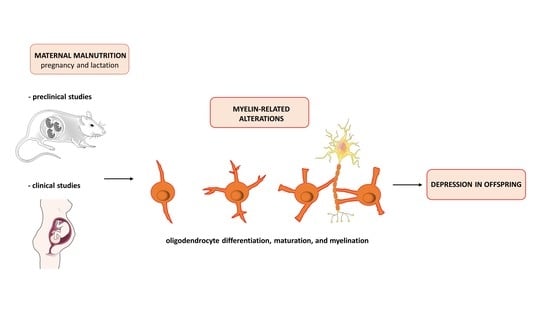
Understanding the Links among Maternal Diet, Myelination, and Depression: Preclinical and Clinical Overview
Abstract
:1. Introduction
2. Myelin—Structure and Function
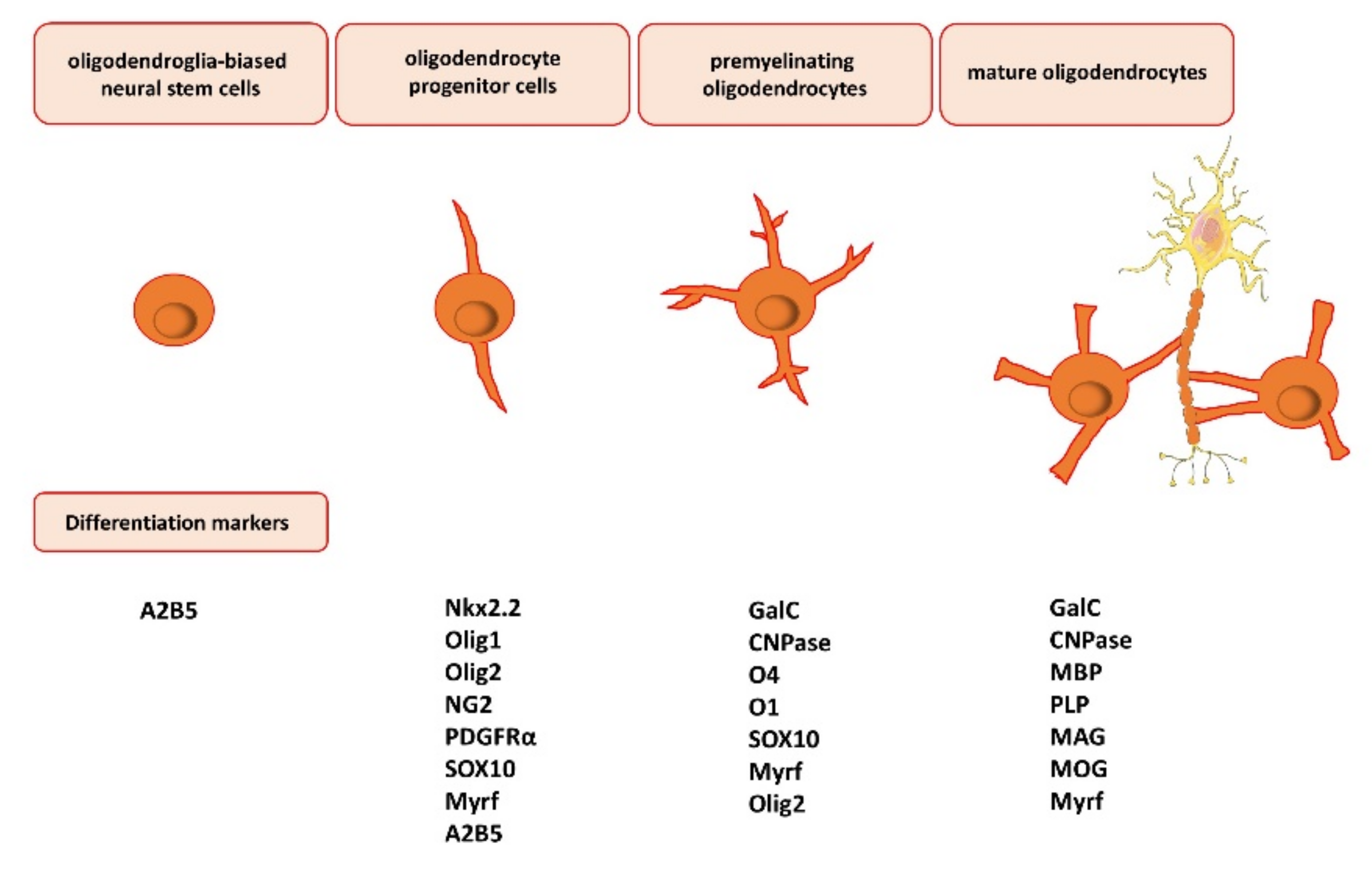
3. Oligodendrocyte Differentiation, Maturation, and Myelination
4. Myelin-Related Changes in Depression
4.1. Preclinical Studies
4.2. Human Studies
5. Crosstalk among Maternal Malnutrition, Myelination, and Depression
5.1. Preclinical Studies
5.2. Human Studies
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, E. Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci. 2019, 53, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3550. [Google Scholar] [CrossRef]
- Gawlinska, K.; Gawlinski, D.; Przegalinski, E.; Filip, M. Maternal high-fat diet during pregnancy and lactation provokes depressive-like behavior and influences the irisin/brain-derived neurotrophic factor axis and inflammatory factors in male and female offspring in rats. J. Physiol. Pharmacol. 2019, 70, 407–417. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal dietary patterns are associated with susceptibility to a depressive-like phenotype in rat offspring. Dev. Cogn. Neurosci. 2020, 47, 100879. [Google Scholar] [CrossRef]
- Gawliński, D.; Gawlińska, K.; Smaga, I. Maternal high-fat diet modulates Cnr1 gene expression in male rat offspring. Nutrients 2021, 13, 2885. [Google Scholar] [CrossRef]
- Bayandor, P.; Farajdokht, F.; Mohaddes, G.; Diba, R.; Hosseindoost, M.; Mehri, K.; Zavvari Oskuye, Z.; Babri, S. The effect of troxerutin on anxiety- and depressive-like behaviours in the offspring of high-fat diet fed dams. Arch. Physiol. Biochem. 2019, 125, 156–162. [Google Scholar] [CrossRef]
- Giriko, C.; Andreoli, C.A.; Mennitti, L.V.; Hosoume, L.F.; Souto Tdos, S.; Silva, A.V.; Mendes-da-Silva, C. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Dev. Neurosci. 2013, 31, 731–739. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef]
- Budday, S.; Steinmann, P.; Kuhl, E. Physical biology of human brain development. Front. Cell Neurosci. 2015, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Nardelli, J. Cellular and molecular introduction to brain development. Neurobiol. Dis. 2016, 92, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Bakhti, M.; Aggarwal, S.; Simons, M. Myelin architecture: Zippering membranes tightly together. Cell Mol. Life Sci. 2014, 71, 1265–1277. [Google Scholar] [CrossRef]
- Laule, C.; Vavasour, I.M.; Kolind, S.H.; Li, D.K.; Traboulsee, T.L.; Moore, G.R.; MacKay, A.L. Magnetic resonance imaging of myelin. Neurotherapeutics 2007, 4, 460–484. [Google Scholar] [CrossRef] [Green Version]
- Snaidero, N.; Simons, M. Myelination at a glance. J. Cell Sci. 2014, 127, 2999–3004. [Google Scholar] [CrossRef] [Green Version]
- Maheras, K.J.; Peppi, M.; Ghoddoussi, F.; Galloway, M.P.; Perrine, S.A.; Gow, A. Absence of claudin 11 in CNS myelin perturbs behavior and neurotransmitter levels in mice. Sci. Rep. 2018, 8, 3798. [Google Scholar] [CrossRef]
- Tomassy, G.S.; Berger, D.R.; Chen, H.H.; Kasthuri, N.; Hayworth, K.J.; Vercelli, A.; Seung, H.S.; Lichtman, J.W.; Arlotta, P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 2014, 344, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb. Cortex 2017, 27, 5001–5013. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Brady, S.T.; Witt, A.S.; Kirkpatrick, L.L.; de Waegh, S.M.; Readhead, C.; Tu, P.H.; Lee, V.M. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J. Neurosci. 1999, 19, 7278–7288. [Google Scholar] [CrossRef]
- Jang, M.; Gould, E.; Xu, J.; Kim, E.J.; Kim, J.H. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. Elife 2019, 8, e42156. [Google Scholar] [CrossRef]
- Monje, M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 2018, 41, 61–76. [Google Scholar] [CrossRef]
- Zemmar, A.; Chen, C.C.; Weinmann, O.; Kast, B.; Vajda, F.; Bozeman, J.; Isaad, N.; Zuo, Y.; Schwab, M.E. Oligodendrocyte- and neuron-specific Nogo-A restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb. Cortex 2018, 28, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef] [Green Version]
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Janowska, J.; Sypecka, J. Therapeutic strategies for leukodystrophic disorders resulting from perinatal asphyxia: Focus on myelinating oligodendrocytes. Mol. Neurobiol. 2018, 55, 4388–4402. [Google Scholar] [CrossRef] [Green Version]
- Warf, B.C.; Fok-Seang, J.; Miller, R.H. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 1991, 11, 2477–2488. [Google Scholar] [CrossRef] [Green Version]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, C.; Yaqubi, M.; Bilodeau, P.A.; Cui, Q.L.; Pernin, F.; Larochelle, C.; Ghadiri, M.; Xu, Y.K.T.; Kennedy, T.E.; Hall, J.; et al. Distinct function-related molecular profile of adult human A2B5-positive pre-oligodendrocytes versus mature oligodendrocytes. J. Neuropathol. Exp. Neurol. 2019, 78, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cai, J.; Wu, Y.; Wu, R.; Lee, J.; Fu, H.; Rao, M.; Sussel, L.; Rubenstein, J.; Qiu, M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 2001, 128, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Tatsumi, K.; Okuda, H.; Shiosaka, S.; Wanaka, A. Olig2-expressing progenitor cells preferentially differentiate into oligodendrocytes in cuprizone-induced demyelinated lesions. Neurochem. Int. 2009, 54, 192–198. [Google Scholar] [CrossRef]
- Hornig, J.; Fröb, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Hermann, A.; Brandt, M.D.; Loewenbrück, K.F.; Storch, A. “Silenced” polydendrocytes: A new cell type within the oligodendrocyte progenitor cell population? Cell Tissue Res. 2010, 340, 45–50. [Google Scholar] [CrossRef]
- Sommer, I.; Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: An immunocytological study in the central nervous system. Dev. Biol. 1981, 83, 311–327. [Google Scholar] [CrossRef]
- Brunner, C.; Lassmann, H.; Waehneldt, T.V.; Matthieu, J.M.; Linington, C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the CNS of adult rats. J. Neurochem. 1989, 52, 296–304. [Google Scholar] [CrossRef]
- Inouye, H.; Kirschner, D.A. Evolution of myelin ultrastructure and the major structural myelin proteins. Brain Res. 2016, 1641, 43–63. [Google Scholar] [CrossRef]
- Snaidero, M.W.; Czopka, T.; Hekking, L.H.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A.; Nave, K.A.; et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Zuchero, J.B.; Fu, M.M.; Sloan, S.A.; Ibrahim, A.; Olson, A.; Zaremba, A.; Dugas, J.C.; Wienbar, S.; Caprariello, A.V.; Kantor, C.; et al. CNS myelin wrapping is driven by actin disassembly. Dev. Cell 2015, 34, 152–167. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, S.; Sánchez, P.; Schmitt, S.; Snaidero, N.; Mitkovski, M.; Velte, C.; Brückner, B.R.; Alexopoulos, I.; Czopka, T.; Jung, S.Y.; et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 2015, 34, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Snaidero, N.; Velte, C.; Myllykoski, M.; Raasakka, A.; Ignatev, A.; Werner, H.B.; Erwig, M.S.; Möbius, W.; Kursula, P.; Nave, K.A.; et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017, 18, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Furusho, M.; Ishii, A.; Bansal, R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 2017, 37, 2931–2946. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.N. Glial cells promote myelin formation and elimination. Front. Cell Dev. Biol. 2021, 9, 661486. [Google Scholar] [CrossRef]
- Vancamp, P.; Demeneix, B.A.; Remaud, S. Monocarboxylate transporter 8 deficiency: Delayed or permanent hypomyelination? Front. Endocrinol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Thornton, M.A.; Hughes, E.G. Neuron-oligodendroglia interactions: Activity-dependent regulation of cellular signaling. Neurosci. Lett. 2020, 727, 134916. [Google Scholar] [CrossRef]
- Mangin, J.M.; Kunze, A.; Chittajallu, R.; Gallo, V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J. Neurosci. 2008, 28, 7610–7623. [Google Scholar] [CrossRef] [Green Version]
- Balia, M.; Vélez-Fort, M.; Passlick, S.; Schäfer, C.; Audinat, E.; Steinhäuser, C.; Seifert, G.; Angulo, M.C. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 2015, 25, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, P.P.; Angulo, M.C. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 2015, 21, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.J.; Kula, B.; Nagy, B.; Barzan, R.; Gall, A.; Ehrlich, I.; Kukley, M. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 2018, 25, 852–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kougioumtzidou, E.; Shimizu, T.; Hamilton, N.B.; Tohyama, K.; Sprengel, R.; Monyer, H.; Attwell, D.; Richardson, W.D. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 2017, 6, e28080. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, F.; Luo, Y.; Chen, L.; Chao, F.; Tan, C.; Gao, Y.; Huang, C.; Zhang, L.; Liang, X.; et al. Exercise protects myelinated fibers of white matter in a rat model of depression. J. Comp. Neurol. 2018, 526, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, Q.; Wang, J.; Jiang, L.; Hu, M.; Jiang, Y.; Tang, J.; Liang, X.; Qi, Y.; Dou, X.; et al. Running exercise protects oligodendrocytes in the medial prefrontal cortex in chronic unpredictable stress rat model. Transl. Psychiatry 2019, 9, 322. [Google Scholar] [CrossRef]
- Banasr, M.; Valentine, G.W.; Li, X.Y.; Gourley, S.L.; Taylor, J.R.; Duman, R.S. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psychiatry 2007, 62, 496–504. [Google Scholar] [CrossRef]
- Tang, J.; Liang, X.; Zhang, Y.; Chen, L.; Wang, F.; Tan, C.; Luo, Y.; Xiao, Q.; Chao, F.; Zhang, L.; et al. The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress. Brain Res. Bull. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Surget, A.; Wang, Y.; Leman, S.; Ibarguen-Vargas, Y.; Edgar, N.; Griebel, G.; Belzung, C.; Sibille, E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 2009, 34, 1363–1380. [Google Scholar] [CrossRef]
- Sibille, E.; Wang, Y.; Joeyen-Waldorf, J.; Gaiteri, C.; Surget, A.; Oh, S.; Belzung, C.; Tseng, G.C.; Lewis, D.A. A molecular signature of depression in the amygdala. Am. J. Psychiatry 2009, 166, 1011–1024. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Luo, F.; Li, B. Chronic stress regulates NG2⁺ cell maturation and myelination in the prefrontal cortex through induction of death receptor 6. Exp. Neurol. 2016, 277, 202–214. [Google Scholar] [CrossRef]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dietz, K.; Hodes, G.E.; Russo, S.J.; Casaccia, P. Widespread transcriptional alternations in oligodendrocytes in the adult mouse brain following chronic stress. Dev. Neurobiol. 2018, 78, 152–162. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef] [Green Version]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cheng, Z.; Tang, H.; Jiao, H.; Sun, X.; Cui, Q.; Luo, F.; Pan, H.; Ma, C.; Li, B. Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of Wnt signaling. Cereb. Cortex 2017, 27, 2871–2884. [Google Scholar] [CrossRef] [Green Version]
- Czéh, B.; Müller-Keuker, J.I.; Rygula, R.; Abumaria, N.; Hiemke, C.; Domenici, E.; Fuchs, E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 2007, 32, 1490–1503. [Google Scholar] [CrossRef] [Green Version]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.L.; Weigel, T.K.; Elkahloun, A.G.; Herkenham, M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci. Rep. 2017, 7, 46548. [Google Scholar] [CrossRef] [Green Version]
- Bonnefil, V.; Dietz, K.; Amatruda, M.; Wentling, M.; Aubry, A.V.; Dupree, J.L.; Temple, G.; Park, H.J.; Burghardt, N.S.; Casaccia, P.; et al. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife 2019, 8, e40855. [Google Scholar] [CrossRef]
- Kurokawa, K.; Tsuji, M.; Takahashi, K.; Miyagawa, K.; Mochida-Saito, A.; Takeda, H. Leukemia inhibitory factor participates in the formation of stress adaptation via hippocampal myelination in mice. Neuroscience 2020, 446, 1–13. [Google Scholar] [CrossRef]
- Takahashi, K.; Kurokawa, K.; Hong, L.; Miyagawa, K.; Mochida-Saito, A.; Takeda, H.; Tsuji, M. Disturbance of prefrontal cortical myelination in olfactory bulbectomized mice is associated with depressive-like behavior. Neurochem. Int. 2021, 148, 105112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dupree, J.L.; Gacias, M.; Frawley, R.; Sikder, T.; Naik, P.; Casaccia, P. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 2016, 36, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkowska, G.; Mahajan, G.; Maciag, D.; Sathyanesan, M.; Iyo, A.H.; Moulana, M.; Kyle, P.B.; Woolverton, W.L.; Miguel-Hidalgo, J.J.; Stockmeier, C.A.; et al. Oligodendrocyte morphometry and expression of myelin-related mRNA in ventral prefrontal white matter in major depressive disorder. J. Psychiatr. Res. 2015, 65, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchet, M.D.; Gotlib, I.H. Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Sci. Rep. 2017, 7, 2200. [Google Scholar] [CrossRef]
- Smagula, S.F.; Aizenstein, H.J. Brain structural connectivity in late-life major depressive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Takahashi, S.; Ukai, S.; Tsuji, T.; Iwatani, J.; Tsuda, K.; Kita, A.; Sakamoto, Y.; Yamamoto, M.; Terada, M.; et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: A tract-specific analysis study. J. Affect. Disord. 2015, 174, 542–548. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Yamamoto, A.; Kiuchi, K.; Kosaka, J.; Nagatsuka, K.; Iida, H.; Kudo, T. Microstructural differences in the corpus callosum in patients with bipolar disorder and major depressive disorder. J. Clin. Psychiatry 2017, 78, 99–104. [Google Scholar] [CrossRef]
- Zeng, L.L.; Liu, L.; Liu, Y.; Shen, H.; Li, Y.; Hu, D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS ONE 2012, 7, e44248. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gupta, R.C.; Albert Thomas, M.; Alger, J.; Wyckoff, N.; Hwang, S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004, 130, 131–140. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Hoptman, M.J.; Lim, K.O.; Murphy, C.F.; Klimstra, S.; Latoussakis, V.; Majcher-Tascio, M.; Hrabe, J.; Ardekani, B.A.; Alexopoulos, G.S. Macromolecular white matter abnormalities in geriatric depression: A magnetization transfer imaging study. Am. J. Geriatr. Psychiatry 2008, 16, 255–262. [Google Scholar] [CrossRef]
- Jia, Z.; Peng, W.; Chen, Z.; Sun, H.; Zhang, H.; Kuang, W.; Huang, X.; Lui, S.; Gong, Q. Magnetization transfer imaging of treatment-resistant depression. Radiology 2017, 284, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreescu, C.; Tudorascu, D.L.; Butters, M.A.; Tamburo, E.; Patel, M.; Price, J.; Karp, J.F.; Reynolds, C.F., III; Aizenstein, H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013, 214, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grangeon, M.C.; Seixas, C.; Quarantini, L.C.; Miranda-Scippa, A.; Pompili, M.; Steffens, D.C.; Wenzel, A.; Lacerda, A.L.; de Oliveira, I.R. White matter hyperintensities and their association with suicidality in major affective disorders: A meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lake, E.M.R.; Steffler, E.A.; Rowley, C.D.; Sehmbi, M.; Minuzzi, L.; Frey, B.N.; Bock, N.A. Altered intracortical myelin staining in the dorsolateral prefrontal cortex in severe mental illness. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 369–376. [Google Scholar] [CrossRef]
- Regenold, W.T.; Phatak, P.; Marano, C.M.; Gearhart, L.; Viens, C.H.; Hisley, K.C. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007, 151, 179–188. [Google Scholar] [CrossRef]
- Ongür, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.R.; Sharma, P.; Fung, K.L.; Pearce, R.K.; Hirsch, S.R.; Maier, M. Axonal myelin increase in the callosal genu in depression but not schizophrenia. Psychol Med. 2015, 45, 2145–2155. [Google Scholar] [CrossRef]
- Williams, M.R.; Sharma, P.; Macdonald, C.; Pearce, R.K.B.; Hirsch, S.R.; Maier, M. Axonal myelin decrease in the splenium in major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Uranova, N.A.; Vostrikov, V.M.; Orlovskaya, D.D.; Rachmanova, V.I. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004, 67, 269–275. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A.; Orlovskaya, D.D. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr. Res. 2007, 94, 273–280. [Google Scholar] [CrossRef]
- Kim, S.; Webster, M.J. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol. Psychiatry 2010, 15, 326–336. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nihonmatsu-Kikuchi, N.; Yu, X.; Ishimoto, K.; Hisanaga, S.I.; Tatebayashi, Y. A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol. Psychiatry 2011, 16, 1155–1158. [Google Scholar] [CrossRef] [Green Version]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: A postmortem study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef]
- Hamidi, M.; Drevets, W.C.; Price, J.L. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol. Psychiatry 2004, 55, 563–569. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr. Res. 2020, 215, 211–216. [Google Scholar] [CrossRef]
- Honer, W.G.; Falkai, P.; Chen, C.; Arango, V.; Mann, J.J.; Dwork, A.J. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 1999, 91, 1247–1255. [Google Scholar] [CrossRef]
- Aston, C.; Jiang, L.; Sokolov, B.P. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry 2005, 10, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Novak, G.; Tallerico, T. Nogo A, B and C expression in schizophrenia, depression and bipolar frontal cortex, and correlation of Nogo expression with CAA/TATC polymorphism in 3’-UTR. Brain Res. 2006, 1120, 161–171. [Google Scholar] [CrossRef]
- Klempan, T.A.; Ernst, C.; Deleva, V.; Labonte, B.; Turecki, G. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol. Psychiatry 2009, 66, 824–831. [Google Scholar] [CrossRef]
- Seney, M.L.; Huo, Z.; Cahill, K.; French, L.; Puralewski, R.; Zhang, J.; Logan, R.W.; Tseng, G.; Lewis, D.A.; Sibille, E. Opposite molecular signatures of depression in men and women. Biol. Psychiatry 2018, 84, 18–27. [Google Scholar] [CrossRef]
- Tanti, A.; Kim, J.J.; Wakid, M.; Davoli, M.A.; Turecki, G.; Mechawar, N. Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol. Psychiatry 2018, 23, 2018–2028. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Sikes-Keilp, C.; Rubinow, D.R. In search of sex-related mediators of affective illness. Biol. Sex Differ. 2021, 12, 55. [Google Scholar] [CrossRef]
- Graf, A.E.; Lallier, S.W.; Waidyaratne, G.; Thompson, M.D.; Tipple, T.E.; Hester, M.E.; Trask, A.J.; Rogers, L.K. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav. Immun. 2016, 58, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Greminger, A.R.; Lee, D.L.; Shrager, P.; Mayer-Pröschel, M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J. Nutr. 2014, 144, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, B.C.; Dimova, J.G.; Siddappa, A.J.; Tran, P.V.; Gewirtz, J.C.; Georgieff, M.K. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency in rats. J. Nutr. 2014, 144, 1858–1865. [Google Scholar] [CrossRef] [Green Version]
- Bordeleau, M.; Fernández de Cossío, L.; Lacabanne, C.; Savage, J.C.; Vernoux, N.; Chakravarty, M.; Tremblay, M. Maternal high-fat diet modifies myelin organization, microglial interactions, and results in social memory and sensorimotor gating deficits in adolescent mouse offspring. Brain Behav. Immun. Health 2021, 15, 100281. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duart-Castells, L.; Cantacorps, L.; López-Arnau, R.; Montagud-Romero, S.; Puster, B.; Mera, P.; Serra, D.; Camarasa, J.; Pubill, D.; Valverde, O.; et al. Effects of high-fat diet and maternal binge-like alcohol consumption and their influence on cocaine response in female mice offspring. Int. J. Neuropsychopharmacol. 2021, 24, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Fan, C.; Liu, X.; Xu, F.; Qi, K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011, 30, 659–667. [Google Scholar] [CrossRef]
- Tuzun, F.; Kumral, A.; Dilek, M.; Ozbal, S.; Ergur, B.; Yesilirmak, D.C.; Duman, N.; Yilmaz, O.; Ozkan, H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J. Matern. Fetal Neonatal Med. 2012, 25, 849–854. [Google Scholar] [CrossRef]
- Haubner, L.; Sullivan, J.; Ashmeade, T.; Saste, M.; Wiener, D.; Carver, J. The effects of maternal dietary docosahexaenoic acid intake on rat pup myelin and the auditory startle response. Dev. Neurosci. 2007, 29, 460–467. [Google Scholar] [CrossRef]
- Vallet, J.L.; Rempel, L.A.; Miles, J.R.; Webel, S.K. Effect of essential fatty acid and zinc supplementation during pregnancy on birth intervals, neonatal piglet brain myelination, stillbirth, and preweaning mortality. J. Anim. Sci. 2014, 92, 2422–2432. [Google Scholar] [CrossRef]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation reduces lipopolysaccharide-induced brain injury. Biofactors 2016, 42, 323–336. [Google Scholar] [CrossRef]
- Trujillo-Villarreal, L.A.; Romero-Díaz, V.J.; Marino-Martínez, I.A.; Fuentes-Mera, L.; Ponce-Camacho, M.A.; Devenyi, G.A.; Mallar Chakravarty, M.; Camacho-Morales, A.; Garza-Villarreal, E.E. Maternal cafeteria diet exposure primes depression-like behavior in the offspring evoking lower brain volume related to changes in synaptic terminals and gliosis. Transl. Psychiatry 2021, 11, 53. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Gleiser, P.M.; Bernal, V.; Hallgrímsson, B.; Gonzalez, P.N. Brain structural networks in mouse exposed to chronic maternal undernutrition. Neuroscience 2018, 380, 14–26. [Google Scholar] [CrossRef]
- Almeida, M.F.; Silveira, A.C.; Guedes, R.C.; Hokoç, J.N.; Martinez, A.M. Quantitative ultrastructural evidence of myelin malformation in optic nerves of rats submitted to a multideficient diet. Nutr. Neurosci. 2005, 8, 91–99. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Y.; Dong, J.; Min, H.; Song, B.; Shan, Z.; Teng, W.; Xi, Q.; Chen, J. Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ. Toxicol 2015, 30, 1264–1274. [Google Scholar] [CrossRef]
- Bousselamti, A.; El Hasbaoui, B.; Echahdi, H.; Krouile, Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr. Med. J. 2018, 30, 152. [Google Scholar] [CrossRef]
- Guez, S.; Chiarelli, G.; Menni, F.; Salera, S.; Principi, N.; Esposito, S. Severe vitamin B12 deficiency in an exclusively breastfed 5-month-old Italian infant born to a mother receiving multivitamin supplementation during pregnancy. BMC Pediatr. 2012, 12, 85. [Google Scholar] [CrossRef] [Green Version]
| Animal Model of Depression | Animal Sex | Molecular Effect/Antidepressant Effect (Dose, Treatment, Route) | References |
|---|---|---|---|
| CUS | Sprague–Dawley rats; males | - ↓ number of oligodendrocytes—prelimbic cortex | [56] |
| Sprague–Dawley rats; males | white matter:
| [54] | |
| Sprague–Dawley rats; males | HIP:
| [57] | |
| Sprague–Dawley rats; males | mPFC:
| [55] | |
| CUMS | BALB/c mice; males | amygdala:
| [58] |
| fluoxetine (20 mg/kg; 36 days; i.p.): reversed | |||
| BALB/c mice; males | altered gene expression
| [59] | |
fluoxetine (20 mg/kg; 36 days; i.p.):
| |||
| chronic stress | C57BL/6N mice; males | mPFC:
| [60] |
| C57/BL6 mice; males | corpus callosum:
| [61] | |
| C57B1/6 mice; males | 4 weeks of stress:
| [62] | |
| social isolation | C57B1/6J mice; males | PFC: 8 weeks of isolation:
| [63] |
| crossing male FVB/N mice and female C57Bl/6 mice; males | mPFC: isolation from PND 21 to PND 65:
- Ø oligodendrocyte density
| [64] | |
| maternal separation | Sprague–Dawley rats; females and males | mPFC:
| [65] |
| chronic social defeat stress | Wistar rats; males | mPFC:
| [66] |
| fluoxetine (10 mg/kg; 28 days; p.o.): Ø | |||
| C57BL/6J mice; males | PFC:
| [67] | |
| C57BL/6 mice; males | mPFC:
| [68] | |
| C57B1/6J mice; males | susceptible mice mPFC:
NAc:
| [69] | |
| chronic restraint stress | ICR mice; males | HIP: stress-maladaptive mice:
| [70] |
| learned helplessness | Sprague–Dawley rats; males | ↓ NG2 glia density (PDGFRα+ cell number)—PFC | [67] |
| olfactory bulbectomy | ddY mice; males | 14 days after surgery: PFC:
21 days after surgery: PFC:
| [71] |
| imipramine (20 mg/kg; 14 days; i.p.): PFC: reversed ↓ MBP, ↓ MAG, ↓ CNPase, ↓ Caspr, ↓ number of nodes, ↓ NG2+/Olig2+, ↓ CC1+/Olig2+, ↓ Olig2+ cells |
| Disease | Study Sample Size | Molecular Effect | References |
|---|---|---|---|
| MDD, BPD | 9 MDD; 14 BPD; 11 C | ↓ glial number—familial subgroup—subgenual PFC | [86] |
| depression with suicide | 11 (10 C) | ↓ MBPD (protein)—anterior PFC | [96] |
| MDD, BPD | 15 MDD; 15 BPD; 15 C | ↓ density of oligodendroglial cells—layer VI dorsolateral PFC | [89] |
| MDD, BPD | 8 MDD; 9 BPD; 10 C | ↓ density of glia and oligodendrocytes—MDD—amygdala | [94] |
| MDD | 12 MDD (14 C) | ↓ expression of genes for:
| [97] |
| MDD, BPD | 11 MDD; 10 BPD; 14 C | ↓ NOGO-B (mRNA)—frontal cortex | [98] |
| MDD, BPD | 15 MDD; 15 BPD; 15 C | ↓ number of perineuronal oligodendrocytes—sublayers IIIa, IIIb, IIIc dorsolateral PFC | [90] |
| BPD, unipolar MD | 15 MDD; 15 BPD; 15 C | ↓ mean deep white matter myelin staining—dorsolateral PFC | [85] |
| MDD | 16 MDD; 13 C | ↓ QKI (mRNA)—cortex, hippocampus, amygdala | [99] |
| MDD | 14 MDD; 14 C | ↓ CNPase (mRNA, protein) ↓ MBPD, PLLP, MOBPD, GPR37, ENPP2 (mRNA)—amygdala | [59] |
| MDD, BPD | 12 MDD; 13 BPD; 15 C | ↓ MOG, OMG, PLP1 (mRNA) ↓ number of perineuronal oligodendrocytes—dorsolateral PFC BA9 | [91] |
| MDD | 11 MDD; 12 C | ↓ oligodendroglial cells—frontopolar cortices BA10 | [92] |
| MDD, BPD | 9 MDD; 6 BPD; 13 C | MDD, BPD: ↓ density of S100B-immunopositive astrocytes—CA1 piramidal layer BPD: ↓ density of S100B-immunopositive oligodendrocytes—left alveus | [93] |
| MDD | 16 MDD; 20 C | ↑ mean myelin cross-sectional area ↑ myelin thickness—corpus callosum genu | [87] |
| MDD | 12 MDD; 8 C | ↓ cell numbers of NG2 glia ↓ PDGFRα (protein)—frontal cortex | [67] |
| MDD | 20 MDD; 16 C | ↓ oligodendrocyte soma size ↓ size of oligodendrocyte cell bodies—ventral PFC gyral white matter ↓ PLP1 (mRNA) ↑ CNPase, MOG, Olig1 (mRNA) ↓ CNPase (protein)—ventral PFC white matter | [73] |
| MDD, BPD | 15 MDD; 15 BPD; 15 C | MDD: ↓ intracortical myelin staining—dorsolateral PFC | [84] |
| MDD | 26 MDD men; 24 MDD women; 50 C | men: ↑ oligodendrocyte-related genes women: ↓ oligodendrocyte-related genes—dorsolateral PFC, anterior cingulate cortex, basolateral amygdala | [100] |
| depression | 18 depression; 18 C | ↓ MBPD (protein)—ventromedial PFC white matter | [101] |
| MDD | 16 MDD; 20 C | ↓ mean myelin cross-sectional area—corpus callosum splenium | [88] |
| MDD, BPD | 15 MDD; 15 BPD; 15 C | BPD: ↓ numerical density of oligodendrocytes and oligodendrocyte clusters—caudate nucleus | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaga, I. Understanding the Links among Maternal Diet, Myelination, and Depression: Preclinical and Clinical Overview. Cells 2022, 11, 540. https://doi.org/10.3390/cells11030540
Smaga I. Understanding the Links among Maternal Diet, Myelination, and Depression: Preclinical and Clinical Overview. Cells. 2022; 11(3):540. https://doi.org/10.3390/cells11030540
Chicago/Turabian StyleSmaga, Irena. 2022. "Understanding the Links among Maternal Diet, Myelination, and Depression: Preclinical and Clinical Overview" Cells 11, no. 3: 540. https://doi.org/10.3390/cells11030540
APA StyleSmaga, I. (2022). Understanding the Links among Maternal Diet, Myelination, and Depression: Preclinical and Clinical Overview. Cells, 11(3), 540. https://doi.org/10.3390/cells11030540