Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
3. Results
3.1. Study Selection
3.1.1. Screening of Preclinical Studies
3.1.2. Recruitment Status of GBE Clinical Trials
3.2. Article Characteristics
3.2.1. Analysis of Included Preclinical Studies
3.2.2. Analysis of Included Clinical Studies
3.3. Meta-Analysis Results
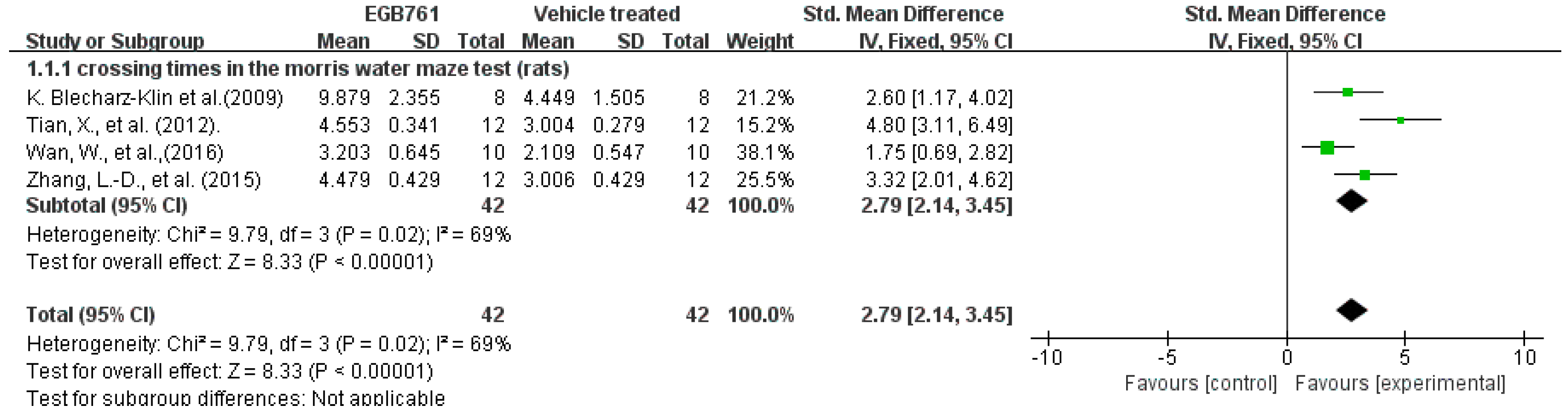
3.3.1. Behavioral Test Analysis in Preclinical Studies
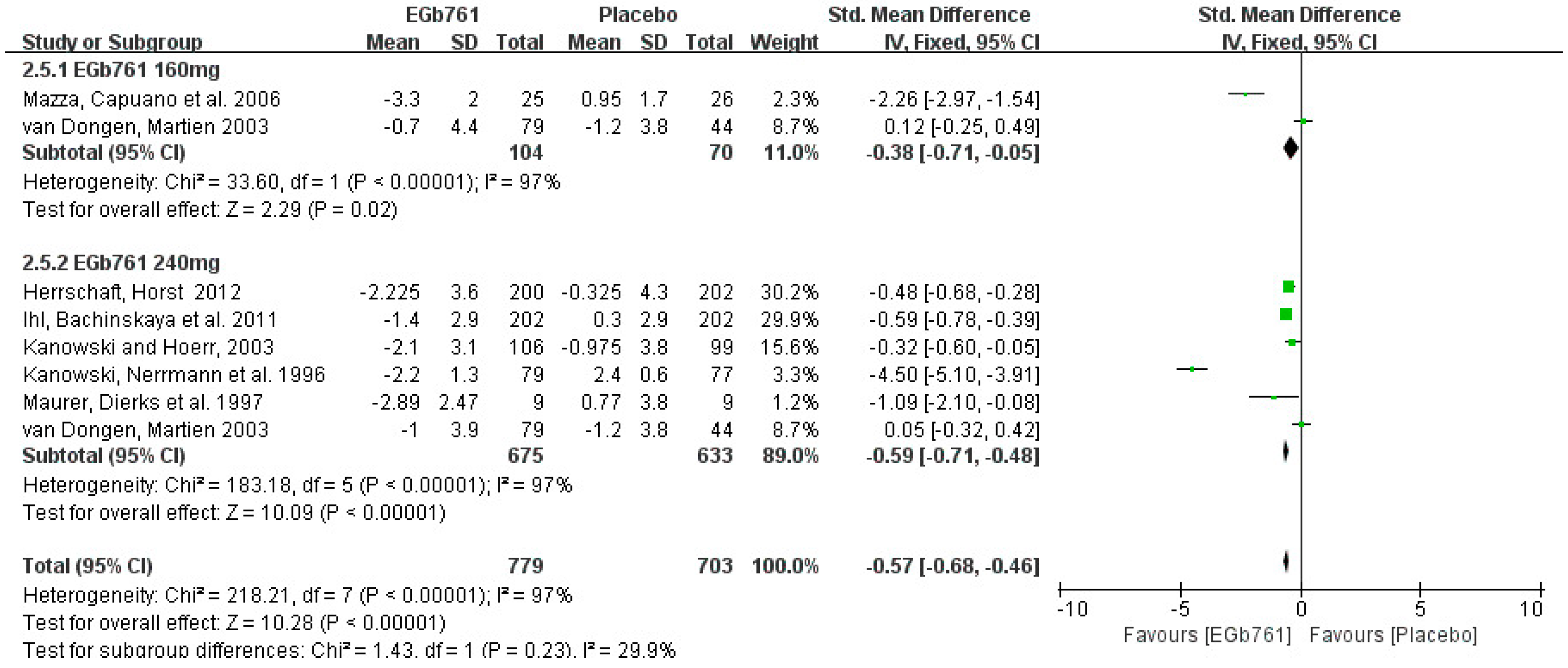
3.3.2. Cognition Improvement Analysis in Clinical Studies
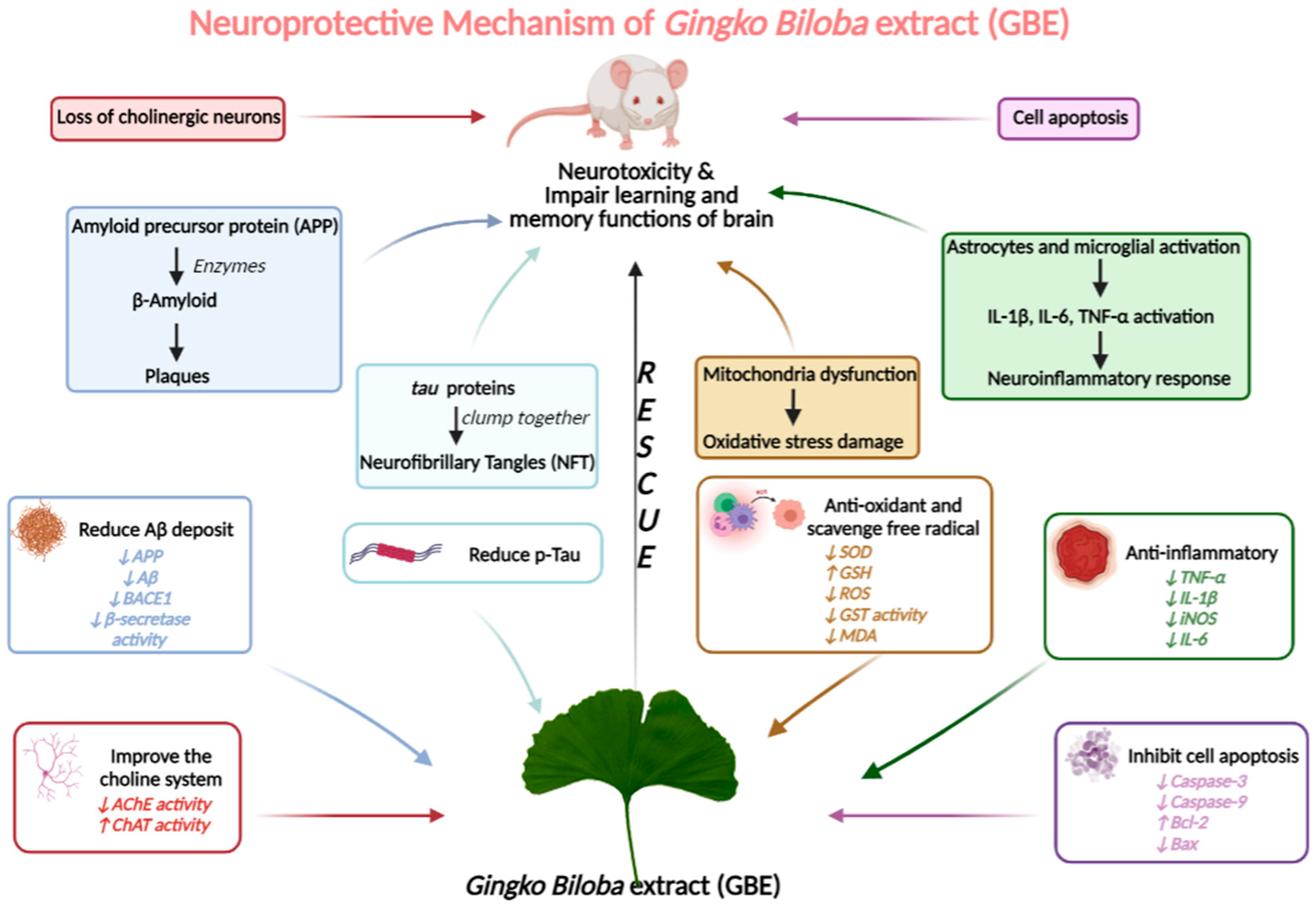
3.4. Neuroprotective Mechanism Analysis
3.4.1. GBE Can Significantly Reduce Aβ Deposits and p-Tau in AD Models
3.4.2. GBE Displays the Antioxidant Activity
3.4.3. GBE Inhibits Cell Apoptosis
3.4.4. GBE Has Anti-Inflammatory Activity
3.4.5. GBE Significantly Improves the Choline System
3.5. Methodological Quality Analysis
3.5.1. Preclinical Studies
3.5.2. Clinical Studies
4. Discussion
4.1. Active Components in Gingko biloba Extract with Anti-AD Properties
4.2. Article Characteristics
4.3. Future Perspective of GBE
4.3.1. Nanomedicine Application
4.3.2. Drug Combination
4.3.3. Application in Other Neurodegenerative Diseases
4.3.4. Comparison of GBE Effects in Rodents and in Humans
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khachaturian, Z.S. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. Alzheimer’s Dement. 2008, 4, 315. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- Guillozet, A.L.; Weintraub, S.; Mash, D.C.; Mesulam, M.M. Neurofibrillary Tangles, Amyloid, and Memory in Aging and Mild Cognitive Impairment. Arch. Neurol. 2003, 60, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AsI, M. Researching Alzheimer’s Medicines: Setbacks and Stepping Stones Summer. PhMRA 2015, 2015, 1–20. [Google Scholar]
- Seo, D.-O.; Boros, B.D.; Holtzman, D.M. The microbiome: A target for Alzheimer disease? Cell Res. 2019, 29, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Drieu, K.; Jaggy, H. History, Development and Constituents of EGb 761; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000. [Google Scholar]
- EGb 761. Drugs R D 2003, 4, 188–193. [CrossRef]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Maclennan, K.; Darlington, C.L. The neuroprotective properties of the Ginkgo biloba leaf: A review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 1996, 50, 131–139. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Ward, C.P.; Redd, K.; Williams, B.M.; Caler, J.R.; Luo, Y.; McCoy, J.G. Ginkgo biloba extract: Cognitive enhancer or antistress buffer. Pharmacol. Biochem. Behav. 2002, 72, 913–922. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Zhu, Q.; Zhang, S.; Ouyang, D.; Lu, J.-H. Resveratrol in experimental Alzheimer’s disease models: A systematic review of preclinical studies. Pharmacol. Res. 2019, 150, 104476. [Google Scholar] [CrossRef] [PubMed]
- Stackman, R.W.; Eckenstein, F.; Frei, B.; Kulhanek, D.; Nowlin, J.; Quinn, J.F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp. Neurol. 2003, 184, 510–520. [Google Scholar] [CrossRef]
- Gong, Q.-H.; Wu, Q.; Huang, X.-N.; Sun, A.-S.; Shi, J.-S. Protective effects of Ginkgo biloba leaf extract on aluminum-induced brain dysfunction in rats. Life Sci. 2005, 77, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wu, J.; Cai, J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. J. Cereb. Blood Flow Metab. 2006, 148, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Qi-Hai, G.; Qin, W.; Xie-Nan, H.; An-Sheng, S.; Jing, N.; Jing-Shan, S. Protective effect of Ginkgo biloba leaf extract on learning and memory deficit induced by aluminum in model rats. Chin. J. Integr. Med. 2006, 12, 37–41. [Google Scholar] [CrossRef]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007, 21, 2400–2408. [Google Scholar] [CrossRef] [Green Version]
- Blecharz-Klin, K.; Piechal, A.; Joniec, I.; Pyrzanowska, J.; Widy-Tyszkiewicz, E. Pharmacological and biochemical effects of Ginkgo biloba extract on learning, memory consolidation and motor activity in old rats. Acta Neurobiol. Exp. 2009, 69, 217–231. [Google Scholar]
- Flaks, M.K.; Forlenza, O.V.; Pereira, F.S.; Viola, L.F.; Yassuda, M.S. Short Cognitive Performance Test: Diagnostic Accuracy and Education Bias in Older Brazilian Adults. Arch. Clin. Neuropsychol. 2009, 24, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Wang, J.; Dai, J.; Yang, L.; Zhang, L.; Shen, S.; Huang, P. Hyperbaric Oxygen and Ginkgo Biloba Extract Inhibit Aβ25-35-induced Toxicity and Oxidative Stress in vivo: A Potential Role in Alzheimer’s Disease. Int. J. Neurosci. 2012, 122, 563–569. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, L.; Wang, J.; Dai, J.; Shen, S.; Yang, L.; Huang, P. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25–35-induced oxidative stress and neuronal apoptosis in rats. Behav. Brain Res. 2013, 242, 1–8. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Nickmahzar, E.G.; Babakordi, F. The effect of Ginkgo biloba extract on scopolamine-induced apoptosis in the hippocampus of rats. Anat. Sci. Int. 2013, 88, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.-D.; Ma, L.; Dai, J.-G.; Chang, L.-G.; Huang, P.-L.; Tian, X.-Q. Hyperbaric Oxygen and Ginkgo Biloba Extract Ameliorate Cognitive and Memory Impairment via Nuclear Factor Kappa-B Pathway in Rat Model of Alzheimer’s Disease. Chin. Med. J. 2015, 128, 3088–3093. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schötz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhang, C.; Danielsen, M.; Li, Q.; Chen, W.; Chan, Y.; Li, Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016, 81, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, M.; Xinghua, L.; Mahaman, Y.A.R.; Bao, J.; Huang, F.; Xiaochuan, W.; Liu, X.; Wang, Q.; Wang, J.-Z.; et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr. Alzheimer Res. 2017, 15, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Ranawat, P.; Sharma, N.; Nehru, B. Ginkgo biloba attenuates aluminum lactate-induced neurotoxicity in reproductive senescent female rats: Behavioral, biochemical, and histopathological study. Environ. Sci. Pollut. Res. 2019, 26, 27148–27167. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Sharma, S.; Ranawat, P.; Nehru, B. Modulatory Effects of Ginkgo biloba Against Amyloid Aggregation Through Induction of Heat Shock Proteins in Aluminium Induced Neurotoxicity. Neurochem. Res. 2020, 45, 465–490. [Google Scholar] [CrossRef]
- Schaffler, K.; Reeh, P. Double blind study of the hypoxia protective effect of a standardized Ginkgo biloba preparation after repeated administration in healthy subjects. Arzneimittelforschung 1985, 35, 1283–1286. [Google Scholar]
- Wesnes, K.; Simmons, D.; Rook, M.; Simpson, P. A double-blind placebo-controlled trial of tanakan in the treatment of idiopathic cognitive impairment in the elderly. Hum. Psychopharmacol. Clin. Exp. 1987, 2, 159–169. [Google Scholar] [CrossRef]
- Rai, G.S.; Shovlin, C.; Wesnes, K.A. A double-blind, placebo controlled study of Ginkgo biloba extract (‘Tanakan’) in elderly outpatients with mild to moderate memory impairment. Curr. Med. Res. Opin. 1991, 12, 350–355. [Google Scholar] [CrossRef]
- Kanowski, S.; Herrmann, W.; Stephan, K.; Wierich, W.; Hörr, R. Proof of Efficacy of the Ginkgo Biloba Special Extract EGb 761 in Outpatients Suffering from Mild to Moderate Primary Degenerative Dementia of the Alzheimer Type or Multi-infarct Dementia. Pharmacopsychiatry 1996, 29, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Ihl, R.; Dierks, T.; Frölich, L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. J. Psychiatr. Res. 1997, 31, 645–655. [Google Scholar] [CrossRef]
- BBarsa, P.L.L.; Kieserc, M.; Itilb, K.Z. A 26-week analysis of a double-blind, placebo-controlled trial of the ginkgo biloba extract EGb 761® in Dementia. Dement. Geriatr. Cogn. Disord. 2000, 11, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kanowski, S.; Hoerr, R. Ginkgo biloba Extract EGb 761® in Dementia: Intent-to-treat Analyses of a 24-week, Multi-center, Double-blind, Placebo-controlled, Randomized Trial. Pharmacopsychiatry 2003, 36, 297–303. [Google Scholar] [CrossRef]
- Mazza, M.; Capuano, A.; Bria, P.; Mazza, S. Ginkgo biloba and donepezil: A comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur. J. Neurol. 2006, 13, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Napryeyenko, O.; Borzenko, I. Ginkgo biloba Special Extract in Dementia with Neuropsychiatric Features. Arzneimittelforschung 2007, 57, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ihl, R.; Bachinskaya, N.; Korczyn, A.D.; Vakhapova, V.; Tribanek, M.; Hoerr, R.; Napryeyenko, O. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2010, 26, 1186–1194. [Google Scholar] [CrossRef]
- Amieva, H.; Meillon, C.; Helmer, C.; Barberger-Gateau, P.; Dartigues, J.F. Ginkgo Biloba Extract and Long-Term Cognitive Decline: A 20-Year Follow-Up Population-Based Study. PLoS ONE 2013, 8, e52755. [Google Scholar] [CrossRef] [Green Version]
- Canevelli, M.; Adali, N.; Kelaiditi, E.; Cantet, C.; Ousset, P.-J.; Cesari, M. Effects of Gingko biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: Data from the ICTUS study. Phytomedicine 2014, 21, 888–892. [Google Scholar] [CrossRef] [Green Version]
- Hoerr, R.; Nacu, A. Neuropsychiatric symptoms in dementia and the effects of Ginkgo biloba extract EGb 761® treatment: Additional results from a 24-week randomized, placebo-controlled trial. Open Access J. Clin. Trials 2016, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Subhan, Z.; Hindmarch, I. The psychopharmacological effects of Ginkgo biloba extract in normal healthy volunteers. Int. J. Clin. Pharmacol. Res. 1984, 4, 89–93. [Google Scholar] [PubMed]
- Lon, S.; Schneider, S.T.; DeKosky, M.R.; Farlow, P.N.; Tariot, H.R.; Kieser, M. A randomized, double-blind, placebo-controlled trial of two doses of ginkgo biloba extract in dementia of the Alzheimer’s type. Curr. Alzheimer Res. 2005, 2, 541–551. [Google Scholar]
- McCarney, R.; Fisher, P.; Iliffe, S.; van Haselen, R.; Griffin, M.; Van Der Meulen, J.; Warner, J. Ginkgo biloba for mild to moderate dementia in a community setting: A pragmatic, randomised, parallel-group, double-blind, placebo-controlled trial. Int. J. Geriatr. Psychiatry 2008, 23, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Snitz, B.E.; O’Meara, E.S.; Carlson, M.C.; Arnold, A.M.; Ives, D.G.; Rapp, S.R.; Saxton, J.; Lopez, O.L.; Dunn, L.O.; Sink, K.; et al. Ginkgo biloba for Preventing Cognitive Decline in Older AdultsA Randomized Trial. JAMA J. Am. Med. Assoc. 2009, 302, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Vellas, B.; Coley, N.; Ousset, P.-J.; Berrut, G.; Dartigues, J.-F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef]
- Nasab, N.M.; Bahrammi, M.A.; Nikpour, M.R.A.; Rahim, F.; Naghibis, S.N. Efficacy of rivastigmine in comparison to ginkgo for treating Alzheimer’s dementia. J. Pak. Med. Assoc. 2012, 62, 677–680. [Google Scholar]
- Zhu, Q.; Zhuang, X.; Lu, J. Neuroprotective effects of baicalein in animal models of Parkinson’s disease: A systematic review of experimental studies. Phytomedicine 2019, 55, 302–309. [Google Scholar] [CrossRef]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.-L.; Manaye, K.; Khan, I.; Luo, Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993.
- Tombaugh, T.; McDowell, I.; Kristjansson, B.; Hubley, A. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychol. Assess. 1996, 8, 48. [Google Scholar] [CrossRef]
- Kueper, J.K.; Speechley, M.; Montero-Odasso, M. The Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in Pre-Dementia Populations. A Narrative Review. J. Alzheimer’s Dis. 2018, 63, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Paul, R.; Solomon, P.; Felicity Adams, B.; Amanda Silver, B.; Jill Zimmer, B.; Richard DeVeaux, P. Ginkgo for Memory Enhancement: A Randomized Controlled Trial; American Medical Association: Chicago, IL, USA, 2002. [Google Scholar]
- Schoenfeld, R.; Schiffelholz, T.; Beyer, C.; Leplow, B.; Foreman, N. Variants of the Morris water maze task to comparatively assess human and rodent place navigation. Neurobiol. Learn. Mem. 2017, 139, 117–127. [Google Scholar] [CrossRef]
- Griggs, B. The History and Evolution of Western Herbal Medicine; Healing Arts Press: London, UK; Rochester, VT, USA, 1997; p. 448. [Google Scholar]
- Yue, T.L.; Feuerstein, G.Z. Platelet-activating factor: A putative neuromodulator and mediator in the pathophysiology of brain injury. Crit. Rev. Neurobiol. 1994, 8, 11–24. [Google Scholar] [PubMed]
- Kaur, N.; Dhiman, M.; Perez-Polo, J.R.; Mantha, A.K. Ginkgolide B revamps neuroprotective role of apurinic/apyrimidinic endonuclease 1 and mitochondrial oxidative phosphorylation against Aβ25-35-induced neurotoxicity in human neuroblastoma cells. J. Neurosci. Res. 2015, 93, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.; Kaur, S.; Kaur, N.; Dhiman, M.; Mantha, A.K. Phytochemical Ginkgolide B Attenuates Amyloid-β1-42 Induced Oxidative Damage and Altered Cellular Responses in Human Neuroblastoma SH-SY5Y Cells. J. Alzheimer’s Dis. 2017, 60, S25–S40. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef] [Green Version]
- Gohil, K.; Moy, R.K.; Farzin, S.; Maguire, J.J.; Packer, L. mRNA expression profile of a human cancer cell line in response to Ginkgo biloba extract: Induction of antioxidant response and the Golgi system. Free Radic. Res. 2000, 33, 831–849. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.; Kromhout, D.; Hollman, P.C.H. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Alzheimer’s disease: Recent progress and prospects—Part III. Harv. Ment. Health Lett. 2001, 18, 1–4.
- Shen, Y.; Wang, H.; Sun, Q.; Yao, H.; Keegan, A.P.; Mullan, M.; Wilson, J.; Lista, S.; Leyhe, T.; Laske, C.; et al. Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer’s Disease Dementia in Individuals With Mild Cognitive Impairment. Biol. Psychiatry 2018, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P.; Censi, R.; Gigliobianco, M.R.; Zerrillo, L.; Magnoni, F.; Agas, D.; Quaglia, W.; Lupidi, G. Nano-medicine Improving the Bioavailability of Small Molecules for the Prevention of Neurodegenerative Diseases. Curr. Pharm. Des. 2017, 23, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, C.; Fan, G.; Li, T.; Gong, H.; Cao, F. Ginkgo biloba extracts-loaded starch nano-spheres: Preparation, characterization, and in vitro release kinetics. Int. J. Biol. Macromol. 2018, 106, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fu, Y.; Cole, A.J.; Liu, J.; Wang, J. Co-encapsulation and sustained-release of four components in ginkgo terpenes from injectable PELGE nanoparticles. Fitoterapia 2012, 83, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Hopfeld, J.; Lau, H.; Klein, J. Effects of Ginkgo biloba Extract EGb 761, Donepezil and their Combination on Central Cholinergic Function in Aged Rats. J. Pharm. Pharm. Sci. 2015, 18, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Hashiguchi, M.; Ohta, Y.; Shimizu, M.; Maruyama, J.; Mochizuki, M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ihl, R. Effects of Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: Review of recently completed randomised, controlled trials. Int. J. Psychiatry Clin. Pract. 2013, 17, 8–14. [Google Scholar] [CrossRef]
- Rojas, P.; Montes, S.; Serrano-García, N.; Rojas-Castañeda, J. Effect of EGb761 supplementation on the content of copper in mouse brain in an animal model of Parkinson’s disease. Nutrition 2009, 25, 482–485. [Google Scholar] [CrossRef]
- Rojas, P.; Serrano-García, N.; Mares-Sámano, J.J.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ögren, S.O. EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: Role of oxidative stress. Eur. J. Neurosci. 2008, 28, 41–50. [Google Scholar] [CrossRef]
- Ahmad, M.; Saleem, S.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Chaturvedi, R.K.; Agrawal, A.K.; Islam, F. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: Neurobehavioural, neurochemical and immunohistochemical evidences. J. Neurochem. 2005, 93, 94–104. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, P.; Li, J.; Liu, T.; Zhang, Y.; Wang, Q.; Zhang, J.; Lu, X.; Fan, X. Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease. J. Pharm. Anal. 2021, 11, 220–231. [Google Scholar] [CrossRef] [PubMed]







| Inclusion Criteria: |
| 1. Parallel experiments were conducted to evaluate the effects of EGB761 on AD protection in vivo. |
| 2. Laboratory animals of any species, age, sex, or strain to induce AD models were included. |
| 3. Any kind of EGB761 intervention compared with a control group was included. Dosages, methods of treatment, and curative times were not limited. |
| Exclusion Criteria: |
| Duplicated references; articles with incorrect and incomplete data; no access to the databases; review articles, comments, letters, and case reports. |
| Inclusion Criteria: |
| 1. The clinical trials were designed as double-blind randomized placebo-controlled trials. |
| 2. The patients, by age, sex, administration route and duration, dosage, were included in the trials. |
| 3. Specific and reliable criteria for the AD assessment, such as the SKT and MMSE, were included. |
| Exclusion Criteria: |
| Duplicated references; repetitive clinical data; articles with incorrect and incomplete data; no access to the databases; review articles, comments, letters, and case reports. |
| Author | Animal Model | Treatment | Method | Result | Mechanism of Action |
|---|---|---|---|---|---|
| Ward, C. P. et al. (2002) [12] | C57BL/6 mice, male, 20 months old | EGb 761 (100 mg/kg/day), orally for 82 consecutive days | 1. Morris water maze test | ↑the time of hidden platform ↓platform crossings (probe test) | Improved the learning and memory cognition |
| 2. Elevated plus-maze test | ↓time on the open arms | ||||
| 3. Protein levels of CREB | no significant differences | Antioxidant properties | |||
| Stackman, R. W. et al. (2003) [14] | Tg2576 mice, female, 8 months old | EGb 761 (70 mg/kg/day), orally for 6 months | 1. Morris water maze test | ↓average distance to the platform ↑search ratio | Alleviated the spatial learning impairment |
| 2. Fibrillar and soluble β-amyloid and protein oxidation products (ELISA) | ↓soluble β-amyloid | N/A | |||
| 3. Histological assessment | ↓β-amyloid | ||||
| 4. Protein carbonyl | ↑protein carbonyls | Antioxidant properties | |||
| Gong, Q. H. et al. (2005) [15] | Wistar rats, male, 8–12 weeks old, daily AlCl3 solution, (500 mg/kg, i.g, 0.5 mL/100 g), gavage for 1 month | EGb761 (50 mg/kg/day, 100 mg/kg/day, 200 mg/kg/day), orally for 2 months | 1. Morris water maze test | ↓searching distance ↓escape latency | Ameliorated the learning and memory abilities |
| 2. Level of caspase-3 | ↓caspase-3 | Antiapoptosis | |||
| 3. Level of APP (immunohistochemistry) | ↓APP | N/A | |||
| Wang, Y. et al. (2006) [16] | 1. Wistar rats, male, 12–13 weeks old 2. Wistar rats, male, 74–78 weeks old (aged) | 1. EGb761 (30 mg/kg/day) 2. EGb761 (60 mg/kg/day), orally for 30 consecutive days | 1. Morris water maze test | ↓escape latency ↑search time | Improved spatial learning in aged animals |
| 2. Changes in synaptic plasticity | ↑hippocampal LTP | N/A | |||
| Gong, Q. H. et al. (2006) [17] | Wistar rats, male, 8–12 weeks old, daily 50 g/L AICI3, gavage for 2 months | EGb761 (50 mg/kg/day, 100 mg/kg/day, 200 mg/kg/day), orally for 2 months | 1. Morris water maze test | ↓escape latency ↓searching distance | Reduced learning and memory deficits |
| 2. Effect of AChE activity | ↓AChE activity | Cholinergic improvement | |||
| Tchantchou, F. et al. (2007) [18] | 1. TgAPP/PS1 founder mice, 6 months old 2. TgAPP/PS1 founder mice, 22 months old | EGb761 (100 mg/kg/day), orally for 1 month | 1. Determine the neurogenic potential | ↑cell proliferation in the hippocampus | Induced neurogenesis as compensation |
| 2. Levels of Aβ and CREB/pCREB | ↓Aβ oligomers ↑pCREB levels in the hippocampus | N/A | |||
| Blecharz-Klin, K. et al. (2009) [19] | Wistar rats, male 18 months old | 1. EGb761 (50 mg/kg b.w./day); 2. EGb761 (100 mg/kg b.w./day); 3. EGb761 (150 mg/kg b.w./day); orally for 3 months | 1. Morris water maze test | ↓crossings ↓escape latency ↓mean swimming speed | Improved spatial memory |
| 2. Hole-board test | ↑motor activity | ||||
| 3. HPLC detects the levels of DA, 5-HT, NA, and HVA | ↑NA in prefrontal cortex and hippocampus ↓DA in prefrontal cortex and hippocampus ↑DOPAC in the prefrontal cortex ↓DOPAC in hippocampus ↑5-HT in the striatum | Neurotransmitter balance regulation | |||
| Hou, Y. et al. (2010) [20] | TgAPP/PS1 mice, male, 8 months old | 1. Ginkgo biloba extract (50 mg/kg/day),gavage for 4 months; 2. flavonol (50 mg/kg/day), i.p. for 7 days | 1. Morris water maze test | ↓time needed to find the platform | Improved impaired spatial learning |
| 2. Levels of BDNF, pCREB, and Aβ | ↑BDNF in neurons and hippocampus ↓both intracellular and medium Aβ levels | NMDA receptor Antagonist Anti-inflammatory activity | |||
| 3. Immunohistochemistry of Aβ deposition | ↓Aβ deposition and plaque formation in hippocampus | N/A | |||
| Tian, X. et al. (2012) [21] | Sprague–Dawley rats, male, 3–4 months old, Aβ25-35 (1 µg/µL), i.c.v. | EGb761 (40 mg/tablets), gavage for 20 days | 1. Morris water maze test | ↓escape latencies ↑platform crossing times ↑percentage of swimming time in Quadrant 1 | Improved the learning and memory cognition |
| 2. Histopathological changes in Aβ | ↓density of the damaged neurons ↑neuronal number | Anti-inflammatory activity | |||
| 3. Activity of SOD, MDA, and NO | ↓SOD ↓MDA ↓NO | Antioxidant properties | |||
| Tian, X. et al. (2013) [22] | Sprague–Dawley rats, male, 4–5 months old, Aβ25–35 (1 µg/µL), i.c.v. | EGB761 (20 mg/kg/day), gavage for 20 days | 1. Morris water maze test | ↓escape latency ↑platform crossings | Improved the learning and memory cognition |
| 2. Levels of SOD, GSH, and MDA | ↓SOD ↑GSH ↓MDA | Antioxidant properties | |||
| 3. Levels of caspase-9 and caspase-3 | ↓caspase-9 ↓caspase-3 | Inhibited cell apoptosis | |||
| 4. TUNEL staining | ↓neuronal apoptosis | ||||
| 5. RT-PCR of Bcl-2 | and Bax ↑Bcl-2 ↓Bax | Inhibited cell apoptosis | |||
| Jahanshahi, M. et al. (2013) [23] | Wistar rats, male, Scopolamine (3 mg/kg), intraperitoneal injection | Ginkgo biloba extract (40 and 80 mg/kg, IP), everyday injection for a week | 1. TUNEL staining | ↓apoptotic cells in the hippocampus | Antioxidant and hydroxyl radical scavenging activity |
| Zhang, L.-D. et al. (2015) [24] | Sprague–Dawley rats, male, 5–6 months old, Aβ25–35 (10 μL; 1 g/L), i.c.v. | EGB761 (20 mg/kg/day), gavage for 20 days | 1. Morris water maze test | ↑times of crossing the former platform ↑percentage of time spent in the quadrant | Improved cognitive and memory capacities |
| 2. TUNEL staining | ↓brown precipitate (apoptosis identification) | Inhibited cell apoptosis | |||
| 3. Levels of p-IKKα/β, p-IκBα, and p-NFκB | ↑p-IKKα/β ↑p-IκBα ↑p-NFκB | Anti-inflammatory activity | |||
| Liu, X. et al. (2015) [25] | TgCRND8 APP-transgenic mice, female, 2 months old | EGb761 (600 mg/kg/day) (0.6%), orally for 5 months | 1. Barnes maze test | ↓time and ↓distance to reach the escape chamber | Improved cognitive function |
| 2. Level of Aβ (ELISA) (%) | ↓Aβ | N/A | |||
| 3. Immunofluorescent staining of Aβ | |||||
| 4. Histological analysis of Iba1 | ↓Iba1 positive cell number | Neuroinflammatory inhibition | |||
| 5. Levels of tnf-α, il-1β, ccl-2, and IL-10 | ↓TNF-α, IL-1β, ccl-2, iNOS, and IL-10 | ||||
| Wan, W. et al. (2016) [26] | APP/PS1 transgenic mice, male, 2 months old | EGb761 (50 mg/kg/day), orally for 6 months | 1. Morris water maze test | ↓escape latency ↓time of passing the platform ↑crossing times | Improved cognitive function |
| 2. Level of Aβ (ELISA) | ↓Aβ | N/A | |||
| 3. Ratio of fluorescence intensity | ↑microglia around the plaque | Attenuated inflammatory reactions | |||
| Zeng, K. et al. (2018) [27] | Sprague–Dawley rats, male, 8 weeks old, Hhcy (400 μg/kg/day), for 14 days i.p. | EGb761 (400 mg/kg/day), gavage for 7 days | 1. Morris water maze test | ↓escape latency | Ameliorated memory deficits |
| 2. Levels of SOD and MDA | ↓SOD↓MDA | Antioxidant properties | |||
| 3. Levels of tau phosphorylation, PSD95, and synapsin-1 | ↓tau phosphorylation ↑PSD95 ↑synapsin-1 | Attenuated oxidative damage | |||
| Verma, S. et al. (2019) [28] | Sprague–Dawley rats, female, 12 months old, Al(lac)3 (10 mg/kg b.wt), daily for 6 weeks | Ginkgo biloba extract, EGb761 (100 mg/kg/day), orally for 6 weeks | 1. Morris water maze test | ↓time to find the platform ↓escape latency | Improved spatial memory |
| 2. Histopathological changes in Aβ | ↓ThT positive cells in hippocampusand cortex ↓Congo red | Antioxidative stress | |||
| 3. Levels of 5-HT, GSH, GST, and SOD | ↑5-HT↓SOD↑GSH↓GST | ||||
| 4. AChE activity | ↓AChE activity in the hippocampus and cortex | ||||
| Verma, S. et al. (2020) [29] | Sprague–Dawley rats, female, 12 months old, Al(lac)3 (10 mg/kg b.wt), daily for 6 weeks | Ginkgo biloba extract, EGb761 (100 mg/kg/day), orally for 6 weeks | 1. Morris water maze test | ↓escape latency | Prevented behavioral impairments |
| 2. Level of ROS | ↓ROS | Antioxidative stress | |||
| 3. Protein level of APP, Aβ, and p-Tau (ELISA) | ↓APP ↓Aβ ↓p-Tau | N/A | |||
| 4. Histopathological changes | ↓silver positive deposits in CA1, CA3 ↓congo red positive deposits in CA1, CA3 ↓ThT positive deposits | Antioxidative stress | |||
| 5. AchE activity | ↓AChE enzyme activity | Cholinergic improvement Neurotransmitter balance regulation | |||
| 6. Level of MAO-B | ↓MAO-B enzyme activity | ||||
| 7. Immunohistochemistry of Aβ (17–23) | ↓Aβ (17–23) | N/A | |||
| Study Author, Date | Country | Inclusion Criteria | Setting | Duration | Treatment | Groups | Age | Baseline Scale | Withdrawal Rate | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | Age | Female (%) | ||||||||||
| Effective | ||||||||||||
| Schaffler and Reeh, 1985 [30] | United Kingdom | / | Normal healthy volunteers | 2 weeks | EGB (Tebonin) 80 mg/day | EGB: n = 4 Placebo: n = 4 | 27 | / | / | 27.3 ± 2.6 | 0 | / |
| Wesnes et al., 1987 [31] | United Kingdom | Crichton geriatric behavioral scale > 14 | Outpatient | 12 weeks | EGB (Tanakan) 120 mg/day | EGB: n = 27 Placebo: n = 27 | 62~85 | / | / | 70.7 ± 7.1 71.3 ± 6.6 | 30% 44% | 7% |
| Rai et al., 1991 [32] | United Kingdom | NINCDS-ADRDA diagnostic criteria | Outpatient | 6 months | EGB (Tanakan) 120 mg/day | EGB: n = 12 Placebo: n = 15 | >50 | MMSE | 26.8 24.3 | 73.4 ± 7.3 78.3 ± 5.9 | 67% 80% | 13% |
| Kendrick digit copying task | 106.6 94.53 | |||||||||||
| Kendrick object learning task | 93.17 87.27 | |||||||||||
| Kanowski, Nerrmann et al., 1996 [33] | Germany | SKT: 6~18; MMSE: 13~25 | Outpatient | 24 weeks | EGb761 240 mg/day | EGb761: n = 79 Placebo: n = 77 | >55 | SKT | 10.2 ± 3.0 11.2 ± 3.4 | 70 ± 10 68 ± 10 | 66% 69% | 30% |
| Maurer, Dierks et al., 1997 [34] | Germany | DSM-III-R and ICD-10 criteria; Hachinski ischemic score < 4 mean; BCRS score 3–5 | Outpatient | 12 weeks | EGb761 240 mg/day | EGb761: n = 10 Placebo: n = 10 | 50~80 | SKT | 19.7 ± 6.4 18.1 ± 9.4 | 68.5 ± 6 60.6 ± 8.2 | 56% 45% | 10% |
| ADAS-Cog | 31.2 ± 12.6 36.1 ± 15.2 | |||||||||||
| Barsa, Kieserc et al., 2000 [35] | United States | DSM-III-R and ICD-10 criteria; MMSE: 9~26; global deterioration scale: 3~6 | Outpatient | 26 weeks | EGb761 120 mg/day | EGb761: n = 166 Placebo: n = 161 | >45 | MMSE | 21.1 ± 5.8 21.2 ± 5.5 | 69 ± 10 69 ± 10 | 51% 56% | 21% |
| ADAS-Cog | 20.0 ± 16.0 20.5 ± 14.7 | |||||||||||
| Kanowski and Hoerr, 2003 [36] | Germany | DSM-III-R and ICD-10 criteria; SKT: 6~18; MMSE: 13~25 | Outpatient | 24 weeks | EGb761 240 mg/day | EGb761: n = 106 Placebo: n = 99 | >55 | MMSE | 21.6 ± 2.6 21.5 ± 2.4 | 72 ± 10 72 ± 10 | 68% 71% | 7.65% |
| SKT | 10.5 ± 3.2 11.2 ± 3.3 | |||||||||||
| ADAS-Cog | 19.0 ± 4.1 19.9 ± 4.3 | |||||||||||
| Mazza, Capuano et al., 2006 [37] | Italy | Brief cognitive rating scale: 3~5; Hachinski ischemic score < 4; SKT: 8~23; MMSE: 13~25 | Outpatient | 24 weeks | EGb761 160 mg/day | EGb761: n = 25 donepezil: n = 25 Placebo: n = 16 | 50~80 | MMSE | 18.8 ± 3.6 18.8 ± 3.6 | 66.2 ± 6 64.5 ± 6 69.8 ± 3 | 52% 48% 61% | 19.70% |
| SKT | 16.5 ± 3.1 15.9 ± 3.9 | |||||||||||
| Napryeyenko and Borzenko, 2007 [38] | Ukraine | NINCDS/ADRDA diagnostic criteria: SKT: 9~23; MMSE: 14~25; ADAS-Cog: 17~35 | Outpatient | 22 weeks | EGb761 240 mg/day | EGb761: n = 198 Placebo: n = 197 | >50 | SKT | 15.6 ± 3.9 15.4 ± 3.7 | 65 ± 8 63 ± 8 | 72% 72% | 1.25% |
| Ihl, Bachinskaya et al., 2011 [39] | Ukraine | NINCDS-ADRDA criteria; SKT: 9~23; MMSE: 14~25; ADAS-Cog: 17~35 | Outpatient | 24 weeks | EGb761 240 mg/day | EGb761: n = 206 Placebo: n = 204 | >50 | SKT | 16.7 ± 3.9 17.2 ± 3.7 | 65 ± 10 65 ± 9 | 69% 66% | 6.82% |
| Herrschaft, Nacu et al., 2012 [8] | Republic of Belarus, Republic of Moldova, and Russian Federation | NINCDS-ADRDA criteria; NINDSAIREN criteria; NINDS-AIREN crtteria | Outpatients | 24 weeks | EGb761 240 mg/day | EGb761: n = 206 Placebo: n = 204 | >50 | SKT | 15.1 ± 4.1 15.3 ± 4.2 | 65.1 ± 8.8 64.9 ± 9.4 | 69.5% 69.3% | 2.00% |
| NPI | 16.8 ± 6.9 16.7 ± 6.4 | |||||||||||
| Amieva, Meillon et al., 2013 [40] | France | / | Outpatient | 20 years | EGb761 dosage unclear | EGb761: n = 589 Piracetam: n = 149 Placebo: n = 2874 | >65 | MMSE | 26.3 ± 2.9 25.7 ± 3.9 25.7 ± 3.5 | 74.8 ± 6.6 75.7 ± 6.6 75 ± 6.9 | 73.9% 61.1% 54.1% | 0 |
| Canevelli, Adali et al., 2014 [41] | Europe | NINCDS-ADRDA criteria, MMSE: 10~26 | Outpatients | 1 year | EGb761 120 mg/day | EGb761 + ChEIs: n = 29 ChEIs: n = 799 | 68~84 | MMSE | 21.2 ± 3.5 20.5 ± 3.9 | 76.2 ± 6.87 5.8 ± 7.8 | 62.1% 64.8% | 0 |
| ADAS-Cog | 15.8 ± 7.9 20.6 ± 8.9 | |||||||||||
| Hoerr and Nacu, 2016 [42] | Russian Federation, Republic of Belarus, Republic of Moldova | SKT: 9~23, mild to moderate dementia; test for the early detection of dementia with differentiation from depression ≤ 35 | Outpatient | 24 weeks | EGb761 240 mg/day | EGb761: n = 200 Placebo: n = 202 | >65 | SKT | 15.1 ± 4.1 15.3 ± 4.2 | 65.1 ± 8.8 64.9 ± 9.4 | 69.5% 69.3% | 2% |
| Ineffective | ||||||||||||
| Subhan and Hindmarch, 1984 [43] | United Kingdom | / | Normal healthy volunteers | 1 h | EGb 761 120 mg/240 mg/600 mg | EGb761(120): n = 2 EGb761(240): n = 2 EGb761(600): n = 2 Placebo: n = 2 | 32 | / | / | 32 ± 0 | 100% | / |
| Schneider, DeKosky et al., 2005 [44] | United States | NINCDS/ ADRDA criteria; modified Hachinski ischemic score < 4; MMSE: 10~24 | Outpatients | 26 weeks | EGb761 120/240 mg/day | EGb761(120): n = 169 EGb761(240): n = 170 Placebo: n = 174 | >60 | MMSE | 17.4 ± 3.8 (240) 17.9 ± 4.5 (120) 17.6 ± 3.9 | 78.6 ± 7.0 78.1 ± 7.0 77.5 ± 7.4 | 50% 56% 52% | 20.00% |
| ADAS-Cog | 24.8 ± 11.3 (240) 26.8 ± 13.7 (120) 26.2 ± 11.8 | |||||||||||
| McCarney, Fisher et al., 2008 [45] | United Kingdom | DSM-IV criteria; MMSE: 12~26 | Outpatient | 24 weeks | EGb761 120 mg/day | EGb761: n = 88 Placebo: n = 88 | >55 | MMSE | 23 22 | 79.3 ± 7.7 79.7 ± 7.5 | 58.0% 63.6% | 25.60% |
| ADAS-Cog | 20.4 ± 8.2 25 ± 10.3 | |||||||||||
| Snitz, O’Meara et al., 2009 [46] | United States | MMSE; ADAS-Cog; neuropsychological test | community-dwelling participants | 6.1 years | EGb761 240 mg/day | EGb761: n = 1545 Placebo: n = 1524 | 72~96 | MMSE | 93.4 ± 4.7 93.3 ± 4.7 | 79.1 ± 3.3 79.1 ± 3.3 | 45% 47% | 37.80% |
| ADAS-Cog | 6.5 ± 2.86.4 ± 2.7 | |||||||||||
| Vellas, Coley et al., 2012 [47] | France | MMSE: >25; covianxiety scale <6; geriatric depression scale <15 | Outpatient | 5 years | EGb761 240 mg/day | EGb761: n = 1419 Placebo: n = 1435 | >70 | MMSE | 27.6 ± 1.9 27.6 ± 1.9 | 76.3 ± 4.4 76.3 ± 4.4 | 67% 66% | 31% |
| Nasab, Bahrammi et al., 2012 [48] | Iran | DSM IV criteria; NINCDS-ADRDA criteria; MMSE: 10~24 | Outpatients | 24 weeks | EGb761 120 mg/day | EGb761: n = 25 Rivastigmine: n = 25 | 50–75 | MMSE | 15.6 ± 4.1 16.6 ± 4.0 | 65.7 ± 4.7 66.0 ± 4.6 | 52% 57.7% | 9.00% |
| Methodological Quality Scores of Included Preclinical Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total | |
| Ward, C. P. et al. (2002) [12] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Stackman, R. W. et al. (2003) [14] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Gong, Q. H. et al. (2005) [15] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Wang, Y. et al. (2006) [16] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Gong, Q. H. et al. (2006) [17] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Tchantchou, F. et al. (2007) [18] | ✔ | ✔ | ✔ | 3 | |||||
| Blecharz-Klin, K. et al. (2009) [19] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Hou, Y. et al. (2010) [50] | ✔ | ✔ | ✔ | 3 | |||||
| Tian, X. et al. (2012) [21] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Tian, X. et al. (2013) [22] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Jahanshahi, M. et al. (2013) [23] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Zhang, L.-D. et al. (2015) [24] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Liu, X. et al. (2015) [25] | ✔ | ✔ | ✔ | ✔ | 3 | ||||
| Wan, W. et al. (2016) [26] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Zeng, K. et al. (2018) [27] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Verma, S. et al. (2019) [28] | ✔ | ✔ | ✔ | ✔ | 4 | ||||
| Verma, S. et al. (2020) [29] | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| 1.Was the article published in a peer-reviewed journal? | |||||||||
| 2. Were the animals allocated to the treatment group or control group randomly during the experiment? | |||||||||
| 3. Were the outcomes assessed blindly? | |||||||||
| 4. Was the dose–response relationship assessed during the experiment? | |||||||||
| 5. Was the appropriate animal model used in the experiment? | |||||||||
| 6. Was the necessary sample size calculated to achieve sufficient power? | |||||||||
| 7. Were the animal welfare regulations complied with during the experiment? | |||||||||
| 8. Was the study free of any potential conflicts of interest? | |||||||||
| Methodological Quality Scores of Clinical Studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | |
| Schaffler and Reeh, 1985 [30] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| Wesnes et al., 1987 [31] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| Rai et al., 1991 [32] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| Kanowski, Herrmann et al., 1996 [33] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 6 | |||
| Maurer, Dierks et al., 1997 [34] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| Barsa, Kieserc et al., 2000 [35] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Kanowski and Hoerr, 2003 [36] | ✔ | ✔ | ✔ | ✔ | 5 | |||||
| Mazza, Capuano et al., 2006 [37] | ✔ | ✔ | ✔ | 3 | ||||||
| Napryeyenko and Borzenko, 2007 [38] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Ihl, Bachinskaya et al., 2011 [39] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Herrschaft, Nacu et al., 2012 [8] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 6 | |||
| Amieva, Meillon et al., 2013 [40] | ✔ | ✔ | ✔ | 3 | ||||||
| Canevelli, Adali et al., 2014 [41] | ✔ | ✔ | ✔ | 3 | ||||||
| Hoerr and Nacu, 2016 [42] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Subhan and Hindmarch, 1984 [43] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| Schneider, DeKosky et al., 2005 [44] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| McCarney, Fisher et al., 2008 [45] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Snitz, O’Meara et al., 2009 [46] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 5 | |||
| Vellas, Coley et al., 2012 [47] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 7 | ||
| Nasab, Bahrammi et al., 2012 [48] | ✔ | ✔ | ✔ | ✔ | 4 | |||||
| 1.Was the article published in a peer-reviewed journal? | ||||||||||
| 2.Were the patients allocated randomly during the clinical trial? | ||||||||||
| 3. Were the outcomes assessed blindly? | ||||||||||
| 4. Was the dose–response relationship assessed during the clinical trial? | ||||||||||
| 5. Were the withdrawals per group reported during the clinical trial? | ||||||||||
| 6. Was the necessary sample size calculated to achieve sufficient power? | ||||||||||
| 7. Was the ITT analysis (intent-to-treat analysis) conducted? | ||||||||||
| 8. Was the funding reported for the clinical trial? | ||||||||||
| 9. Was the study free of potential conflicts of interest? | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Zhu, Q.; Lu, J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells 2022, 11, 479. https://doi.org/10.3390/cells11030479
Xie L, Zhu Q, Lu J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells. 2022; 11(3):479. https://doi.org/10.3390/cells11030479
Chicago/Turabian StyleXie, Liming, Qi Zhu, and Jiahong Lu. 2022. "Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies" Cells 11, no. 3: 479. https://doi.org/10.3390/cells11030479
APA StyleXie, L., Zhu, Q., & Lu, J. (2022). Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells, 11(3), 479. https://doi.org/10.3390/cells11030479







