Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms
Abstract
:1. Introduction
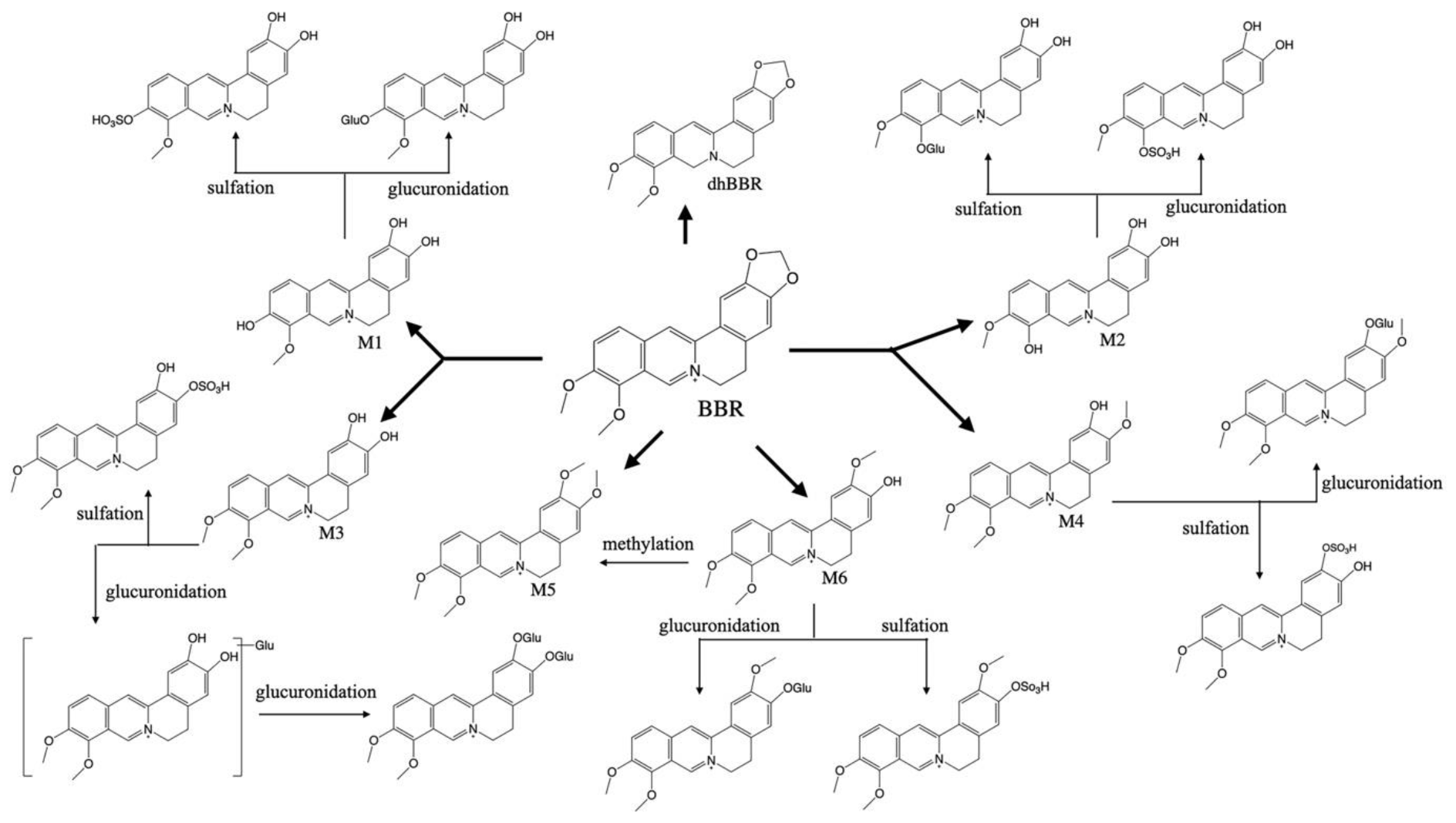
2. Pharmacokinetic Characteristics of BBR
3. Efficacies and Mechanisms of BBR on Neurological Disorders
3.1. BBR on Alzheimer’s Disease
3.1.1. Inhibitory Effect of BBR on ChE
3.1.2. Anti-Aβ and Tau Effects of BBR
3.2. BBR on Parkinson’s Disease
3.3. BBR on Stroke
3.4. BBR on Huntington’s Disease
3.5. BBR on Dementia
3.6. BBR on Psychiatric Disorders and Epilepsy
3.6.1. Schizophrenia, Anxiety, and Depression
3.6.2. Epilepsy
3.7. BBR on Traumatic Brain Injury
3.8. BBR on Tumor
4. Clinic Applications
4.1. Effect of BBR on Stroke Patients
4.2. Effect of BBR on Schizophrenia Patients
5. Concluding Remarks and Future Perspectives
5.1. Cell-Viability-Related Pathway
5.2. Oxidation- and Inflammation-Related Pathways
5.3. Gut-Microbiota-Related Pathway
5.4. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.L.; Vos, T. Global Burden of Neurological Disorders: From Global Burden of Disease Estimates to Actions. Neuroepidemiology 2019, 52, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Espay, A.J.; Aybek, S.; Carson, A.; Edwards, M.J.; Goldstein, L.H.; Hallett, M.; LaFaver, K.; LaFrance, W.C., Jr.; Lang, A.E.; Nicholson, T.; et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol. 2018, 75, 1132–1141. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverria, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Li, S.; Li, X. Therapeutic potential of berberine against neurodegenerative diseases. Sci. China Life Sci. 2015, 58, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Zhao, Z.X.; Ma, S.R.; Guo, F.; Wang, Y.; Jiang, J.D. Gut Microbiota-Regulated Pharmacokinetics of Berberine and Active Metabolites in Beagle Dogs After Oral Administration. Front. Pharmacol. 2018, 9, 214. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.W.; Zhao, Z.X.; He, C.Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.S.; Li, X.Y.; Wen, B.Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Shou, J.W.; Zhao, Z.X.; Li, X.Y.; Zhang, X.F.; Ma, S.R.; He, C.Y.; Lin, Y.; Wen, B.Y.; et al. Gut Microbiota-Mediated Personalized Treatment of Hyperlipidemia Using Berberine. Theranostics 2017, 7, 2443–2451. [Google Scholar] [CrossRef]
- Zhao, H.W.; Shen, Z.; Zhou, B.; Lin, X.; Ye, M. Studies on percutaneous absorption of ruyi jinhuang san patcher with radioisotope tracer. Zhongguo Zhong Yao Za Zhi 1993, 18, 5. [Google Scholar]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS Pharmscitech 2011, 12, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, R.; Xing, D.; Su, H.; Ma, C.; Ding, Y.; Du, L. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci. 2005, 77, 3058–3067. [Google Scholar] [CrossRef]
- Li, Y.; Ren, G.; Wang, Y.X.; Kong, W.J.; Yang, P.; Wang, Y.M.; Li, Y.H.; Yi, H.; Li, Z.R.; Song, D.Q.; et al. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J. Transl. Med. 2011, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Y.; Feng, R.; Tan, X.S.; Ma, C.; Shou, J.W.; Fu, J.; Huang, M.; He, C.Y.; Chen, S.N.; Zhao, Z.X.; et al. Excretion of berberine and its metabolites in oral administration in rats. J. Pharm. Sci. 2013, 102, 4181–4192. [Google Scholar] [CrossRef]
- Wang, K.; Chai, L.; Feng, X.; Liu, Z.; Liu, H.; Ding, L.; Qiu, F. Metabolites identification of berberine in rats using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2017, 139, 73–86. [Google Scholar] [CrossRef]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, neurodegeneration, and depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef]
- Wong, A.W.K.; Lau, S.C.L.; Cella, D.; Lai, J.S.; Xie, G.; Chen, L.; Chan, C.C.H.; Heinemann, A.W. Linking of the quality of life in neurological disorders (Neuro-QoL) to the international classification of functioning, disability and health. Qual. Life Res. 2017, 26, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sanchez-Lopez, E.; Garcia, M.L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016, 2016, 8501693. [Google Scholar] [CrossRef] [Green Version]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: Getting on and staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, J.S.; Abdalla, F.H.; Dornelles, G.L.; Adefegha, S.A.; Palma, T.V.; Signor, C.; da Silva Bernardi, J.; Baldissarelli, J.; Lenz, L.S.; Magni, L.P.; et al. Berberine protects against memory impairment and anxiogenic-like behavior in rats submitted to sporadic Alzheimer’s-like dementia: Involvement of acetylcholinesterase and cell death. Neurotoxicology 2016, 57, 241–250. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. Sci. World J. 2012, 2012, 823201. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Morgan, K.; Kalsheker, N. Amyloid precursor protein (APP) and the biology of proteolytic processing: Relevance to Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2003, 35, 1505–1535. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Durairajan, S.S.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Huang, L.; Li, X.S.; Huang, J.D.; Li, M. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging 2012, 33, 2903–2919. [Google Scholar] [CrossRef]
- Liang, Y.; Ye, C.; Chen, Y.; Chen, Y.; Diao, S.; Huang, M. Berberine Improves Behavioral and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease via Regulation of beta-Amyloid Production and Endoplasmic Reticulum Stress. ACS Chem. Neurosci. 2021, 12, 1894–1904. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; He, W.; Chen, Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting beta/gamma-Secretases Activity and Enhancing alpha-Secretases. Curr. Alzheimer Res. 2018, 15, 1045–1052. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Q.; Wen, B.; Wu, N.; He, B.; Chen, J. Berberine Reduces Abeta42 Deposition and Tau Hyperphosphorylation via Ameliorating Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 640758. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yan, Q.; Huang, Z.T.; Zou, Q.; Li, J.; Yuan, M.H.; Wu, L.Q.; Cai, Z.Y. Ameliorating Ribosylation-Induced Amyloid-beta Pathology by Berberine via Inhibiting mTOR/p70S6K Signaling. J. Alzheimers Dis. 2021, 79, 833–844. [Google Scholar] [CrossRef]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Haghani, M.; Shabani, M.; Tondar, M. The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Abeta neurotoxicity. Eur. J. Pharmacol. 2015, 758, 82–88. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Chen, Y.; He, Y.; Cai, Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-kappaB signaling, oxidative stress and neuroinflammation. Pharmacol. Rep. 2017, 69, 1341–1348. [Google Scholar] [CrossRef]
- Heurtaux, T.; Michelucci, A.; Losciuto, S.; Gallotti, C.; Felten, P.; Dorban, G.; Grandbarbe, L.; Morga, E.; Heuschling, P. Microglial activation depends on beta-amyloid conformation: Role of the formylpeptide receptor 2. J. Neurochem. 2010, 114, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, C.; Xue, X.; Hu, B.; Bao, H. SOCS1 Mediates Berberine-Induced Amelioration of Microglial Activated States in N9 Microglia Exposed to beta Amyloid. Biomed. Res. Int. 2021, 2021, 9311855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Liang, Y.; Chen, H.; Ji, X.; Huang, M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed. Pharmacother. 2020, 121, 109670. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic animal models of Parkinson’s disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Cho, K.H.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Shin, D.H.; Yang, H.J. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int. J. Mol. Med. 2014, 33, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Ma, Z. Protective effects of berberine against MPTP-induced dopaminergic neuron injury through promoting autophagy in mice. Food Funct. 2021, 12, 8366–8375. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Lin, Y.; Liu, M.; Li, Y.; Mao, H.; Zhang, Z.; Zhang, Y.; Ye, P.; Ding, L.; et al. Berberine Protects Against NLRP3 Inflammasome via Ameliorating Autophagic Impairment in MPTP-Induced Parkinson’s Disease Model. Front. Pharmacol. 2020, 11, 618787. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal. Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Bae, J.; Lee, D.; Kim, Y.K.; Gil, M.; Lee, J.Y.; Lee, K.J. Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1. Mol. Cells 2013, 35, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Inden, M.; Kitamura, Y.; Abe, M.; Tamaki, A.; Takata, K.; Taniguchi, T. Parkinsonian rotenone mouse model: Reevaluation of long-term administration of rotenone in C57BL/6 mice. Biol. Pharm. Bull. 2011, 34, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Kysenius, K.; Brunello, C.A.; Huttunen, H.J. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PLoS ONE 2014, 9, e107129. [Google Scholar] [CrossRef]
- Deng, H.; Jia, Y.; Pan, D.; Ma, Z. Berberine alleviates rotenone-induced cytotoxicity by antioxidation and activation of PI3K/Akt signaling pathway in SH-SY5Y cells. Neuroreport 2020, 31, 41–47. [Google Scholar] [CrossRef]
- Chugh, C. Acute Ischemic Stroke: Management Approach. Indian J. Crit. Care Med. 2019, 23, S140–S146. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Kinlay, S. Changes in stroke epidemiology, prevention, and treatment. Circulation 2011, 124, e494–e496. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Yang, Y.; Wu, X.; Fan, H.; Qiao, Y. Identification of berberine as a direct thrombin inhibitor from traditional Chinese medicine through structural, functional and binding studies. Sci. Rep. 2017, 7, 44040. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; McCullough, L.D. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J. Biomed. Biotechnol. 2011, 2011, 464701. [Google Scholar] [CrossRef]
- Manzanero, S.; Santro, T.; Arumugam, T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013, 62, 712–718. [Google Scholar] [CrossRef]
- Majid, A. Neuroprotection in stroke: Past, present, and future. ISRN Neurol. 2014, 2014, 515716. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.R.; Lu, H.D.; Guo, C.; Fang, W.R.; Zhao, H.D.; Zhou, J.S.; Wang, F.; Zhao, Y.L.; Li, Y.M.; Zhang, Y.D.; et al. Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-kappaB nuclear translocation. Acta Pharmacol. Sin. 2018, 39, 1706–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramiro, L.; Simats, A.; Garcia-Berrocoso, T.; Montaner, J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, X.; Wang, J.; Yang, M.; Kong, L. Treatment Effects of Ischemic Stroke by Berberine, Baicalin, and Jasminoidin from Huang-Lian-Jie-Du-Decoction (HLJDD) Explored by an Integrated Metabolomics Approach. Oxid. Med. Cell. Longev. 2017, 2017, 9848594. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, H.; Diya, J.B.; Shashikumar, S.; Rajanikant, G.K. Oxidative stress—Assassin behind the ischemic stroke. Folia Neuropathol. 2012, 50, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wei, S.; Yu, Y.; Xue, H.; Yao, F.; Zhang, M.; Xiao, J.; Hatch, G.M.; Chen, L. Pretreatment of rats with increased bioavailable berberine attenuates cerebral ischemia-reperfusion injury via down regulation of adenosine-5′ monophosphate kinase activity. Eur. J. Pharmacol. 2016, 779, 80–90. [Google Scholar] [CrossRef]
- Hu, J.; Chai, Y.; Wang, Y.; Kheir, M.M.; Li, H.; Yuan, Z.; Wan, H.; Xing, D.; Lei, F.; Du, L. PI3K p55gamma promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. Eur. J. Pharmacol. 2012, 674, 132–142. [Google Scholar] [CrossRef]
- Simoes Pires, E.N.; Frozza, R.L.; Hoppe, J.B.; Menezes Bde, M.; Salbego, C.G. Berberine was neuroprotective against an in vitro model of brain ischemia: Survival and apoptosis pathways involved. Brain Res. 2014, 1557, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qian, Z.; Pan, L.; Li, H.; Zhu, H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol. Hung 2012, 99, 311–323. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H.; Li, S.; Zhang, M. Berberine Ameliorates MCAO Induced Cerebral Ischemia/Reperfusion Injury via Activation of the BDNF-TrkB-PI3K/Akt Signaling Pathway. Neurochem. Res. 2018, 43, 702–710. [Google Scholar] [CrossRef]
- Chai, Y.S.; Yuan, Z.Y.; Lei, F.; Wang, Y.G.; Hu, J.; Du, F.; Lu, X.; Jiang, J.F.; Xing, D.M.; Du, L.J. Inhibition of retinoblastoma mRNA degradation through Poly (A) involved in the neuroprotective effect of berberine against cerebral ischemia. PLoS ONE 2014, 9, e90850. [Google Scholar] [CrossRef] [Green Version]
- Maleki, S.N.; Aboutaleb, N.; Souri, F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J. Chem. Neuroanat. 2018, 87, 54–59. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, C.; Li, Y.; Dong, L.; Cui, L.; Wang, L.; Liu, Z.; Qiao, H.; Zhu, C.; et al. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: Up-regulated pAkt, pGSK and pCREB, down-regulated NF-kappaB expression, ameliorated BBB permeability. Brain Res. 2012, 1459, 61–70. [Google Scholar] [CrossRef]
- Shou, J.W.; Li, X.X.; Tang, Y.S.; Lim-Ho Kong, B.; Wu, H.Y.; Xiao, M.J.; Cheung, C.K.; Shaw, P.C. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARdelta for antioxidant action. Free Radic. Biol. Med. 2022, 181, 62–71. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, D.; Guo, C.; Liu, M.; Tao, Y.; Zhou, J.; Wang, F.; Zhao, Y.; Wei, J.; Zhang, Y.; et al. Berberine Facilitates Angiogenesis Against Ischemic Stroke through Modulating Microglial Polarization via AMPK Signaling. Cell. Mol. Neurobiol. 2019, 39, 751–768. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, W.; Gaertig, M.A.; Li, S.; Li, X.J. Therapeutic Effect of Berberine on Huntington’s Disease Transgenic Mouse Model. PLoS ONE 2015, 10, e0134142. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jonsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [Green Version]
- Aski, M.L.; Rezvani, M.E.; Khaksari, M.; Hafizi, Z.; Pirmoradi, Z.; Niknazar, S.; Mehrjerdi, F.Z. Neuroprotective effect of berberine chloride on cognitive impairment and hippocampal damage in experimental model of vascular dementia. Iran. J. Basic Med. Sci. 2018, 21, 53–58. [Google Scholar] [CrossRef]
- Yin, S.; Bai, W.; Li, P.; Jian, X.; Shan, T.; Tang, Z.; Jing, X.; Ping, S.; Li, Q.; Miao, Z.; et al. Berberine suppresses the ectopic expression of miR-133a in endothelial cells to improve vascular dementia in diabetic rats. Clin. Exp. Hypertens. 2019, 41, 708–716. [Google Scholar] [CrossRef]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef]
- Shaker, F.H.; El-Derany, M.O.; Wahdan, S.A.; El-Demerdash, E.; El-Mesallamy, H.O. Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 2021, 269, 119078. [Google Scholar] [CrossRef]
- Zhan, P.Y.; Peng, C.X.; Zhang, L.H. Berberine rescues D-galactose-induced synaptic/memory impairment by regulating the levels of Arc. Pharmacol. Biochem. Behav. 2014, 117, 47–51. [Google Scholar] [CrossRef]
- Krystal, J.H.; State, M.W. Psychiatric disorders: Diagnosis to therapy. Cell 2014, 157, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.J. New approaches to psychiatric diagnostic classification. Neuron 2014, 84, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Cabral, P.; Meyer, H.B.; Ames, D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: A meta-analysis. Prim. Care Companion CNS Disord. 2011, 13, 26290. [Google Scholar] [CrossRef]
- Hesdorffer, D.C.; Ishihara, L.; Mynepalli, L.; Webb, D.J.; Weil, J.; Hauser, W.A. Epilepsy, suicidality, and psychiatric disorders: A bidirectional association. Ann. Neurol. 2012, 72, 184–191. [Google Scholar] [CrossRef]
- LaFrance, W.C., Jr.; Kanner, A.M.; Hermann, B. Psychiatric comorbidities in epilepsy. Int. Rev. Neurobiol. 2008, 83, 347–383. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Ghotbi Ravandi, S.; Shabani, M.; Bashiri, H.; Saeedi Goraghani, M.; Khodamoradi, M.; Nozari, M. Ameliorating effects of berberine on MK-801-induced cognitive and motor impairments in a neonatal rat model of schizophrenia. Neurosci. Lett. 2019, 706, 151–157. [Google Scholar] [CrossRef]
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef]
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety disorders. Lancet 2021, 397, 914–927. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J. Physiol. Pharmacol. 2012, 16, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Rezaeian, L.; Kalalian-Moghaddam, H.; Mohseni, F.; Khaksari, M.; Rafaiee, R. Effects of berberine hydrochloride on methamphetamine-induced anxiety behaviors and relapse in rats. Iran. J. Basic Med. Sci. 2020, 23, 1480–1488. [Google Scholar] [CrossRef]
- Alavijeh, M.M.; Vaezi, G.; Khaksari, M.; Hojati, V. Berberine hydrochloride attenuates voluntary methamphetamine consumption and anxiety-like behaviors via modulation of oxytocin receptors in methamphetamine addicted rats. Physiol. Behav. 2019, 206, 157–165. [Google Scholar] [CrossRef]
- Muhlethaler, M.; Charpak, S.; Dreifuss, J.J. Contrasting effects of neurohypophysial peptides on pyramidal and non-pyramidal neurones in the rat hippocampus. Brain Res. 1984, 308, 97–107. [Google Scholar] [CrossRef]
- Mulhall, S.; Andel, R.; Anstey, K.J. Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas 2018, 108, 7–12. [Google Scholar] [CrossRef]
- Cyranowski, J.M. Practice considerations for behavioral therapies for depression and anxiety in midlife women. Menopause 2022, 29, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Li, B.; Ge, T.; Zhang, Z.; Lv, J.; Zhao, J.; Wang, P.; Liu, W.; Wang, X.; Mlyniec, K.; et al. Berberine produces antidepressant-like effects in ovariectomized mice. Sci. Rep. 2017, 7, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Zhang, J.; Zhu, S.; He, M.; Ma, S.; Jia, Q.; Sun, Q.; Song, L.; Wang, Y.; Duan, L. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol. Res. 2021, 165, 105439. [Google Scholar] [CrossRef]
- Shneker, B.F.; Fountain, N.B. Epilepsy. Dis. Mon. 2003, 49, 426–478. [Google Scholar] [CrossRef]
- Schubert, J.; Siekierska, A.; Langlois, M.; May, P.; Huneau, C.; Becker, F.; Muhle, H.; Suls, A.; Lemke, J.R.; de Kovel, C.G.; et al. Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat. Genet. 2014, 46, 1327–1332. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Chen, B.; Jiang, J.D.; Zhang, J.P. Syntaxin 1B Mediates Berberine’s Roles in Epilepsy-Like Behavior in a Pentylenetetrazole-Induced Seizure Zebrafish Model. Front. Mol. Neurosci. 2018, 11, 378. [Google Scholar] [CrossRef]
- Ambrogini, P.; Torquato, P.; Bartolini, D.; Albertini, M.C.; Lattanzi, D.; Di Palma, M.; Marinelli, R.; Betti, M.; Minelli, A.; Cuppini, R.; et al. Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1098–1112. [Google Scholar] [CrossRef]
- Sedaghat, R.; Taab, Y.; Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: Underlying mechanisms. Biomed. Pharmacother. 2017, 87, 200–208. [Google Scholar] [CrossRef]
- Gao, F.; Gao, Y.; Liu, Y.F.; Wang, L.; Li, Y.J. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr. Dis. Treat. 2014, 10, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Mojarad, T.B.; Roghani, M. The Anticonvulsant and Antioxidant Effects of Berberine in Kainate-induced Temporal Lobe Epilepsy in Rats. Basic Clin. Neurosci. 2014, 5, 124–130. [Google Scholar]
- Guna, V.; Saha, L.; Bhatia, A.; Banerjee, D.; Chakrabarti, A. Anti-Oxidant and Anti-Apoptotic Effects of Berberine in Pentylenetetrazole-Induced Kindling Model in Rat. J. Epilepsy Res. 2018, 8, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, L.; Ji, X.; Zhang, S.; Sik, A.; Liu, K.; Jin, M. Anti-Inflammation Associated Protective Mechanism of Berberine and its Derivatives on Attenuating Pentylenetetrazole-Induced Seizures in Zebrafish. J. Neuroimmune Pharmacol. 2020, 15, 309–325. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.X.; Qiu, G.; Cheng, F.R.; Pei, Z.; Yang, Z.; Deng, X.H.; Zhu, J.H.; Chen, L.; Chen, C.C.; Lin, W.F.; et al. Berberine Protects Secondary Injury in Mice with Traumatic Brain Injury Through Anti-oxidative and Anti-inflammatory Modulation. Neurochem. Res. 2018, 43, 1814–1825. [Google Scholar] [CrossRef]
- Chen, C.C.; Hung, T.H.; Lee, C.Y.; Wang, L.F.; Wu, C.H.; Ke, C.H.; Chen, S.F. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS ONE 2014, 9, e115694. [Google Scholar] [CrossRef] [Green Version]
- Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Cote, D. Epidemiology of Brain Tumors. Neurol. Clin. 2018, 36, 395–419. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 2016, 7, 66944–66958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yu, J.; Liu, X.; Zhang, C.; Cao, J.; Li, G.; Liu, X.; Chen, Y.; Huang, H. Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomed. Pharmacother. 2018, 102, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, P.; Buja, L.M. Oncosis: An important non-apoptotic mode of cell death. Exp. Mol. Pathol. 2012, 93, 302–308. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Zhao, M.; Wei, Z.; Li, X.; Zhang, X.; Liu, Z.; Gong, Y.; Shao, C. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol. Cancer Ther. 2015, 14, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef]
- Jin, F.; Xie, T.; Huang, X.; Zhao, X. Berberine inhibits angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway. Pharm. Biol. 2018, 56, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Song, X.; Song, Z.; Jiang, X.; Gao, X.; Bai, L.; Wu, J.; Na, L.; Yao, Z. Berberine reduces temozolomide resistance by inducing autophagy via the ERK1/2 signaling pathway in glioblastoma. Cancer Cell Int. 2020, 20, 592. [Google Scholar] [CrossRef]
- Chai, M.J.; Li, X.; Yang, H.; Li, H.S.; Wang, P.; Zhao, J. Effects of Berberine on Oxidative Stress and Neurological Function in Patients with Acute Ischemic Stroke. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 2017, 31, 4. [Google Scholar] [CrossRef]
- Chai, M.J.; Wang, P. Effect of berberine hydrochloride on neurological function and serum malondialdehyde in patients with acute ischemic stroke. Clin. Focus 2016, 31, 4. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Chai, M.J.; Yang, F.; Li, H.S.; Zhao, J.; Wang, H.; Lu, D.D. Effects of berberine on serum inflammatory factors and carotid atherosclerotic plaques in patients with acute cerebral ischemic stroke. Zhongguo Zhong Yao Za Zhi 2016, 41, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Wang, P.P.; Zhang, Y.; Han, Y.Z. A multicenter study effect of berberine hydrochloride on serum HIF-1α and Caspase-3 levels in patients with acute cerebral infarction. Chin. J. Biol. Pharm. 2014, 7, 3. [Google Scholar]
- Jia, Q.; Li, J.; Zhang, J.; Liu, Y.; Zhao, Y.P.; Li, M.J.; Li, J.G. A randomized double blind study of the effect of berberine on improvement of cognitive ability in patients with schizophrenia. Chin. Ment. Health J. 2016, 6, 677–682. [Google Scholar]
- Dong, W.; Gu, W.X.; Tang, X.W.; Liu, P.; Zhao, J.T.; Chu, X. Effect of berberine combined with risperidone therapy on endocrine hormones and oxidative stress in patients with schizophrenia. J. Hainan Med. Univ. 2017, 23, 4. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Liu, Y.; Qiu, Y.; Li, M.; Jia, Q.; Zhang, J.; Li, J. The impact of berberine on insulin resistance and cytokines in patients with schizophrenia. Tianjin Med. J. 2016, 44, 4. [Google Scholar] [CrossRef]
- Penninx, B.W.; Lange, S.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci 2018, 20, 63–73. [Google Scholar]
- Qiu, Y.; Li, M.; Zhang, Y.; Liu, Y.; Zhao, Y.; Zhang, J.; Jia, Q.; Li, J. Berberine treatment for weight gain in patients with schizophrenia by regulating leptin rather than adiponectin. Asian J. Psychiatry 2021, 67, 102896. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Qiu, Y.; Zhang, J.; Zhang, Y.; Zhao, Y.; Jia, Q.; Li, J. The effect of berberine adjunctive treatment on glycolipid metabolism in patients with schizophrenia: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Res. 2021, 300, 113899. [Google Scholar] [CrossRef]
- Smith, S.W.; Hauben, M.; Aronson, J.K. Paradoxical and bidirectional drug effects. Drug Saf. 2012, 35, 173–189. [Google Scholar] [CrossRef]
- Fu, W.R.; Chen, J.L.; Li, X.Y.; Dong, J.X.; Liu, Y. Bidirectional Regulatory Mechanisms of Jaceosidin on Mitochondria Function: Protective Effects of the Permeability Transition and Damage of Membrane Functions. J. Membr. Biol. 2020, 253, 25–35. [Google Scholar] [CrossRef]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Fang, J.; Zhang, J.; Ding, S.; Gan, D. Curcumin Inhibited Podocyte Cell Apoptosis and Accelerated Cell Autophagy in Diabetic Nephropathy via Regulating Beclin1/UVRAG/Bcl2. Diabetes Metab. Syndr. Obes. 2020, 13, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Xue, Q.; Zhang, Q.; Li, C.; Liu, X.; Liu, J.; Li, H.; Yang, J. How Ginsenosides Trigger Apoptosis in Human Lung Adenocarcinoma Cells. Am. J. Chin. Med. 2019, 47, 1737–1754. [Google Scholar] [CrossRef]
- Liu, M.; Bai, X.; Yu, S.; Zhao, W.; Qiao, J.; Liu, Y.; Zhao, D.; Wang, J.; Wang, S. Ginsenoside Re Inhibits ROS/ASK-1 Dependent Mitochondrial Apoptosis Pathway and Activation of Nrf2-Antioxidant Response in Beta-Amyloid-Challenged SH-SY5Y Cells. Molecules 2019, 24, 2687. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Chen, B.; Ren, Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1alpha/BNIP3 pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3657–3663. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Zhang, J.; Peng, J.; Zhang, Y.; Wang, L.; Wu, J.; Ye, L.; Fang, C. Effect of baicalin on proliferation and apoptosis in pancreatic cancer cells. Am. J. Transl. Res. 2019, 11, 5645–5654. [Google Scholar]
- El-Deiry, W.S. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001, 8, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woynarowska, B.A.; Woynarowski, J.M. Preferential targeting of apoptosis in tumor versus normal cells. Biochim. Biophys. Acta 2002, 1587, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Leszek, J.; Barreto, G.E.; Gasiorowski, K.; Koutsouraki, E.; Avila-Rodrigues, M.; Aliev, G. Inflammatory Mechanisms and Oxidative Stress as Key Factors Responsible for Progression of Neurodegeneration: Role of Brain Innate Immune System. CNS Neurol. Disord. Drug Targets 2016, 15, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christofidou-Solomidou, M.; Muzykantov, V.R. Antioxidant strategies in respiratory medicine. Treat. Respir. Med. 2006, 5, 47–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An insight into intestinal mucosal microbiota disruption after stroke. Sci. Rep. 2018, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenit, M.C.; Sanz, Y.; Codoner-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Ambrosini, Y.M.; Borcherding, D.; Kanthasamy, A.; Kim, H.J.; Willette, A.A.; Jergens, A.; Allenspach, K.; Mochel, J.P. The Gut-Brain Axis in Neurodegenerative Diseases and Relevance of the Canine Model: A Review. Front. Aging Neurosci. 2019, 11, 130. [Google Scholar] [CrossRef]
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut Microbiota in Neurological Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Stefano, G.B.; Pilonis, N.; Ptacek, R.; Raboch, J.; Vnukova, M.; Kream, R.M. Gut, Microbiome, and Brain Regulatory Axis: Relevance to Neurodegenerative and Psychiatric Disorders. Cell Mol. Neurobiol. 2018, 38, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, Y.; Tan, Z.R.; Klaassen, C.D.; Zhou, H.H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur. J. Clin. Pharmacol. 2012, 68, 213–217. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Shou, J.W.; Cheung, C.K.; Gao, J.; Shi, W.W.; Shaw, P.C. Berberine Protects C17.2 Neural Stem Cells from Oxidative Damage Followed by Inducing Neuronal Differentiation. Front. Cell. Neurosci. 2019, 13, 395. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Lee, Y.J.; Kim, Y.H.; Lee, S.H.; Park, J.H.; Kim, M.O.; Suh, H.N.; Ryu, J.M.; Yun, S.P.; Jang, M.W.; et al. Role of Peroxisome Proliferator-Activated Receptor (PPAR)delta in Embryonic Stem Cell Proliferation. Int. J. Stem Cells 2009, 2, 28–34. [Google Scholar] [CrossRef]
- Wei, S.; Wang, K.; Zou, X.; Hu, S.; Xu, L.; Jiang, S.; Li, J.; Lu, F. Comparative study on dynamic changes of berberine content in blood and brain tissue and bioavailability by nasal drug delivery and oral administration in rats. Chin. Arch. Tradit. Chin. Med. 2018, 36, 5. [Google Scholar] [CrossRef]
- Heng, X.; Qi, Y.Y.; Qu, S.Y.; Ge, P.Y.; Yang, N.Y.; Guo, R.; Zhang, Q.C.; Zhu, H.X. Distribution characteristics of nine main components of Huanglian Jiedu Decoction in rat brain. Chin. Tradit. Herb Drugs 2020, 51, 8. [Google Scholar] [CrossRef]
- Peng, S.L.L. Effect of formula compatibility on berberine pharmacokinetics in brain tissue of rats. Chin. Tradit. Herb Drugs 2016, 47, 2877–2882. [Google Scholar]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef]
- Wang, Y.; Shou, J.W.; Li, X.Y.; Zhao, Z.X.; Fu, J.; He, C.Y.; Feng, R.; Ma, C.; Wen, B.Y.; Guo, F.; et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 2017, 70, 72–84. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]





| Disease | No. of Patients | Dosage /Duration | Outcome | Ref. |
|---|---|---|---|---|
| Acute cerebral ischemic stroke | 55 | 300 mg (tid) /14 days | Improved neural function; decreased MDA level; increased GSH-Px level | [131] |
| Acute cerebral ischemic stroke | 52 | 500 mg (tid) /14 days | Improved neural function; decreased MDA level | [132] |
| Acute cerebral ischemic stroke | 60 | 300 mg (tid) /14 days | Decreased levels of MIF and IL-6 | [133] |
| Acute cerebral infarction | 63 | 700 mg (tid) /7 days | Reduced serum HIF-1α, caspase-3 level, and fatality rate | [134] |
| Schizophrenia | 31 | 300 mg (tid) /12 weeks | Improved learning memory function and information processing | [135] |
| Schizophrenia | 43 | 300 mg (tid) /2 months | Increased prolactin, SOD, GSH-Px, and CAT levels; decreased MDA and triiodothyronine levels | [136] |
| Schizophrenia | 34 | 300 mg (tid) /12 weeks | Decreased IL-1β, IL-6, and TNFα levels | [137] |
| Schizophrenia | 27 | 300 mg (tid) /8 weeks | Decline in weight gain | [138] |
| Schizophrenia | 27 | 300 mg (tid) /8 weeks | Decreased levels of total cholesterol, low-density lipoprotein cholesterol, fasting serum insulin, and insulin resistance | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shou, J.-W.; Shaw, P.-C. Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells 2022, 11, 796. https://doi.org/10.3390/cells11050796
Shou J-W, Shaw P-C. Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells. 2022; 11(5):796. https://doi.org/10.3390/cells11050796
Chicago/Turabian StyleShou, Jia-Wen, and Pang-Chui Shaw. 2022. "Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms" Cells 11, no. 5: 796. https://doi.org/10.3390/cells11050796
APA StyleShou, J.-W., & Shaw, P.-C. (2022). Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells, 11(5), 796. https://doi.org/10.3390/cells11050796







