Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Issues
2.2. oAECs Culture
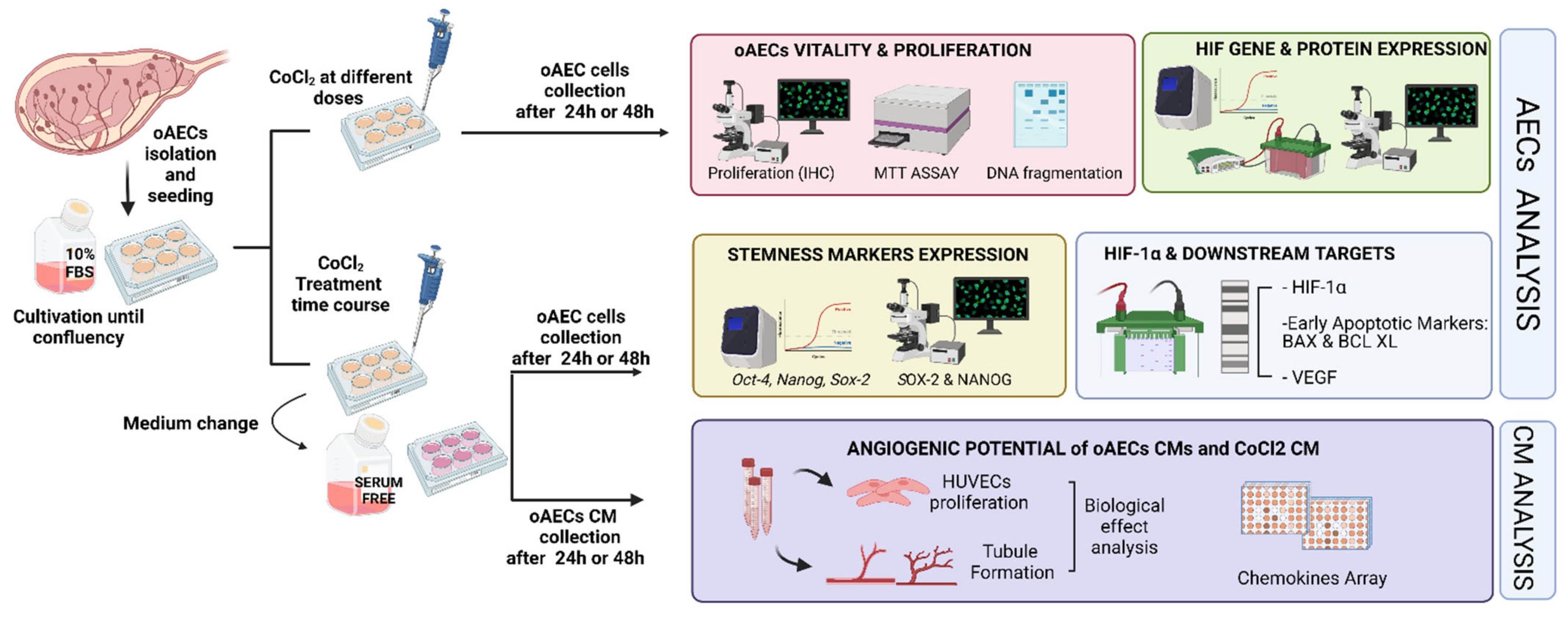
2.3. Experimental Plan Designed to Study the Effect of Chemical-Induced Hypoxia on oAECs
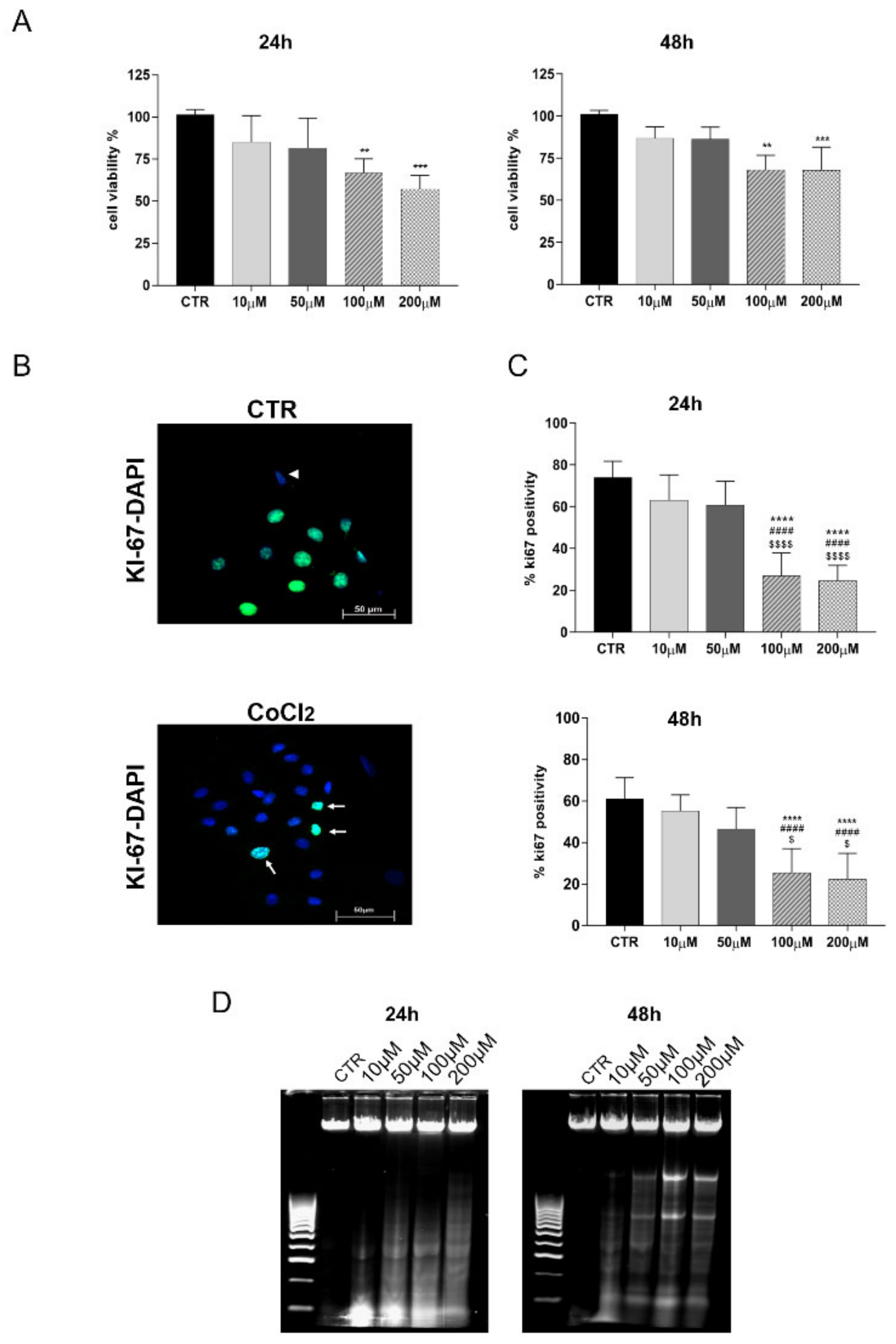
2.4. CoCl2 Influence on Cell Viability and Proliferation
- MTT Assay: MTT assay (M5655-1G, Sigma-Aldrich, St. Louis, MO, USA) was performed to evaluate the effect of different CoCl2 doses on cell viability, according to the manufacturer’s instructions. Briefly, oAECs were seeded into 96-well plates (0.2 × 105 cells/well) until reaching 70% confluence. After 24 h or 48 h from CoCl2 treatments, 20 µL MTT (5 mg/mL in PBS) was added to each well/sample and incubated at 37 °C for 3.5 h. Afterwards, the formazan crystals were dissolved in DMSO. Absorbance was measured at 595 nm with a micro-plate reader (Multi-mode Plate Reader EnSpire, Perkin Elmer). For each sample, the relative blank values were subtracted (i.e., the culture medium without cells). Data viability was normalized for the controls and expressed in percentage [23,31].
- DNA Fragmentation: to exclude any toxic effect of CoCl2 on oAECs, the degrees of apoptosis induced at 24 and 48 h after treatments were evaluated. The analysis of DNA fragmentation was performed according to Kasibhatla et al. [43]. The detection of DNA fragments was conducted via agarose gel electrophoresis. After DNA extraction [43], 5 μg of each sample was suspended in 2 µL of sample buffer (0.25% bromophenol blue, 30% glycerol) and loaded into 1.8% agarose gel containing 1 µg/mL of GelRed (Bioline, Thomas Scientific, Swedesboro, NJ, USA) and run at a constant 70 V current. DNA fragments were visualized by an ultra-violet trans-illuminator (E-Gel Imager, Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA).
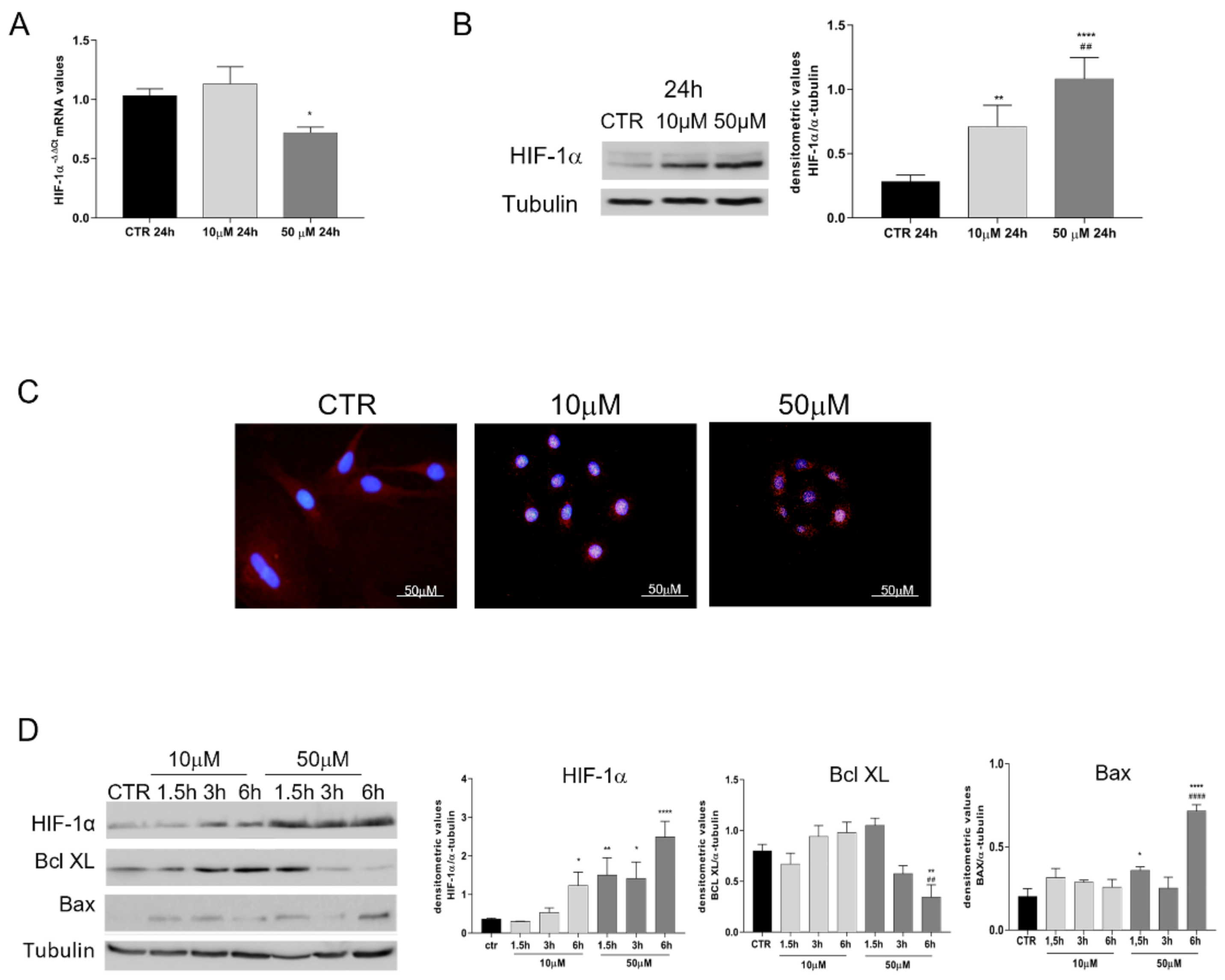
2.5. Influence of Chemical-Induced Hypoxia on oAEC Genes’ Expression
2.6. Analyses of Protein Profile Promoted by oAECs after Exposure to CoCl2
- Immunohistochemistry (IHC).
- b.
- Western blot (WB) analysis.
2.7. Pro-Angiogenic Influence of CM Derived from Naïve and Hypoxia-Induced oAECs
- a.
- Angiogenic protein markers’ array.
- b.
- HUVEC Proliferation Assay.
- c.
- In Vitro Tubule-Like Formation.
2.8. Statistical Analysis
3. Results
3.1. Doses Effect of CoCl2 Treatment on oAECs Properties
3.2. HIF-1α Pathway Is Activated by CoCl2-Treated oAECs
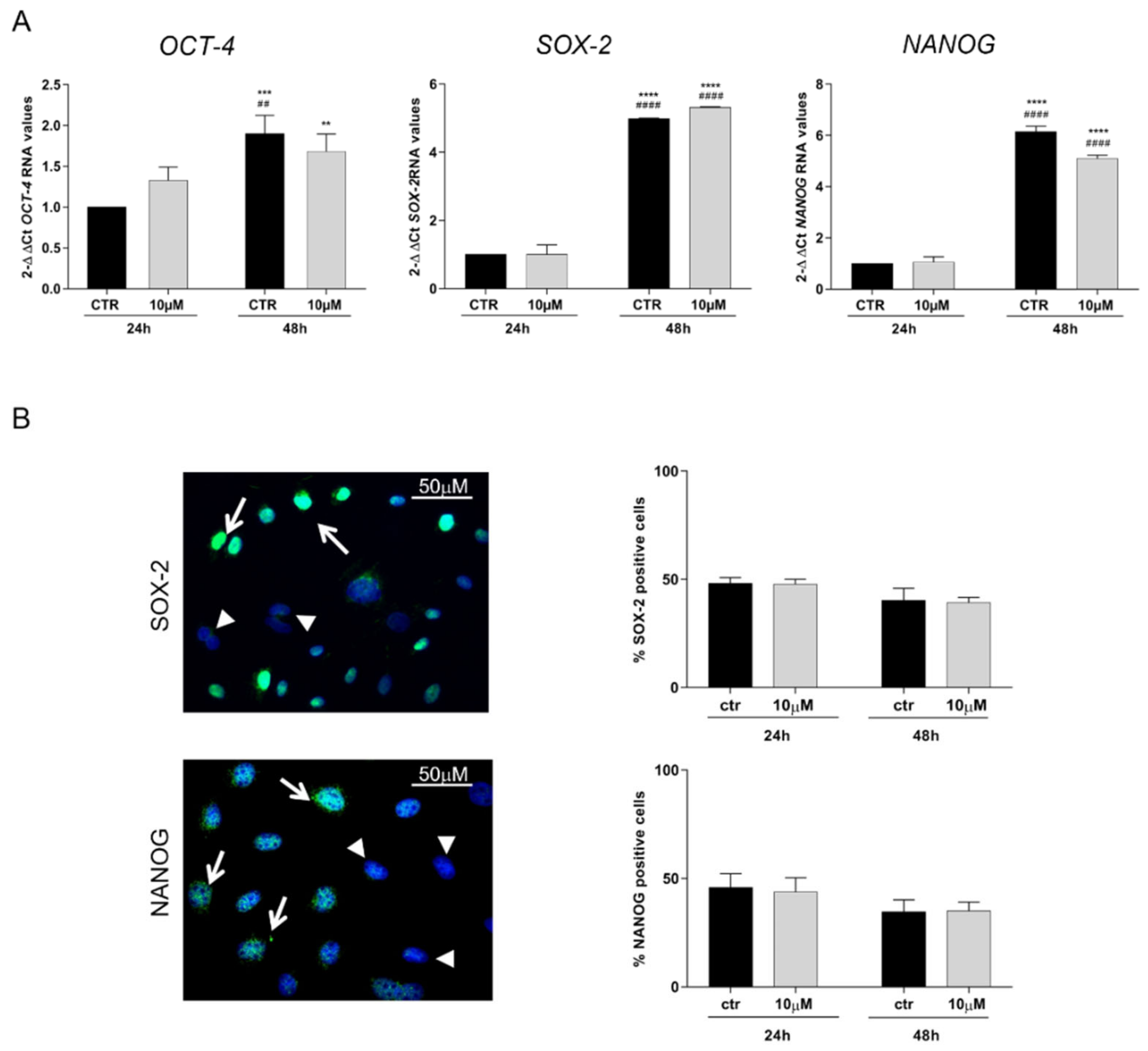
3.3. CoCl2 Effects on Pluripotency Markers
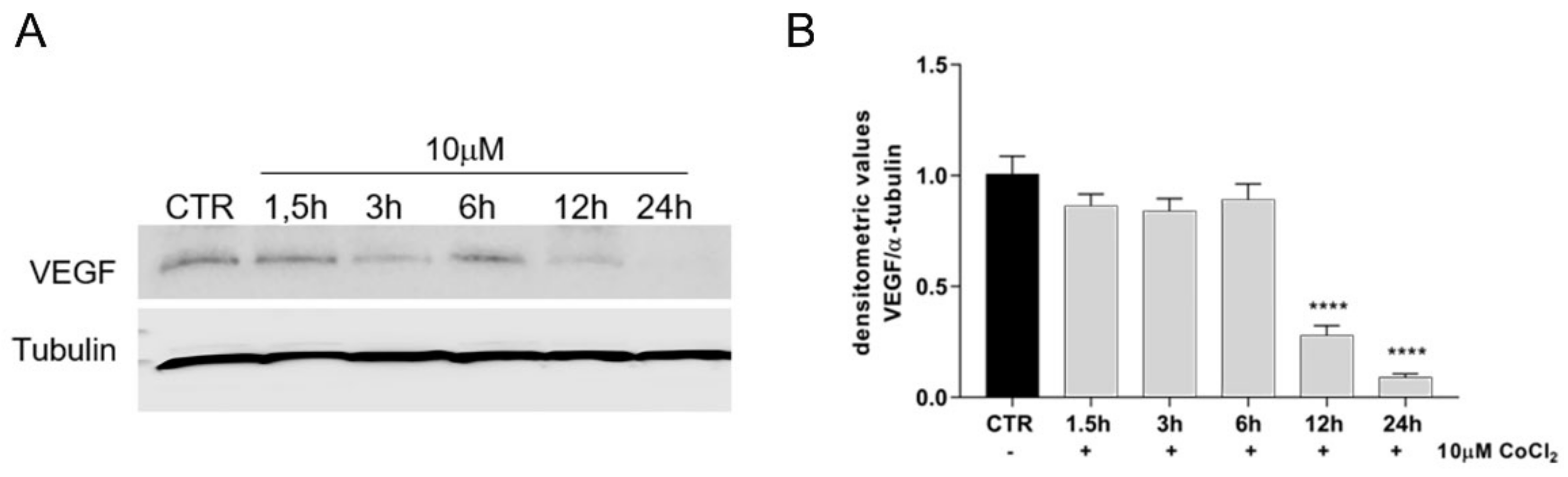
3.4. Growth Factor Expression in CoCl2-Treated oAECs Conditioned Media
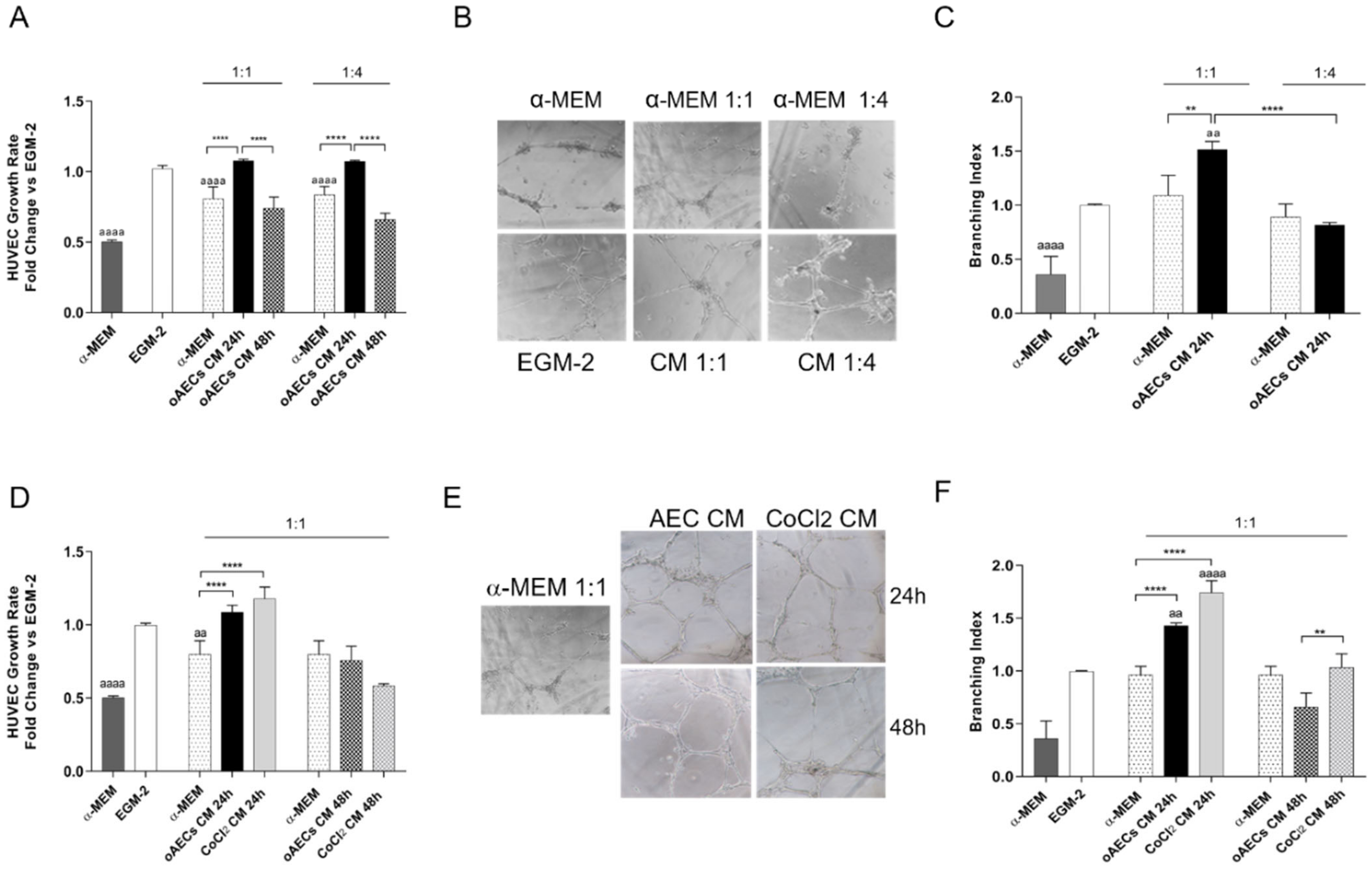
3.5. Pro-Angiogenic Effect Induced by Chemical Hypoxia on AECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic Membrane and Amniotic Fluid-Derived Cells: Potential Tools for Regenerative Medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, A.; Bersinger, N.A. Human Placental Stem Cells: Biomedical Potential and Clinical Relevance. J. Stem Cells 2011, 6, 75–92. [Google Scholar]
- Miki, T. Amnion-Derived Stem Cells: In Quest of Clinical Applications. Stem Cell Res. Ther. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboni, B.; Curini, V.; Russo, V.; Mauro, A.; Di Giacinto, O.; Marchisio, M.; Alfonsi, M.; Mattioli, M. Indirect Co-Culture with Tendons or Tenocytes Can Program Amniotic Epithelial Cells towards Stepwise Tenogenic Differentiation. PLoS ONE 2012, 7, e30974. [Google Scholar] [CrossRef] [Green Version]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic Membrane: From Structure and Functions to Clinical Applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Mangano, C.; Valbonetti, L.; Marruchella, G.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mauro, A.; Bedini, R.; Turriani, M.; et al. Synthetic Bone Substitute Engineered with Amniotic Epithelial Cells Enhances Bone Regeneration after Maxillary Sinus Augmentation. PLoS ONE 2013, 8, e63256. [Google Scholar] [CrossRef] [Green Version]
- Barboni, B.; Russo, V.; Berardinelli, P.; Mauro, A.; Valbonetti, L.; Sanyal, H.; Canciello, A.; Greco, L.; Muttini, A.; Gatta, V.; et al. Placental Stem Cells from Domestic Animals: Translational Potential and Clinical Relevance. Cell Transplant. 2018, 27, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, A.; Curini, V.; Russo, V.; Mauro, A.; Bernabò, N.; Marchisio, M.; Alfonsi, M.; Muttini, A.; Mattioli, M.; Barboni, B. Characterization, GFP Gene Nucleofection, and Allotransplantation in Injured Tendons of Ovine Amniotic Fluid-Derived Stem Cells. Cell Transplant. 2013, 22, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.V.; Parolini, O. Amniotic Membrane and Amniotic Cells: Potential Therapeutic Tools to Combat Tissue Inflammation and Fibrosis? Placenta 2011, 32 (Suppl. 4), S320–S325. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.; Gloria, A.; Turriani, M.; Mauro, A.; Curini, V.; Russo, V.; Tetè, S.; Marchisio, M.; Pierdomenico, L.; Berardinelli, P.; et al. Stemness Characteristics and Osteogenic Potential of Sheep Amniotic Epithelial Cells. Cell Biol. Int. 2012, 36, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of Amniotic Stem Cells. Cell. Reprogram. 2014, 16, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange-Consiglio, A.; Corradetti, B.; Bizzaro, D.; Magatti, M.; Ressel, L.; Tassan, S.; Parolini, O.; Cremonesi, F. Characterization and Potential Applications of Progenitor-Like Cells Isolated from Horse Amniotic Membrane. J. Tissue Eng. Regen. Med. 2012, 6, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Curini, V.; Martelli, A.; Berardinelli, P.; Mauro, A.; Mattioli, M.; Marchisio, M.; Bonassi Signoroni, P.; Parolini, O.; et al. Gestational Stage Affects Amniotic Epithelial Cells Phenotype, Methylation Status, Immunomodulatory and Stemness Properties. Stem Cell Rev. Rep. 2014, 10, 725–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.-Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human Amniotic Epithelial Cell Transplantation Induces Markers of Alternative Macrophage Activation and Reduces Established Hepatic Fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef] [Green Version]
- Moodley, Y.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.; Trounson, A.; Manuelpillai, U. Human Amnion Epithelial Cell Transplantation Abrogates Lung Fibrosis and Augments Repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles Tendon Regeneration Can Be Improved by Amniotic Epithelial Cell Allotransplantation. Cell Transplant. 2012, 21, 2377–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muttini, A.; Salini, V.; Valbonetti, L.; Abate, M. Stem Cell Therapy of Tendinopathies: Suggestions from Veterinary Medicine. Muscles Ligaments Tendons J. 2012, 2, 187–192. [Google Scholar]
- Barboni, B.; Russo, V.; Gatta, V.; Bernabò, N.; Berardinelli, P.; Mauro, A.; Martelli, A.; Valbonetti, L.; Muttini, A.; Di Giacinto, O.; et al. Therapeutic Potential of HAECs for Early Achilles Tendon Defect Repair through Regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1594–e1608. [Google Scholar] [CrossRef]
- Mauro, A.; Russo, V.; Di Marcantonio, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mattioli, M.; Barboni, B. M1 and M2 Macrophage Recruitment during Tendon Regeneration Induced by Amniotic Epithelial Cell Allotransplantation in Ovine. Res. Vet. Sci. 2016, 105, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Muttini, A.; Russo, V.; Rossi, E.; Mattioli, M.; Barboni, B.; Tosi, U.; Maffulli, N.; Valbonetti, L.; Abate, M. Pilot Experimental Study on Amniotic Epithelial Mesenchymal Cell Transplantation in Natural Occurring Tendinopathy in Horses. Ultrasonographic and Histological Comparison. Muscles Ligaments Tendons. J. 2015, 5, 5–11. [Google Scholar] [CrossRef]
- Muttini, A.; Valbonetti, L.; Abate, M.; Colosimo, A.; Curini, V.; Mauro, A.; Berardinelli, P.; Russo, V.; Cocciolone, D.; Marchisio, M.; et al. Ovine Amniotic Epithelial Cells: In Vitro Characterization and Transplantation into Equine Superficial Digital Flexor Tendon Spontaneous Defects. Res. Vet. Sci. 2013, 94, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Di Germanio, C.; Bernier, M.; de Cabo, R.; Barboni, B. Amniotic Epithelial Cells: A New Tool to Combat Aging and Age-Related Diseases? Front. Cell Dev. Biol. 2016, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Sanyal, H.; Canciello, A.; Berardinelli, P.; Russo, V.; Bernabò, N.; Valbonetti, L.; Barboni, B. In Vitro Effect of Estradiol and Progesterone on Ovine Amniotic Epithelial Cells. Stem Cells Int. 2019, 2019, 8034578. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of Antiangiogenic and Antiinflammatory Proteins in Human Amniotic Membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Steed, D.L.; Trumpower, C.; Duffy, D.; Smith, C.; Marshall, V.; Rupp, R.; Robson, M. Amnion-Derived Cellular Cytokine Solution: A Physiological Combination of Cytokines for Wound Healing. Eplasty 2008, 8, e18. [Google Scholar] [PubMed]
- Gu, Q.; Gu, Y.; Shi, Q.; Yang, H. Hypoxia Promotes Osteogenesis of Human Placental-Derived Mesenchymal Stem Cells. Tohoku J. Exp. Med. 2016, 239, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development: Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, M.; Mauro, A.; Citeroni, M.R.; Dufrusine, B.; Peserico, A.; Russo, V.; Berardinelli, P.; Dainese, E.; Cimini, A.; Barboni, B. Insight into Hypoxia Stemness Control. Cells 2021, 10, 2161. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [Green Version]
- Canciello, A.; Russo, V.; Berardinelli, P.; Bernabò, N.; Muttini, A.; Mattioli, M.; Barboni, B. Progesterone Prevents Epithelial-Mesenchymal Transition of Ovine Amniotic Epithelial Cells and Enhances Their Immunomodulatory Properties. Sci. Rep. 2017, 7, 3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, V.; El Khatib, M.; di Marcantonio, L.; Ancora, M.; Wyrwa, R.; Mauro, A.; Walter, T.; Weisser, J.; Citeroni, M.R.; Lazzaro, F.; et al. Tendon Biomimetic Electrospun PLGA Fleeces Induce an Early Epithelial-Mesenchymal Transition and Tenogenic Differentiation on Amniotic Epithelial Stem Cells. Cells 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citeroni, M.R.; Mauro, A.; Ciardulli, M.C.; Di Mattia, M.; El Khatib, M.; Russo, V.; Turriani, M.; Santer, M.; Della Porta, G.; Maffulli, N.; et al. Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model. Front. Bioeng. Biotechnol. 2021, 9, 649288. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The Use of Cobalt Chloride as a Chemical Hypoxia Model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt Inhibits the Interaction between Hypoxia-Inducible Factor-α and von Hippel-Lindau Protein by Direct Binding to Hypoxia-Inducible Factor-α. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef] [Green Version]
- Oladipupo, S.; Hu, S.; Kovalski, J.; Yao, J.; Santeford, A.; Sohn, R.E.; Shohet, R.; Maslov, K.; Wang, L.V.; Arbeit, J.M. VEGF Is Essential for Hypoxia-Inducible Factor-Mediated Neovascularization but Dispensable for Endothelial Sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 13264–13269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, S.; Semenza, G.L. Hypoxia-Inducible Factor-1-Dependent Mechanisms of Vascularization and Vascular Remodelling. Cardiovasc. Res. 2010, 86, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, Q.; Yang, X.; Yu, X.; Yu, D.; Zhao, W. Effects of Cobalt Chloride on the Stem Cell Marker Expression and Osteogenic Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth. Cell Stress Chaperones 2019, 24, 527–538. [Google Scholar] [CrossRef]
- Laksana, K.; Sooampon, S.; Pavasant, P.; Sriarj, W. Cobalt Chloride Enhances the Stemness of Human Dental Pulp Cells. J. Endod. 2017, 43, 760–765. [Google Scholar] [CrossRef]
- Chen, R.; Forsyth, N. Editorial: The Development of New Classes of Hypoxia Mimetic Agents for Clinical Use. Front. Cell Dev. Biol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Canciello, A.; Greco, L.; Russo, V.; Barboni, B. Amniotic Epithelial Cell Culture. In Epithelial Cell Culture; Baratta, M., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1817, pp. 67–78. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Induction and Testing of Hypoxia in Cell Culture. J. Vis. Exp. 2011, e2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Analysis of DNA Fragmentation Using Agarose Gel Electrophoresis. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot4429. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Galzio, R.; Cristiano, L.; Fidoamore, A.; Cifone, M.G.; Benedetti, E.; Cinque, B.; Menghini, P.; Raysi Dehcordi, S.; Ippoliti, R.; Giordano, A.; et al. Hypoxia Modulation of Peroxisome Proliferator-Activated Receptors (PPARs) in Human Glioblastoma Stem Cells. Implications for Therapy. J. Cell. Biochem. 2012, 113, 3342–3352. [Google Scholar] [CrossRef]
- Mauro, A.; Martelli, A.; Berardinelli, P.; Russo, V.; Bernabò, N.; Di Giacinto, O.; Mattioli, M.; Barboni, B. Effect of Antiprogesterone RU486 on VEGF Expression and Blood Vessel Remodeling on Ovarian Follicles before Ovulation. PLoS ONE 2014, 9, e95910. [Google Scholar] [CrossRef] [Green Version]
- Fathi, E.; Farahzadi, R.; Valipour, B. Alginate/Gelatin Encapsulation Promotes NK Cells Differentiation Potential of Bone Marrow Resident C-Kit+ Hematopoietic Stem Cells. Int. J. Biol. Macromol. 2021, 177, 317–327. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, S.; Du, L.; Liu, J.; Xu, X.; Lu, X.; Ma, L.; Zhu, H.; Wei, J.; Yu, Y. Conditioned Medium from Primary Cytotrophoblasts, Primary Placenta-Derived Mesenchymal Stem Cells, or Sub-Cultured Placental Tissue Promoted HUVEC Angiogenesis In Vitro. Stem Cell Res. Ther. 2021, 12, 141. [Google Scholar] [CrossRef]
- Delle Monache, S.; Martellucci, S.; Clementi, L.; Pulcini, F.; Santilli, F.; Mei, C.; Piccoli, L.; Angelucci, A.; Mattei, V. In Vitro Conditioning Determines the Capacity of Dental Pulp Stem Cells to Function as Pericyte-Like Cells. Stem Cells Dev. 2019, 28, 695–706. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef]
- Bresson, C.; Darolles, C.; Carmona, A.; Gautier, C.; Sage, N.; Roudeau, S.; Ortega, R.; Ansoborlo, E.; Malard, V. Cobalt Chloride Speciation, Mechanisms of Cytotoxicity on Human Pulmonary Cells, and Synergistic Toxicity with Zinc. Met. Integr. Biometal Sci. 2013, 5, 133–143. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt Metabolism and Toxicology—A Brief Update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lison, D.; De Boeck, M.; Verougstraete, V.; Kirsch-Volders, M. Update on the Genotoxicity and Carcinogenicity of Cobalt Compounds. Occup. Environ. Med. 2001, 58, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and Its Potential for Therapeutic Intervention in Malignancy and Ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar] [PubMed]
- Salceda, S.; Caro, J. Hypoxia-Inducible Factor 1α (HIF-1α) Protein Is Rapidly Degraded by the Ubiquitin-Proteasome System under Normoxic Conditions. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, R.H. Cellular Adaptation to Hypoxia: O2-Sensing Protein Hydroxylases, Hypoxia-Inducible Transcription Factors, and O2-Regulated Gene Expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Chamboredon, S.; Ciais, D.; Desroches-Castan, A.; Savi, P.; Bono, F.; Feige, J.-J.; Cherradi, N. Hypoxia-Inducible Factor-1α MRNA: A New Target for Destabilization by Tristetraprolin in Endothelial Cells. Mol. Biol. Cell 2011, 22, 3366–3378. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.J.; Song, C.H.; Ahn, Y.K.; Han, H.J. Role of FAK Phosphorylation in Hypoxia-Induced HMSCS Migration: Involvement of VEGF as Well as MAPKS and ENOS Pathways. Am. J. Physiol. Cell Physiol. 2010, 298, C847–C856. [Google Scholar] [CrossRef]
- Chacko, S.M.; Ahmed, S.; Selvendiran, K.; Kuppusamy, M.L.; Khan, M.; Kuppusamy, P. Hypoxic Preconditioning Induces the Expression of Prosurvival and Proangiogenic Markers in Mesenchymal Stem Cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1562–C1570. [Google Scholar] [CrossRef]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and Function of Mesenchymal Stem Cells (MSCs) Depend on Glucose to Overcome Exposure to Long-Term, Severe and Continuous Hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Andreeva, E.R.; Gogvadze, V.; Zhivotovsky, B. Mesenchymal Stem Cells and Hypoxia: Where Are We? Mitochondrion 2014, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Greijer, A.E. The Role of Hypoxia Inducible Factor 1 (HIF-1) in Hypoxia Induced Apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.D.; Espinosa, J.M. Transcriptional Regulation by Hypoxia Inducible Factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelucci, A.; Delle Monache, S.; Cortellini, A.; Di Padova, M.; Ficorella, C. “Vessels in the Storm”: Searching for Prognostic and Predictive Angiogenic Factors in Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lai, D. Application of Human Amniotic Epithelial Cells in Regenerative Medicine: A Systematic Review. Stem Cell Res. Ther. 2020, 11, 439. [Google Scholar] [CrossRef]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins and Their Impact on the Blood Vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular Vesicles Derived from Hypoxia-Preconditioned Olfactory Mucosa Mesenchymal Stem Cells Enhance Angiogenesis via MiR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef]
- Mesquita, J.; Castro-de-Sousa, J.P.; Vaz-Pereira, S.; Neves, A.; Passarinha, L.A.; Tomaz, C.T. Vascular Endothelial Growth Factors and Placenta Growth Factor in Retinal Vasculopathies: Current Research and Future Perspectives. Cytokine Growth Factor Rev. 2018, 39, 102–115. [Google Scholar] [CrossRef]
- Cunningham, F.; Van Bergen, T.; Canning, P.; Lengyel, I.; Feyen, J.H.M.; Stitt, A.W. The Placental Growth Factor Pathway and Its Potential Role in Macular Degenerative Disease. Curr. Eye Res. 2019, 44, 813–822. [Google Scholar] [CrossRef]
- Jacobo, S.M.P.; Kazlauskas, A. Insulin-Like Growth Factor 1 (IGF-1) Stabilizes Nascent Blood Vessels. J. Biol. Chem. 2015, 290, 6349–6360. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Bråkenhielm, E.; Li, X.; Pietras, K.; Widenfalk, J.; Östman, A.; Eriksson, U.; Cao, Y. Angiogenesis Stimulated by PDGF-CC, a Novel Member in the PDGF Family, Involves Activation of PDGFR-aa and -ap Receptors. FASEB J. 2002, 16, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R.; Vietor, I.; Javanmardi, S. Cardiac Differentiation of Bone-Marrow-Resident c-Kit+ Stem Cells by L-Carnitine Increases through Secretion of VEGF, IL6, IGF-1, and TGF-β as Clinical Agents in Cardiac Regeneration. J. Biosci. 2020, 45, 92. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamabe, H.; Osawa, H.; Nakamura, N.; Shimada, M.; Kumasaka, R.; Murakami, R.; Fujita, T.; Osanai, T.; Okumura, K. Hypoxic Conditions Stimulate the Production of Angiogenin and Vascular Endothelial Growth Factor by Human Renal Proximal Tubular Epithelial Cells in Culture. Nephrol. Dial. Transplant. 2006, 21, 1489–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandi, S.; Al Kahtani, A.; Mashyakhy, M.; Alsofi, L.; Maganur, P.C.; Vishwanathaiah, S.; Testarelli, L.; Del Giudice, A.; Mehta, D.; Vyas, N.; et al. Modulation of the Dental Pulp Stem Cell Secretory Profile by Hypoxia Induction Using Cobalt Chloride. J. Pers. Med. 2021, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Rodriguez, L.A.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-Term Physiological Hypoxia Potentiates the Therapeutic Function of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Forward Sequence | Reverse Sequence | References |
|---|---|---|---|
| GAPDH | 5′-TCGGAGTGAACGGATTTGGC-3′ | 5′-CCGTTCTCTGCCTTGACTGT-3′ | [23,31] |
| HIF-1α | 5′-TGCTCATCAGTTGCCACTTC-3′ | 5′-TTTCCTCATGGTCACATGG-3′ | [33] |
| OCT-4 | 5′-CTGCAGAAGTGGGTGGAGGAA-3′ | 5′-CTGCAGTGTGGGTTTCGGGCA-3′ | [23,31] |
| SOX-2 | 5′-CACCCGCATGTACAACATGAT-3′ | 5′-TCTTAGGATTCTCTTGGGCCA-3 | [23,31] |
| NANOG | 5′-TGGATCTGCTTATTCAGGACAG-3′ | 5′-TGCTGGAGGCTGAGGTATTTC-3′ | [23,31] |
| Antibodies Used for IHC Analysis | |||
| Primary Antibody (Company Information) | Primary Antibody Dilution | Secondary Antibody (Company Information) | Secondary Antibody Dilution |
| KI-67 (Santa Cruz, sc-101861) | 1:50 | Mouse Alexa Fluor 488 (Abcam, ab 150113) | 1:100 |
| HIF-1α (Novus Bio, 100-123) | 1:100 | Mouse CY Conjugated (Millipore, AP124C) | 1:500 |
| SOX-2 (Abcam, ab69893) | 1:200 | Rabbit Alexa Fluor (Abcam, ab 150077) | 1:500 |
| NANOG (Millipore, AB9220) | 1:1000 | Rabbit Alexa Fluor (Abcam, ab 150077) | 1:500 |
| Antibodies Used for WB Analysis | |||
| HIF-1α (Novus Bio, 100-123) | 1:500 | Anti-mouse HRP conjugated (Santa Cruz, sc-516102) | 1:10000 |
| VEGF (Calbiochem, PC37) | 1:500 | Anti-rabbit HRP conjugated (Santa Cruz, sc-2357) | 1:10000 |
| Bax (Santa Cruz, sc 23959) | 1:250 | Anti-mouse HRP conjugated (Santa Cruz, sc-516102) | 1:10000 |
| Bcl XL (Santa Cruz, sc 8392) | 1:250 | Anti-mouse HRP conjugated (Santa Cruz, sc-516102) | 1:10000 |
| Tubulin (Sigma, T5168) | 1:1000 | Anti-mouse HRP conjugated (Santa Cruz, sc-516102) | 1:10000 |
| TGF-β1 (Abcam, ab27969) | 1:250 | Anti-mouse HRP conjugated (Santa Cruz, sc-516102) | 1:10000 |
| TIMP-1 (Millipore, AB770) | 1:250 | Anti-rabbit HRP conjugated (Santa Cruz, sc-2357) | 1:10000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mattia, M.; Mauro, A.; Delle Monache, S.; Pulcini, F.; Russo, V.; Berardinelli, P.; Citeroni, M.R.; Turriani, M.; Peserico, A.; Barboni, B. Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium. Cells 2022, 11, 461. https://doi.org/10.3390/cells11030461
Di Mattia M, Mauro A, Delle Monache S, Pulcini F, Russo V, Berardinelli P, Citeroni MR, Turriani M, Peserico A, Barboni B. Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium. Cells. 2022; 11(3):461. https://doi.org/10.3390/cells11030461
Chicago/Turabian StyleDi Mattia, Miriam, Annunziata Mauro, Simona Delle Monache, Fanny Pulcini, Valentina Russo, Paolo Berardinelli, Maria Rita Citeroni, Maura Turriani, Alessia Peserico, and Barbara Barboni. 2022. "Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium" Cells 11, no. 3: 461. https://doi.org/10.3390/cells11030461
APA StyleDi Mattia, M., Mauro, A., Delle Monache, S., Pulcini, F., Russo, V., Berardinelli, P., Citeroni, M. R., Turriani, M., Peserico, A., & Barboni, B. (2022). Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium. Cells, 11(3), 461. https://doi.org/10.3390/cells11030461











