Rheumatoid Arthritis: Novel Perspectives Based on Review of the Literature. Medicine 2015, 94, e1326. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Nagata, Y.; Yamasaki, K.; Ochi, M. A mini-review: microRNA in arthritis. Physiol. Genom. 2011, 43, 566–570. [Google Scholar] [CrossRef]
- Nimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2010, 11, 209. [Google Scholar] [CrossRef] [Green Version]
- Lennert, A.; Fardo, D.W. Detecting novel micro RNAs in rheumatoid arthritis with gene-based association testing. Clin. Exp. Rheum. 2017, 35, 586–592. [Google Scholar]
- Anaparti, V.; Smolik, I.; Meng, X.; Spicer, V.; Mookherjee, N.; El-Gabalawy, H. Whole blood microRNA expression pattern differentiates patients with rheumatoid arthritis, their seropositive first-degree relatives, and healthy unrelated control subjects. Arthritis Res. Ther. 2017, 19, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, X.; Yang, M.; Ruan, W.; Wei, W.; Gu, D.; Wang, J.; Guo, X.; Guo, L.; Yuan, Y. miR-145-5p Increases Osteoclast Numbers In Vitro and Aggravates Bone Erosion in Collagen-Induced Arthritis by Targeting Osteoprotegrin. Med. Sci. Monit. 2018, 24, 5292–5300. [Google Scholar] [CrossRef]
- Rajasekhar, M.; Olsson, A.M.; Steel, K.J.; Georgouli, M.; Ranasinghe, U.; Read, C.B.; Frederiksen, K.S.; Taams, L.S. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. J. Autoimmun. 2017, 79, 53–62. [Google Scholar] [CrossRef] [Green Version]
- ElAtta, A.S.A.; Ali, Y.B.M.; Bassyouni, I.H.; Talaat, R.M. Upregulation of miR-221/222 expression in rheumatoid arthritis (RA) patients: Correlation with disease activity. Clin. Exp. Med. 2019, 19, 47–53. [Google Scholar] [CrossRef]
- Tang, X.; Yin, K.; Zhu, H.; Tian, J.; Shen, D.; Yt, L.; Rui, K.; Ma, J.; Xu, H.; Wang, S. Correlation Between the Expression of MicroRNA-301a-3p and the Proportion of Th17 cells in patients with rheumatoid arthritis. Inflammation 2016, 39, 759–767. [Google Scholar] [CrossRef]
- Dong, L.; Wang, X.; Tan, J.; Li, H.; Qian, W.; Chen, J.; Chen, Q.; Wang, J.; Xu, W.; Tao, C.; et al. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J. Cell. Mol. Med. 2014, 18, 2213–2224. [Google Scholar] [CrossRef]
- Hruskova, V.; Jandova, R.; Vernerova, L.; Mann, H.; Pecha, O.; Prajzlerova, K.; Pavelka, K.; Vencovsky, J.; Filkova, M.; Senlt, L. MicroRNA-125b: Association with disease activity and the treatment response of patients with early rheumatoid arthritis. Arhtirits Res. Ther. 2016, 18, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zheng, F.; Gao, G.; Yan, S.; Zhang, L.; Wang, L.; Cai, X.; Wang, X.; Xu, D.; Wang, J. MiR-548a-3p regulates inflammatory response via TLR4/NF-kappaB signaling pathway in rheumatoid arthritis. J. Cell. Biochem. 2019, 120, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, S.; Zhang, Z.; Lu, Y.; Yang, M.; Chen, P.; Chen, L.; Wang, M. MiRNA-6089 inhibits rheumatoid arthritis fibroblast-like synoviocytes proliferation and induces apoptosis by targeting CCR4. Arch. Physiol. Biochem. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zheid, A.; Saad, M.; Soliman, E. MicroRNA 146a expression I rheumatoid arthritis: Association with tumor necrosis factor-alpha and disease activity. Genet. Test. Mol. Biomark. 2011, 15, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhang, P.; Wang, X.; Tu, J.; Wei, W. The effects of MicroRNAs on key signalling pathways and epigenetic modification in Fibroblast-Luke synoviocytes of rheumatoid arthritis. Mediat. Inflamm. 2018, 10, 9013124. [Google Scholar]
- Zhang, B.; Wang, L.S.; Zhou, Y.H. Elevated microRNA-125b promotes inflammation in rheumatoid arthritis by activation of NF-kappaB pathway. Biomed. Pharm. 2017, 93, 1151–1157. [Google Scholar] [CrossRef]
- Hong, B.K.; You, S.; Yoo, S.A.; Park, D.; Whang, D.; Cho, S.D.; Kim, W.-U. MicroRNA-143 and -145 modulate the phenotype of synovial fibroblasts in rheumatoid arthritis. Exp. Mol. Med. 2017, 49, e363. [Google Scholar] [CrossRef] [Green Version]
- Migita, K.; Iwanaga, N.; Izumi, Y.; Kawahara, C.; Kumagai, K.; Nakamura, T.; Koga, T.; Kawakami, A. TNF-alpha-induced miR-155 regulates IL-6 signaling in rheumatoid synovial fibroblasts. BMC Res. Notes 2017, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fei, D.; Xing, J.; Du, J. MicroRNA-29a inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by repressing STAT3. Biomed. Pharm. 2017, 96, 173–181. [Google Scholar] [CrossRef]
- Iwamoto, N.; Fukui, S.; Takatani, A.; Shimizu, T.; Umeda, M.; Nishino, A.; Igawa, T.; Koga, T.; Kawashiri, S.; Ichinose, K. Osteogenic differentiation of fibroblast-like synovial cells in rheumatoid arthritis is induced by microRNA-218 through a ROBO/Slit pathway. Arthritis Res. Ther. 2018, 20, 189. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cao, X. MiR-19 suppresses fibroblast-like synoviocytes cytokine release by targeting toll like receptor 2 in rheumatoid arthritis. Am. J. Transl. Res. 2016, 8, 5512–5518. [Google Scholar] [PubMed]
- Fuziwara, C.S.; Kimura, E.T. Insights into regulation of the miR-17-92 cluster of miRNAs in cancer. Front. Med. 2015, 2, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtra, N.; Singh, A.K.; Ahmed, S. MicroRNA-17 suppresses TNF-α signaling by interfering with TRAF2 and cIAP2 association in rheumatoid arthritis synovial fibroblasts. J. Immunol. 2016, 197, 2219–2228. [Google Scholar] [CrossRef]
- Li, X.F.; Shen, W.W.; Sun, Y.Y.; Li, W.X.; Sun, Z.H.; Liu, Y.H.; Zhang, L.; Huang, C.; Meng, X.-M.; Li, J. MicroRNA-20a negatively regulates expression of NLRP3-in-flammasome by targeting TXNIP in adjuvant-induced arthritis fibroblast-like-synoviocytes. J. Bone Spine Rev. Rheum. 2016, 83, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Trenkmann, M.; Brock, M.; Gay, R.F.; Michel, B.A.; Gay, S.; Huber, I.C. Tumor necrosis factor α-induced microRNA-18a activates rheumatoid arthritis synovial fibroblast through a feedback loop in NF-κΒ signaling: miR-18a enhances NF—κΒ signaling in RASFs. Arthrits Rheum. 2013, 65, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Lu, H.; Zhou, Q.; Yu, S.M.; Mao, Y.M.; Zhang, H.J.; Zhang, P.-C.; Yan, W.-J. MiR-451 inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion in rheumatoid arthritis through mediating p38MAPK signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 14562–14567. [Google Scholar]
- Chatzikyriakidou, A.; Voulgari, P.V.; Georgiou, I.; Drosos, A.A. A polymorphism in the 3’-YTR of interleukin-1 receptor-associated kinase (IRAK1), a target gene of miR-146a, is associated with rheumatoid arthritis susceptibility. Jt. Bone Spine 2010, 77, 411–413. [Google Scholar] [CrossRef]
- Nakasa, T.; Shibuya, H.; Nagata, Y.; Niimoto, T.; Ochi, M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheumatol. 2011, 63, 1582–1590. [Google Scholar] [CrossRef]
- Sun, W.; Ma, J.; Zhao, H.; Xiao, H.; Ling, H.; Xie, Z.; Tian, Q.; Chen, H.; Zhang, T.; Chen, M.; et al. Resolvin D1 suppresses pannus formation via decreasing connective tissue growth factor caused by upregulation of miRNA-146a-5p in rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 61. [Google Scholar] [CrossRef] [Green Version]
- Philippe, L.; Alsaleh, G.; Suffert, G.; Meyer, A.; Georgel, P.; Sibilia, J.; Wachsmann, D.; Pfeffer, S. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J. Immunol. 2012, 188, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulos, D.; Kavousanaki, M.; Ioannou, M.; Boumpas, D.; Verginis, P. The negative costimulatory molecular PD-1 modulates the balance between immunity and tolerance via miR-21. Eur. J. Immunol. 2011, 41, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, C.; Chen, Z.; Li, J. MicroRNA-323-3p: A new biomarker and potential therapeutic target for rheumatoid arthritis. Rheumatol. Int. 2013, 34, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.X.; Pan, H.F.; Qin, W.Z.; Chen, G.M.; Ye, D.Q. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 2011, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, J.; Pedrioli, D.M.; Brentano, F. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheumatol. 2008, 58, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [Green Version]
- Niederer, F.; Trenkmann, M.; Ospell, C.; Karouzakis, E.; Neidhart, M.; Stancyk, J.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; et al. Down-regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheumatol. 2012, 64, 1771–1779. [Google Scholar] [CrossRef]
- Najm, A.; Blanchard, F.; Goff, B.L. Micro-RNAs in inflammatory arthritis: From physiopathology do diagnosis, prognosis and therapeutic opportunities. Biochem. Pharmocol. 2019, 165, 134–144. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, X.L.; Kong, R.N.; Ji, L.M.; He, L.L.; Zhao, D.B. MicroRNA-126 targeting PIK3R2 promotes rheumatoid arthritis synovial fibro-blasts proliferation and resistance to apoptosis by regulating PI3K/AKT oathway. Exp. Mol. Pathol. 2016, 100, 192–198. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, J.; Deng, J.X.; Zhang, Y.P.; Laing, W.Y.; Jiang, Z.L.; Yu, Q.-H.; Li, J. MicroRNA-126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K-AKT signal pathway. Oncotarget 2016, 7, 74217. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yang, Y. Downregulation of microRNA-221 decreases migration and invasion in fibroblast-like synoviocytes in rheumatoid arthritis. Mol. Med. Rep. 2015, 12, 2395–2401. [Google Scholar] [CrossRef]
- Nagata, Y.; Nakasa, T.; Mochizuki, Y.; Ishikawa, M.; Miyaki, S.; Shikuya, H.; Yamasaki, K.; Adachi, N.; Asahara, H.; Ochi, M. Induction of apoptosis in the synovium of mice with autoantibody-mediated arthritis by the intraarticular injection of double-stranded Micro-RNA-15a. Arthritis Rheumatol. 2009, 60, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, Y.; Kawano, S.; Takenokuchi, M.; Nishimura, K.; Sakai, Y.; Chin, T.; Saura, R.; Kurosaka, M.; Kumagai, S. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheumatol. 2009, 60, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Ding, H.; Jiang, H.; Bao, N.; Zhou, L.; Zhao, J. miR-338-5p regulates the viability, proliferation, apoptosis and migration of rheumatoid arthritis fibroblast-like synoviocytes by targeting NFAT5. Cell. Physiol. Biochem. 2018, 49, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, X.; Liu, Z.; Wang, X.; Li, Y. MiR-338-5p suppresses rheumatoid arthritis synovial fibroblast proliferation and invasion by targeting ADAMTS-9. Clin. Exp. Rheumatol. 2018, 36, 195–202. [Google Scholar]
- Guo, J.; Du, J.; Fei, D.; Xing, J.; Liu, J.; Lu, H. miR-152 inhibits rheumatoid arthritis synovial fibroblast proliferation and induces apoptosis by targeting ADAM10. Int. J. Mol. Med. 2018, 42, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yan, P.; Chen, Y.; Chen, Y.; Yang, J.; Xu, G.; Mao, H.; Qiu, Y. MicroRNA-26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK-3 β/ β-catenin pathway. Diagn. Pathol. 2015, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, F.; Guo, J. miR-137 decreases proliferation, migration and invasion in rheumatoid arthritis fibroblast-like synoviocytes. Mol. Med. Rep. 2018, 17, 3312–3317. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guan, S.B.; Lu, Y.; Wang, F. MiR-140-5p inhibits synovial fibroblast proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed. Pharmacother. 2017, 96, 208–214. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Zhang, Q.; Xu, J.; Shi, Q.; Wang, M. MiR-650 inhibits proliferation, migration and invasion of rheumatoid arthritis synovial fibroblasts by targeting AKT2. Biomed. Pharmacother. 2017, 88, 535–541. [Google Scholar] [CrossRef]
- Ruedel, A.; Dietrich, P.; Schubert, T.; Hofmeister, S.; Hellerbrand, C.; Bosserhoff, A.K. Expression and function of microRNa-188-5p in activated rheumatoid arthritis synovial fibroblasts. Int. J. Clin. Exp. Pathol. 2015, 8, 4953–4962. [Google Scholar]
- Blum, S.; Bonelli, M.; Niederreiter, B.; Puchner, A.; Mayr, G.; Hayer, S.; Koenders, M.U.; van den Berg, W.B.; Smolen, J.; Redlich, K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheumatol. 2011, 63, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Lu, X. MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1. Mol. Cell. Biochem. 2017, 432, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, J.; Ospelt, C.; Karouzakis, E.; Filer, A.; Raza, K.; Kolling, C.; Gay, R.; Buckley, C.D.; Tak, P.P.; Gay, S.; et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheumatol. 2011, 63, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, H.; Nakasa, T.; Adachi, N.; Nagata, Y.; Ishikawa, M.; Deie, M.; Suzuki, O.; Ochi, M. Overexpression of microRNA-223 in rheumatoid arthritis synovium controls osteoclast differentiation. Mod. Rheumatol. 2013, 23, 674–685. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, S.Y.; Wang, C.R.; Liu, C.-C.; Jou, I.-M.; Shiau, A.-L.; Wu, C.-L. Brief report: Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheumatol. 2012, 64, 3240–3245. [Google Scholar] [CrossRef]
- Yang, G.; Wu, D.; Zeng, G.; Jiang, O.; Yuan, P.; Huang, S.; Zhu, J.; Tian, J.; Weng, Y.; Rao, Z. Correlation between miR-126 expression and DNA hypermethylation of CD4+ T cells in rheumatoid arthritis patients. Int. J. Clin. Exp. Pathol. 2015, 8, 8929–8936. [Google Scholar]
- Murata, K.; Furu, M.; Yoshitomi, H.; Ishikawa, M.; Shikuya, H.; Hashimoto, M.; Imura, Y.; Fujii, T.; Ito, H.; Mimori, T.; et al. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS ONE 2013, 8, e69118. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.X.; Massachi, I.; Manickavel, S.; Singh, S.; Rao, N.P.; Hasan, S.; Mc Curdy, D.K.; Sharma, S.; Wong, D.; Hahn, B.H.; et al. The role of miRNA in inflammation and autoimmunity. Autoimmun. Rev. 2013, 12, 1160–1165. [Google Scholar] [CrossRef]
- Duroux-Ruchard, I.; Pers, Y.M.; Fabre, S.; Ammari, M.; Baeten, D.; Carton, G.; Touitou, I.; Jorgensen, C.; Apparailly, F. Circulating miRNA-125b is potential biomarker predicting response to rituximab in rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 342524. [Google Scholar] [CrossRef]
- Jin, F.; Hu, H.; Xu, M.; Zhan, S.; Wang, Y.; Zhang, H.; Chen, X. Serum microRNA Profiles Serve as Novel Biomarkers for Autoimmune Diseases. Front. Immunol. 2018, 19, 2381. [Google Scholar] [CrossRef] [Green Version]
- Filkova, M.; Aradi, B.; Senolt, L.; Ospelt, C.; Vettor, S.; Mann, H.; Filer, A.; Raza, K.; Buckley, C.D.; Snow, M.; et al. Association of circulating miR-223 and miR-16 with disease activity in patients with early rheumatoid arthritis. Ann. Rhem. Dis. 2014, 73, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Villegas, C.; Perez-Sanchez, C.; Escudera, A.; Filipescu, I.; Verdu, M.; Ruiz-Limon, P.; Aguirre, M.A.; Jimenez-Gomez, Y.; Font, P.; Rodriguez-Ariza, A.; et al. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNF—α. Arthritis Res. Ther. 2015, 17, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciechomska, M.; Bonek, K.; Merdas, M.; Zarecki, P.; Swierkot, J.; Gluszko, P.; Bogunia-Kubik, K.; Maslinski, W. Changes in MiRNA-5196 Expression as a Potential Biomarker of Anti-TNF-α Therapy in Rheumatoid Arthritis and Ankylosing Spondylitis Patients. Arch. Immunol. Ther. Exp. 2018, 66, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krintel, S.B.; Dehlendorff, C.; Hetland, M.L.; Hoslev-Petersen, K.; Andersen, K.K.; Junkierm, P.; Pedenphant, J.; Ellingsen, T.; Ahlquist, P.; Lindegaard, H.M.; et al. Prediction of treatment response to adalimumab: A double-blind placebo-controlled study of circulating microRNA in patients with early rheumatoid arthritis. Pharm. J. 2016, 16, 141–146. [Google Scholar] [CrossRef]
- Cunninham, C.C.; Wade, S.; Floudas, A.; Orr, C.; McGarry, T.; Wade, S.; Cregan, S.; Fearon, U.; Veale, D.J. Serum miRNA Signature in Rheumatoid Arthritis and At-Risk Individuals. Front. Immunol. 2021, 12, 633201. [Google Scholar] [CrossRef]
- Guo, D.; Lv, J.; Chen, X.; Yan, X.; Ma, F.; Liu, Y.; Chen, X.; Xie, J.; Zhang, M.; Jin, Z.; et al. Study of miRNA interactome in active rheumatoid arthritis patients reveals key pathogenic roles of dysbiosis in the infection–immune network. Rhematology 2021, 60, 1512–1522. [Google Scholar] [CrossRef]
- Lu, H.; Yao, Y.; Yang, J.; Zhang, H.; Li, L. Microbiome-miRNA interactions in the progress from undifferentiated arthritis to rheumatoid arthritis: Evidence, hypotheses, and opportunities. Rheumatol. Int. 2021, 41, 1567–1575. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Luo, X.; Peng, L.; Zhao, Z.; He, C.; He, Y. Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway. Biomater. Sci. 2020, 12, 3430–3442. [Google Scholar] [CrossRef]
- Kmiołek, T.; Rzeszotarska, E.; Wajda, A.; Walczuk, E.; Kuca-Warnawin, E.; Romanowska-Próchnicka, K.; Stypinska, B.; Majewski, D.; Jagodzinski, P.P.; Pawlik, A.; et al. The interplay between Transcriptional Factors and MicroRNA as an Important Factor for Th17/Treg Balance in RA Patients. Int. J. Mol. Sci. 2020, 21, 7169. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Goldberg, T. Mipomersen (Kynamro). Pharm. Ther. 2014, 39, 119–122. [Google Scholar]
- Mendell, J.R.; Rodino-Klapac, L.R.; Sahenk, Z.; Roush, K.; Bird, L.; Lowes, L.P.; Alfano, L.; Gomez, A.M.; Lewis, S.; Kota, J.; et al. The eteplirsen Study Group, Eteplirsen for the treatment of Duchenne muscular dystrophy: Eteplirsen for DMD. Ann. Neurol. 2013, 74, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Kauppinen, S. Discovering the first microRNA-targeted drug. J. Cell Biol. 2012, 199, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from bench to clinic as next generation medicine. Mol. Ther. Nucl. Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Luck, M.E.; Muljo, S.A.; Colins, C.B. Prospects for therapeutic targeting of microRNAs in human immunological diseases. J. Immunol. 2015, 194, 5047–5052. [Google Scholar] [CrossRef]
- Dhungel, B.; Ramlogan-Steel, C.; Steel, J. MicroRNA-regulated gene delivery systems for research and therapeutic pruposes. Molecules 2018, 23, 1500. [Google Scholar] [CrossRef] [Green Version]
- Saquib, M.; Agnihotri, P.; Biswas, M.; Biswas, S. Exogenous miRNA: A Perspective Role as Therapeutic in Rheumatoid Arthritis. Curr. Rhematol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Weisman, M.H.; Durez, P.; Hallegua, D.; Aranda, R.; Becker, J.-C.; Nuamah, I.; Vratsanos, G.; Zhou, Y.; Moreland, L.W. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J. Rheumatol. 2006, 33, 2162–2166. [Google Scholar]
- Dai, R.; Ahmed, S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation and autoimmune diseases. Transl. Res. 2011, 157, 163–179. [Google Scholar] [CrossRef] [Green Version]


| Increased Level of miRNA | Targets | Subject of Studies |
|---|---|---|
| miR-16 | Unknown | Human |
| miR-103a | AGO2, TP53 | Human |
| miR-132 | Unknown | Human |
| miR-145 | Unknown | Human |
| miR-146a * | IRAK1, TRAF6 | Human |
| miR-155 * | APAF1, CASP10, SHIP-1, SOCS1, | Human and Murine |
| miR-221 | Unknown | Human |
| miR-222 | Unknown | Human |
| miR301a | PIAS3 | Human |
| Decreased Level of miRNA | Targets | Subject of Studies |
|---|---|---|
| let-7a | ERK1, ERK2, K-Ras, JNK | Human |
| miR-21 | Foxp3, STAT3, STAT5 | Human |
| miR-125b | Unknown | Human |
| miR-548 | NF-κB pathway, TLR-4 | Human |
| Increased Level of miRNA | Targets | Subject of Studies |
|---|---|---|
| miR-18a | TNFAIP-3 | Human |
| miR-19 * | TLR2 pathway | Human |
| miR-21 | NF-κB pathway | Murine |
| miR-125b | NF-κB pathway | Human |
| miR-126 * | PIK3R2 | Human |
| miR-143 | IGF1R, IGFBP5, Ras MAPK, p38 MAPK | Human |
| miR-145 | SEMA3A | Human |
| miR-146a * | IRAK-1, TRAF6 | Human and Murine |
| miR-155 * | IKBKE, JAK2, STAT3 | Human and Murine |
| miR-203 * | NF-κB pathway | Human |
| Unknown change to miR-218 | ROBO1, Wnt/β-catenin | Human |
| miR-221 * | Wnt, BMP | Human and Murine |
| miR-222 | Wnt/cadherin | Murine |
| miR-223 * | IL-17RD, NFI-A | Human and Murine |
| miR-323 | Wnt/cadherin | Murine |
| miR-338 * | NFAT5 | Human |
| miR-346 | Btk, TTP | Human |
| miR-522 | SOCS3 | Human |
| miR-663 | APC | Human |
| Decreased Level of miRNA | Targets | Subject of Studies |
|---|---|---|
| miR-10b * | BTRC, IRAK4, TAK1, TBX5 | Human |
| miR-17 | TRAF2 | Human |
| miR-20a * | ASK1, TXNIP | Human and Murine |
| miR-22 | Cyr61 | Human |
| miR-23b | IKK- α, TAB2, TAB3 | Human and Murine |
| miR27a | FSTL1, NF-κB pathway, TR4 pathway | Human |
| miR-29a * | STAT3 | Human |
| miR-30-3p | BAFF | Human |
| miR-34 * | XIAP | Human |
| miR-124a * | CDK2, MCP1 | Human |
| miR-137 | CXCL12 | Murine |
| miR-140-5p | SCDF1, Sirtuin1 | Human |
| miR-152 * | ADAM10, DNMT1 | Human and Murine |
| miR-188-5p | CEMIP | Human |
| miR-192 | Caveolin 1 | Human |
| miR-199a | RB1 | Human |
| miR-204 | ATF2 | Human |
| miR-211 | ATF2 | Human |
| miR-212 | SOX5 | Human |
| miR-375 | Wnt/FZD8 | Murine |
| miR-539 | OPN | Human |
| miR-650 | AKT2 | Human |
| miRNAs | Regulation of T Lymphocytes | Inflammatory Response |
|---|---|---|
| Let-7a | Positive regulator | |
| miR-16 | Positive regulator | |
| miR-17 | Negative regulator | |
| miR-18 | Positive regulator | |
| miR-19a/b | Negative regulator | |
| miR-20 | Negative regulator | |
| miR-21 | Positive regulator | |
| miR-26 | Positive regulator | |
| miR-125b | Positive regulator | |
| miR-132 | Positive regulator | |
| miR-146a | Positive regulator | Positive regulator |
| miR-146b | Positive regulator | |
| miR-150 | Positive regulator | |
| miR-155 | Positive regulator | Positive regulator |
| miR-203 | Positive regulator | |
| miR-223 | Positive regulator | |
| miR-323-3p | Positive regulator | Positive regulator |
| miR-451 | Negative regulator |
| miRNAs | Apoptosis | Cellular Proliferation | Migration |
|---|---|---|---|
| miR-15a | Positive regulator | ||
| miR-26b | Positive regulator | Negative regulator | |
| miR-29a | Positive regulator | Negative regulator | |
| miR-34a | Positive regulator | ||
| miR-124a | Negative regulator | ||
| miR-126 | Negative regulator | Positive regulator | |
| miR-137 | Negative regulator | Negative regulator | |
| miR-140-5p | Negative regulator | ||
| miR-152 | Positive regulator | Negative regulator | Negative regulator |
| miR-188-5p | Negative regulator | ||
| miR-192 | Positive regulator | Negative regulator | |
| miR-221 | Negative regulator | Negative regulator | |
| miR-338-5p | Positive regulator | Negative regulator | Negative regulator |
| miR-650 | Negative regulator | Positive regulator | Positive regulator |
| miRNAs | Osteoclast Generation | Matrix Metalloproteinases |
|---|---|---|
| miR-19a/b | Negative regulator | |
| miR-106b | Positive regulator | |
| miR-146a | Negative regulator | |
| miR-155 | Positive regulator | Negative regulator |
| miR-203 | Positive regulator | |
| miR-223 | Positive/negative regulator |
| Therapeutic siRNAs | Target | Indication | Phase |
|---|---|---|---|
| AGN21174 | VEGFR1 gene | Age-related macular leukemia | Terminated in phase II |
| AGN211745 | VEGFR1 gene | Treatment of age-related macular degeneration | Clinical trial phase II |
| ALN-RSV01 | RSV nucleocapsid | Treatment of RSV infection during lung transplantation | Clinical trial phase IIb |
| ALN-TTR02 | TTR | Treatment of transthyretin-mediated amyloidosis | APOLLO study phase III |
| ALN-VSP | VEGF gene | Treatment of liver cancer | Completed phase I |
| ApoB SNALP | Apolipoprotein B gene | Treatment of hypercholesterolemia | Concluded clinical trial phase I |
| Atu-027 | Protein kinase N3 gene | Treatment of advanced solid tumors | Clinical trial phase I |
| Bevasiranib | VEGF gene | Treatment of AMD or diabetic macular edema | Clinical trial phase III |
| CALAA-01 | M2 subunit of ribonucleotide reductase | Inhibit tumor and cancer therapy | Clinical trial phase Ib |
| Excellair | Syk gene | Treatment of inflammatory disorders | Clinical trial phase II |
| QPI-1002 | p53 | Avoidance of AKI, prophylaxis of DGF | In phase II obtained Orphan drug designation |
| QPI-1007 | Caspase-2 gene | Treatment of nonarthritic anterior ischemic optic neuropathy | |
| PF-04523655 | HIF-1-responsive gene, RTP801 | Treatment of age-related macular degeneration and diabetic macular edema | Clinical trial phase II |
| PF-655 | RTP801 gene | Treatment of age-related macular degeneration | Clinical trial phase II |
| RXI-109 | CTGF gene | Treatment of fibrosis and ocular disorders | Clinical trial phase I |
| SYL040012 | β2-adrenergic receptor gene | Ocular hypertension | Completed phase II |
| Therapeutic miRNAs | Target | Indication | Phase |
|---|---|---|---|
| MGN-1374 | miR-15 and miR-195 | Treatment of post-myocardial infraction remodeling | Preclinical stage |
| MGN-2677 | miR-143/145 | Treatment of vascular disease | Being prepared |
| MGN-4220 | miR-29 | Treatment of cardiac fibrosis | Being prepared |
| MGN-4893 | miR-451 | Treatment of disorders (polycythemia vera) | Being prepared |
| MGN-5804 | miR-378 | Treatment of cardio metabolic disease | Being prepared |
| MGN-6114 | miR-92 | Treatment of peripheral arterial disease | Being prepared |
| MGN-9103 | miR-208 | Treatment of chronic heart failure | Being prepared |
| Miravirsen | HCV infection | Clinical trial phase IIa | |
| MiRX34 | Treatment of variety of cancers | Stopped in clinical trial phase I | |
| RG-012 | Treatment of Alport syndrome | Being prepared for clinical trial phase II | |
| RG-101 | GalNAc-conjugated anti-miR | Treatment of HCV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmiołek, T.; Paradowska-Gorycka, A. miRNAs as Biomarkers and Possible Therapeutic Strategies in Rheumatoid Arthritis. Cells 2022, 11, 452. https://doi.org/10.3390/cells11030452
Kmiołek T, Paradowska-Gorycka A. miRNAs as Biomarkers and Possible Therapeutic Strategies in Rheumatoid Arthritis. Cells. 2022; 11(3):452. https://doi.org/10.3390/cells11030452
Chicago/Turabian StyleKmiołek, Tomasz, and Agnieszka Paradowska-Gorycka. 2022. "miRNAs as Biomarkers and Possible Therapeutic Strategies in Rheumatoid Arthritis" Cells 11, no. 3: 452. https://doi.org/10.3390/cells11030452






