The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA?
Abstract
1. Introduction
2. Mitochondria and the Cell Cycle
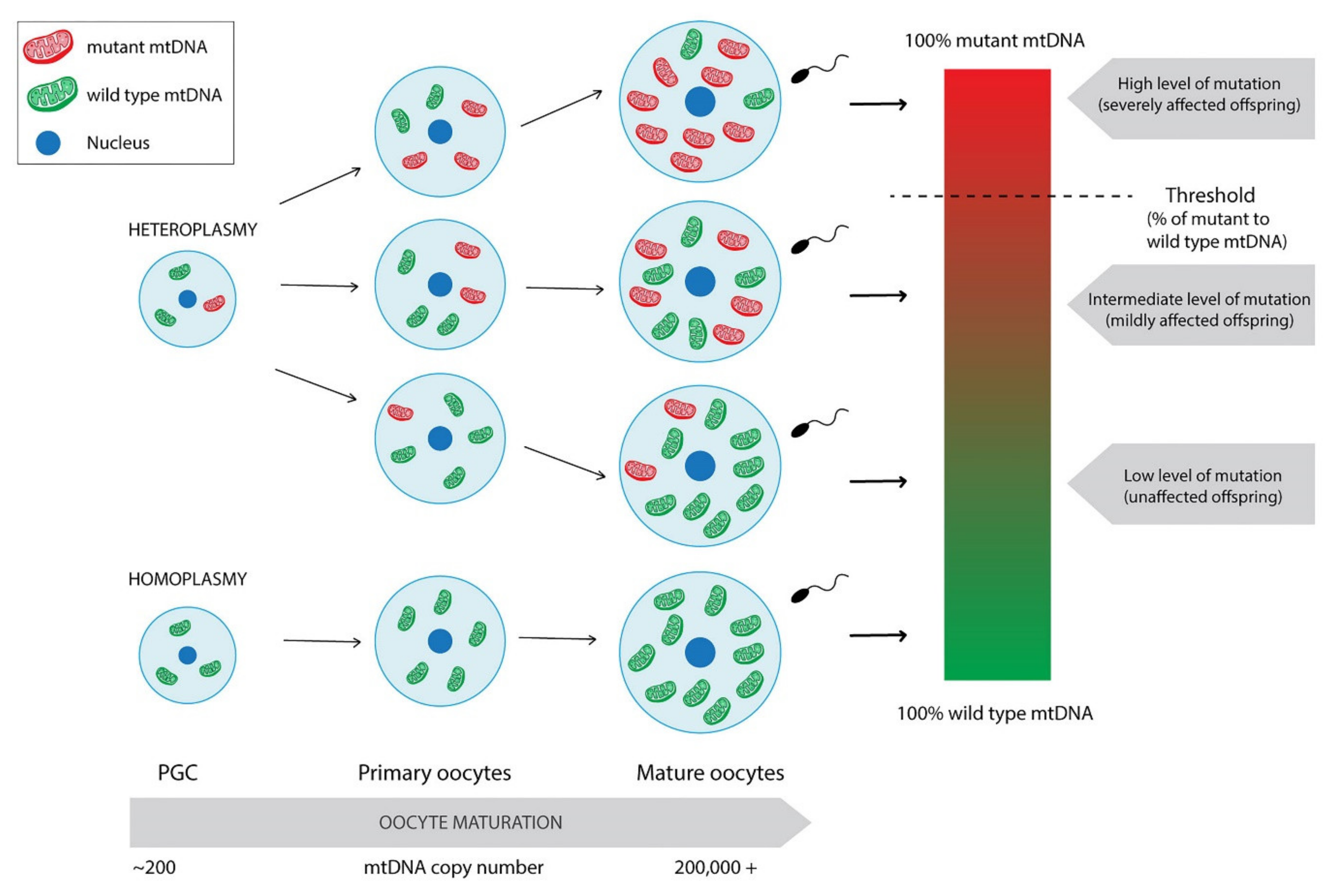
3. Mitochondrial Genetics
4. Mitochondria in Human Gametes and Embryos
4.1. Mitochondrial Distribution in Human Oocytes and Embryos
4.2. Mitochondria and Energy Production in the Embryo
4.3. Mitochondrial Activity versus Fertility and Early Embryogenesis
4.4. Effects of Ageing and Other Factors on Mitochondrial Insufficiency
4.5. Impact of Mitochondrial Insufficiency on Fertility
4.6. Do mtDNA Mutations Influence Early Embryo Development?
5. Clinical Usefulness of Assessing and Improving Mitochondrial DNA Function and Quantity
5.1. Assessment of mtDNA Content in Human Embryos and Its Clinical Significance
5.2. Mitochondrial Score—The Debate under Its Usefulness as Embryo Selection Marker
5.3. Opportunities to Improve Mitochondrial DNA Function
5.4. Mitochondrial DNA Transfer in Improving the Reproductive Potential of Oocytes
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- St John, J.C.; Facucho-Oliveira, J.; Jiang, Y.; Kelly, R.; Salah, R. Mitochondrial DNA transmission, replication and inheritance: A journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum. Reprod. Update 2010, 16, 488–509. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G.; Genova, M.L. Supramolecular Organisation of the Mitochondrial Respiratory Chain: A New Challenge for the Mechanism and Control of Oxidative Phosphorylation. Adv. Exp. Med. Biol. 2012, 748, 107–144. [Google Scholar] [PubMed]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529 Pt 1, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G. Mitochondrial control of apoptosis: An introduction. Biochem. Biophys. Res. Commun. 2003, 304, 433–435. [Google Scholar] [CrossRef]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. (Lausanne). 2017, 8, 296. [Google Scholar] [CrossRef]
- Arciuch, V.G.A.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxidants Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Kurland, C.G.; Andersson, S.G. Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 2000, 64, 786–820. [Google Scholar] [CrossRef]
- Mascanzoni, F.; Ayala, I.; Colanzi, A. Organelle Inheritance Control of Mitotic Entry and Progression: Implications for Tissue Homeostasis and Disease. Front. Cell Dev. Biol. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Pangou, E.; Sumara, I. The Multifaceted Regulation of Mitochondrial Dynamics During Mitosis. Front. Cell Dev. Biol. 2021, 9, 767221. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.F.; Machado, T.S.; Garcia, B.M.; Zangirolamo, A.F.; Macabelli, C.H.; Sugiyama, F.H.C.; Grejo, M.P.; Augusto Neto, J.D.; Tostes, K.; Ribeiro, F.K.S.; et al. Mitofusin 1 is required for oocyte growth and communication with follicular somatic cells. FASEB J. 2020, 34, 7644–7660. [Google Scholar] [CrossRef]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott, R.; Horvath, T.; Seli, E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging (Albany. NY). 2019, 11, 3919–3938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott III, R.; Horvath, T.; Seli, E. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019, 10, 560. [Google Scholar] [CrossRef]
- Katajisto, P.; Döhla, J.; Chaffer, C.L.; Pentinmikko, N.; Marjanovic, N.; Iqbal, S.; Zoncu, R.; Chen, W.; Weinberg, R.A.; Sabatini, D.M. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 2015, 348, 340–343. [Google Scholar] [CrossRef]
- Wei, W.; Chinnery, P.F. Inheritance of mitochondrial DNA in humans: Implications for rare and common diseases. J. Intern. Med. 2020, 287, 634–644. [Google Scholar] [CrossRef]
- Sato, M.; Sato, K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta - Mol. Cell Res. 2013, 1833, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Hayashi, J.I.; Takahama, S.; Taya, C.; Lindahl, K.F.; Yonekawa, H. Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 4542–4546. [Google Scholar] [CrossRef]
- Shitara, H.; Kaneda, H.; Sato, A.; Inoue, K.; Ogura, A.; Yonekawa, H.; Hayashi, J.I. Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout emborogenesis. Genetics 2000, 156, 1277–1284. [Google Scholar] [CrossRef]
- Cummins, J.M.; Wakayama, T.; Yanagimachi, R. Fate of microinjected spermatid mitochondria in the mouse oocyte and embryo. Zygote 1998, 6, 213–222. [Google Scholar] [CrossRef]
- Seli, E.; Wang, T.; Horvath, T.L. Mitochondrial unfolded protein response: A stress response with implications for fertility and reproductive aging. Fertil. Steril. 2019, 111, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Pang, M.G. Mitochondrial functionality in male fertility: From spermatogenesis to fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P.; Moreno, R.D.; Ramalho-Santos, J.; Dominko, T.; Simerly, C.; Schatten, G. Ubiquitin tag for sperm mitochondria. Nature 1999, 402, 371–372. [Google Scholar] [CrossRef]
- St. John, J. The control of mtDNA replication during differentiation and development. Biochim. Biophys. Acta - Gen. Subj. 2014, 1840, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Valencia, C.A.; Zhang, J.; Lee, N.-C.; Slone, J.; Gui, B.; Wang, X.; Li, Z.; Dell, S.; Brown, J.; et al. Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. 2018, 115, 13039 LP–13044. [Google Scholar] [CrossRef]
- Cree, L.M.; Samuels, D.C.; Chinnery, P.F. The inheritance of pathogenic mitochondrial DNA mutations. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 1097–1102. [Google Scholar] [CrossRef]
- Vaught, R.C.; Dowling, D.K. Maternal inheritance of mitochondria: Implications for male fertility? Reproduction 2018, 155, R159–R168. [Google Scholar] [CrossRef]
- Chiaratti, M.R.; Garcia, B.M.; Carvalho, K.F.; Machado, S.; Karina, F.; Habermann, C. The role of mitochondria in the female germline: Implications to fertility and inheritance of mitochondrial diseases. Cell Biol. Int. 2018, 42, 1–39. [Google Scholar] [CrossRef]
- Chatzovoulou, K.; Mayeur, A.; Gigarel, N.; Jabot-Hanin, F.; Hesters, L.; Munnich, A.; Frydman, N.; Bonnefont, J.P.; Steffann, J. Mitochondrial DNA mutations do not impact early human embryonic development. Mitochondrion 2021, 58, 59–63. [Google Scholar] [CrossRef]
- Zhang, R.; Nakahira, K.; Choi, A.M.K.; Gu, Z. Heteroplasmy concordance between mitochondrial DNA and RNA. Sci. Rep. 2019, 9, 12942. [Google Scholar] [CrossRef]
- McCormick, E.M.; Muraresku, C.C.; Falk, M.J. Mitochondrial Genomics: A Complex Field Now Coming of Age. Curr. Genet. Med. Rep. 2018, 6, 52–61. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Gomez-Duran, A. Oldies but Goldies mtDNA Population Variants and Neurodegenerative Diseases. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, R.; Faustin, B.; Rocher, C.; Malgat, M.; Mazat, J.P.; Letellier, T. Mitochondrial threshold effects. Biochem. J. 2003, 370, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.B.; Chinnery, P.F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Davis, P.; Alexander, S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: Relationship to microtubular organization, ATP content and competence. Hum. Reprod. 2000, 15, 2621–2633. [Google Scholar] [CrossRef]
- Squirrell, J.M.; Schramm, R.D.; Paprocki, A.M.; Wokosin, D.L.; Bavister, B.D. Imaging mitochondrial organization in living primate oocytes and embryos using multiphoton microscopy. Microsc. Microanal. 2003, 9, 190–201. [Google Scholar] [CrossRef]
- Bavister, B.D.; Squirrell, J.M. Mitochondrial distribution and function in oocytes and early embryos. Hum. Reprod. 2000, 15 (Suppl. 2), 189–198. [Google Scholar] [CrossRef] [PubMed]
- Podolak, A.; Liss, J.; Kiewisz, J.; Pukszta, S.; Cybulska, C.; Rychlowski, M.; Lukaszuk, A.; Jakiel, G.; Lukaszuk, K. Mitochondrial DNA Copy Number in Cleavage Stage Human Embryos—Impact on Infertility Outcome. Curr. Issues Mol. Biol. 2022, 44, 273–287. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boguenet, M.; Hachem, H.E.; Bouet, P.E.; Reynier, P. Embryo and its mitochondria. Antioxidants 2021, 10, 139. [Google Scholar] [CrossRef]
- Leese, H.J.; Houghton, F.D.; Macmillan, D.A.; Donnay, I. Metabolism of the Early Embryo: Energy Production and Utilization. ART Hum. Blastocyst 2001, 61–68. [Google Scholar] [CrossRef]
- Wilding, M.; Coppola, G.; Dale, B.; Di Matteo, L. Mitochondria and human preimplantation embryo development. Reproduction 2009, 137, 619–624. [Google Scholar] [CrossRef]
- Remi, D.; Duchen, M.J.C. The Role of Mitochondrial Function in the Oocyte and Embryo. Curr. Top. Dev. Biol. 2007, 77. [Google Scholar] [CrossRef]
- Rieger, D. Relationships between energy metabolism and development of early mammalian embryos. Theriogenology 1992, 37, 75–93. [Google Scholar] [CrossRef]
- Chappel, S. The Role of Mitochondria from Mature Oocyte to Viable Blastocyst. Obstet. Gynecol. Int. 2013, 2013, 183024. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.B.; dos Santos, É.C.; Ispada, J.; Fontes, P.K.; Nogueira, M.F.G.; dos Santos, C.M.D.; Milazzotto, M.P. The dynamics between in vitro culture and metabolism: Embryonic adaptation to environmental changes. Sci. Rep. 2020, 10, 15672. [Google Scholar] [CrossRef]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef]
- Konstantinidis, M.; Alfarawati, S.; Hurd, D.; Paolucci, M.; Shovelton, J.; Fragouli, E.; Wells, D. Simultaneous assessment of aneuploidy, polymorphisms, and mitochondrial DNA content in human polar bodies and embryos with the use of a novel microarray platform. Fertil. Steril. 2014, 102, 1385–1392. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Ludaway, T.; Russ, R.B.; Fields, E.J.; Koczor, C.; Lewis, W. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J. Assist. Reprod. Genet. 2012, 29, 637–642. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chrétien, M.F.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef]
- Boucret, L.; Bris, C.; Seegers, V.; Goudenège, D.; Desquiret-Dumas, V.; Domin-Bernhard, M.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Reynier, P.; et al. Deep sequencing shows that oocytes are not prone to accumulate mtDNA heteroplasmic mutations during ovarian ageing. Hum. Reprod. 2017, 32, 2101–2109. [Google Scholar] [CrossRef]
- May-Panloup, P.; Brochard, V.; Hamel, J.F.; Desquiret-Dumas, V.; Chupin, S.; Reynier, P.; Duranthon, V. Maternal ageing impairs mitochondrial DNA kinetics during early embryogenesis in mice. Hum. Reprod. 2019, 34, 1313–1324. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; de la Barca, J.M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier1, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Barritt, J.A.; Kokot, M.; Cohen, J.; Steuerwald, N.; Brenner, C.A. Quantification of human ooplasmic mitochondria. Reprod. Biomed. Online 2002, 4, 243–247. [Google Scholar] [CrossRef]
- Yang, L.; Lin, X.; Tang, H.; Fan, Y.; Zeng, S.; Jia, L.; Li, Y.; Shi, Y.; He, S.; Wang, H.; et al. Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox. Aging Cell 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of ovarian aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Navarra, A.; Levi-Setti, P.E. Qualitative and quantitative ovarian and peripheral blood mitochondrial dna (Mtdna) alterations: Mechanisms and implications for female fertility. Antioxidants 2021, 10, 55. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia 2021, 53, 1–15. [Google Scholar] [CrossRef]
- Menger, K.E.; Rodríguez-Luis, A.; Chapman, J.; Nicholls, T.J. Controlling the topology of mammalian mitochondrial DNA. Open Biol. 2021, 11, 210168. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef]
- Colella, M.; Cuomo, D.; Peluso, T.; Falanga, I.; Mallardo, M.; De Felice, M.; Ambrosino, C. Ovarian Aging: Role of Pituitary-Ovarian Axis Hormones and ncRNAs in Regulating Ovarian Mitochondrial Activity. Front. Endocrinol. (Lausanne) 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Cunarro, J.; Casado, S.; Lugilde, J.; Tovar, S. Hypothalamic Mitochondrial Dysfunction as a Target in Obesity and Metabolic Disease. Front. Endocrinol. (Lausanne). 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility – An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Tiosano, D.; Mears, J.A.; Buchner, D.A. Mitochondrial Dysfunction in Primary Ovarian Insufficiency. Endocrinology 2019, 160, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Boucret, L.; Chao De La Barca, J.M.; Morinière, C.; Desquiret, V.; Ferré-L’Hôtellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef]
- Wei, Y.H.; Kao, S.H. Mitochondrial DNA mutation and depletion are associated with decline of fertility and motility of human sperm. Zool. Stud. 2000, 39, 1–12. [Google Scholar]
- Demain, L.A.M.; Conway, G.S.; Newman, W.G. Genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017, 91, 199–207. [Google Scholar] [CrossRef]
- Machado, T.S.; Macabelli, C.H.; Collado, M.D.; Meirelles, F.V.; Guimarães, F.E.G.; Chiaratti, M.R. Evidence of Selection Against Damaged Mitochondria During Early Embryogenesis in the Mouse. Front. Genet. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Regin, M.; De Munck, N.; De Deckersberg, E.C.; Belva, F.; Sermon, K.; Tournaye, H.; Blockeel, C.; Velde, H. Van De Mitochondrial DNA variants segregate during human preimplantation development into genetically different cell lineages that are maintained postnatally. bioRxiv 2021, 3. [Google Scholar]
- Ritu, G.; Veerasigamani, G.; Ashraf, M.C.; Singh, S.; Laheri, S.; Modi, D. No association of mitochondrial DNA levels in trophectodermal cells with the developmental competence of the blastocyst and pregnancy outcomes. bioRxiv 2019, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; Diao, Z.; Zhu, L.; Zhu, J.; Lin, F.; Jiang, W.; Fang, J.; Xu, Z.; Xing, J.; Zhou, J.; et al. Trophectoderm Mitochondrial DNA Content Associated with Embryo Quality and Day-5 Euploid Blastocyst Transfer Outcomes. DNA Cell Biol. 2021, 40, 643–651. [Google Scholar] [CrossRef]
- Klimczak, A.M.; Pacheco, L.E.; Lewis, K.E.; Massahi, N.; Richards, J.P.; Kearns, W.G.; Saad, A.F.; Crochet, J.R. Embryonal mitochondrial DNA: Relationship to embryo quality and transfer outcomes. J. Assist. Reprod. Genet. 2018, 35, 871–877. [Google Scholar] [CrossRef] [PubMed]
- de los Santos, M.J.; Diez Juan, A.; Mifsud, A.; Mercader, A.; Meseguer, M.; Rubio, C.; Pellicer, A. Variables associated with mitochondrial copy number in human blastocysts: What can we learn from trophectoderm biopsies? Fertil. Steril. 2018, 109, 110–117. [Google Scholar] [CrossRef]
- Victor, A.R.; Brake, A.J.; Tyndall, J.C.; Griffin, D.K.; Zouves, C.G.; Barnes, F.L.; Viotti, M. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil. Steril. 2017, 107, 34–42.e3. [Google Scholar] [CrossRef] [PubMed]
- Fragouli, E.; Spath, K.; Alfarawati, S.; Kaper, F.; Craig, A.; Michel, C.-E.; Kokocinski, F.; Cohen, J.; Munne, S.; Wells, D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015, 11, e1005241. [Google Scholar] [CrossRef]
- Fragouli, E.; McCaffrey, C.; Ravichandran, K.; Spath, K.; Grifo, J.A.; Munné, S.; Wells, D. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: A blinded prospective non-selection study. Hum. Reprod. 2017, 32, 2340–2347. [Google Scholar] [CrossRef]
- Ravichandran, K.; McCaffrey, C.; Grifo, J.; Morales, A.; Perloe, M.; Munne, S.; Wells, D.; Fragouli, E. Mitochondrial DNA quantification as a tool for embryo viability assessment: Retrospective analysis of data from single euploid blastocyst transfers. Hum. Reprod. 2017, 32, 1282–1292. [Google Scholar] [CrossRef]
- Treff, N.R.; Zhan, Y.; Tao, X.; Olcha, M.; Han, M.; Rajchel, J.; Morrison, L.; Morin, S.J.; Scott, R.T.J. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum. Reprod. 2017, 32, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Zhang, Y.; Shu, M.; Wang, W.; Ren, L.; Chen, F.; Shao, L.; Lu, S.; Bo, S.; Ma, S.; et al. Comprehensive chromosomal and mitochondrial copy number profiling in human IVF embryos. Reprod. Biomed. Online 2018, 36, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Diez-Juan, A.; Rubio, C.; Marin, C.; Martinez, S.; Al-Asmar, N.; Riboldi, M.; Díaz-Gimeno, P.; Valbuena, D.; Simón, C. Mitochondrial DNA content as a viability score in human euploid embryos: Less is better. Fertil. Steril. 2015, 104, 534–541.e1. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.T.; Sun, L.; Zhan, Y.; Marin, D.; Tao, X.; Seli, E. Mitochondrial DNA content is not predictive of reproductive competence in euploid blastocysts. Reprod. Biomed. Online 2020, 41, 183–190. [Google Scholar] [CrossRef]
- Wu, F.S.-Y.; Weng, S.-P.; Shen, M.-S.; Ma, P.-C.; Wu, P.-K.; Lee, N.-C. Suboptimal trophectoderm mitochondrial DNA level is associated with delayed blastocyst development. J. Assist. Reprod. Genet. 2021, 38, 587–594. [Google Scholar] [CrossRef] [PubMed]
- El-Damen, A.; Elkhatib, I.; Bayram, A.; Arnanz, A.; Abdala, A.; Samir, S.; Lawrenz, B.; De Munck, N.; Fatemi, H.M. Does blastocyst mitochondrial DNA content affect miscarriage rate in patients undergoing single euploid frozen embryo transfer? J. Assist. Reprod. Genet. 2021, 38, 595–604. [Google Scholar] [CrossRef]
- Lee, Y.X.; Chen, C.H.; Lin, S.Y.; Lin, Y.H.; Tzeng, C.R. Adjusted mitochondrial DNA quantification in human embryos may not be applicable as a biomarker of implantation potential. J. Assist. Reprod. Genet. 2019, 36, 1855–1865. [Google Scholar] [CrossRef]
- De Munck, N.; Liñán, A.; Elkhatib, I.; Bayram, A.; Arnanz, A.; Rubio, C.; Garrido, N.; Lawrenz, B.; Fatemi, H.M. mtDNA dynamics between cleavage-stage embryos and blastocysts. J. Assist. Reprod. Genet. 2019, 36, 1867–1875. [Google Scholar] [CrossRef]
- Arnanz, A.; De Munck, N.; Bayram, A.; El-Damen, A.; Abdalla, A.; ElKhatib, I.; Melado, L.; Lawrenz, B.; Fatemi, H.M. Blastocyst mitochondrial DNA (mtDNA) is not affected by oocyte vitrification: A sibling oocyte study. J. Assist. Reprod. Genet. 2020, 37, 1387–1397. [Google Scholar] [CrossRef]
- Boynukalin, F.K.; Gultomruk, M.; Cavkaytar, S.; Turgut, E.; Findikli, N.; Serdarogullari, M.; Coban, O.; Yarkiner, Z.; Rubio, C.; Bahceci, M. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. PLoS ONE 2020, 15, e0227619. [Google Scholar] [CrossRef]
- Du, S.; Huang, Z.; Lin, Y.; Sun, Y.; Chen, Q.; Pan, M.; Zheng, B. Mitochondrial DNA Copy Number in Human Blastocyst: A Novel Biomarker for the Prediction of Implantation Potential. J. Mol. Diagn. 2021, 23, 637–642. [Google Scholar] [CrossRef]
- MitoScore: Mitochondrial Biomarker Developed by Igenomix Will Increase Implantation Rate of In Vitro Fertilization. Available online: https://carlos-simon.com/mitoscore-mitochondrial-biomarker-developed-by-igenomix-will-increase-implantation-rate-of-in-vitro-fertilization/ (accessed on 10 January 2022).
- Fu, L.; Luo, Y.X.; Liu, Y.; Liu, H.; Li, H.Z.; Yu, Y. Potential of Mitochondrial Genome Editing for Human Fertility Health. Front. Genet. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Yahata, N.; Matsumoto, Y.; Omi, M.; Yamamoto, N.; Hata, R. TALEN-mediated shift of mitochondrial DNA heteroplasmy in MELAS-iPSCs with m.13513G>A mutation. Sci. Rep. 2017, 7, 15557. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Kang, X.; Liang, Y.; Lan, T.; Li, T.; Tan, T.; Peng, J.; Zhang, Q.; An, G.; et al. Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell 2018, 9, 283–297. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Wang, Y.; Wang, Z.; Liu, Q.; Lu, B.; Liu, Y.; Gu, F. CRISPR/Cas9-mediated mutagenesis at microhomologous regions of human mitochondrial genome. Sci. China. Life Sci. 2021, 64, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Tang, M.X.; Gorman, G.S.; De Sutter, P.; Heindryckx, B. Novel reproductive technologies to prevent mitochondrial disease. Hum. Reprod. Update 2017, 23, 501–519. [Google Scholar] [CrossRef]
- Reznichenko, A.S.; Huyser, C.; Pepper, M.S. Mitochondrial transfer: Implications for assisted reproductive technologies. Appl. Transl. Genomics 2016, 11, 40–47. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Humaidan, P.; Coetzee, K. Mitochondria and reproduction: Possibilities for testing and treatment. Panminerva Med. 2019, 82–96. [Google Scholar] [CrossRef]
- Mobarak, H.; Heidarpour, M.; Tsai, P.S.J.; Rezabakhsh, A.; Rahbarghazi, R.; Nouri, M.; Mahdipour, M. Autologous mitochondrial microinjection; A strategy to improve the oocyte quality and subsequent reproductive outcome during aging. Cell Biosci. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.G.; Pors, S.E.; Andersen, C.Y. Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum. Reprod. 2017, 32, 725–732. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, H. Mitochondria: Emerging therapeutic strategies for oocyte rescue. Reprod. Sci. 2021, 29, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Scott, R.; Schimmel, T.; Levron, J.; Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet (London, England) 1997, 350, 186–187. [Google Scholar] [CrossRef]
- Cohen, J.; Alikani, M.; Garrisi, J.G.; Willadsen, S. Micromanipulation of human gametes and embryos: Ooplasmic donation at fertilization VIDEO. Hum. Reprod. Update 1998, 4, 195–196. [Google Scholar] [CrossRef][Green Version]
- Cohen, J.; Scott, R.; Alikani, M.; Schimmel, T.; Munné, S.; Levron, J.; Wu, L.; Brenner, C.; Warner, C.; Willadsen, S. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod. 1998, 4, 269–280. [Google Scholar] [CrossRef]
- Woods, D.C.; Tilly, J.L. Autologous Germline Mitochondrial Energy Transfer (AUGMENT) in Human Assisted Reproduction. Semin. Reprod. Med. 2015, 33, 410–421. [Google Scholar] [CrossRef]
- Woods, D.C.; Tilly, J.L. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat. Protoc. 2013, 8, 966–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. An ethical and legal analysis of ovascience – A publicly traded fertility company and its lead product AUGMENT. Am. J. Law Med. 2018, 44, 508–528. [Google Scholar] [CrossRef]
- Powell, K. Born or made? Debate on mouse eggs reignites. Nature 2006, 441, 795. [Google Scholar] [CrossRef] [PubMed]
- Grieve, K.M.; McLaughlin, M.; Dunlop, C.E.; Telfer, E.E.; Anderson, R.A. The controversial existence and functional potential of oogonial stem cells. Maturitas 2015, 82, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G.; Johnson, M.H. Can oocyte quality be augmented? Reprod. Biomed. Online 2016, 32, 551–555. [Google Scholar] [CrossRef][Green Version]
- Troubled OvaScience cuts half of workforce. Available online: https://www.biopharmadive.com/news/troubled-ovascience-cuts-half-of-workforce/514160/ (accessed on 10 January 2022).
- OvaScience Sued by Shareholders for Over-Hyped Fertility Treatment. Available online: https://www.courthousenews.com/ovascience-sued-by-shareholders-for-over-hyped-fertility-treatment/ (accessed on 10 January 2022).
- Clinical Application of Autologous Mitochondria Transplantation for Improving Oocyte Quality. Available online: https://clinicaltrials.gov/ct2/show/NCT03639506?term=Mitochondrial+transfer&draw=2&rank=7 (accessed on 10 January 2022).

| Material | N | Aneploidy | Morphology | Implantation | Live Birth | Maternal Age | |
|---|---|---|---|---|---|---|---|
| Ritu et al., 2019 | TE | 287 | • | • | • | • | • |
| Scott et al., 2020 | TE | 615 | - | • | • | • | • |
| Wu et al., 2021 | TE | 1301 | - | • | • | - | • |
| El-Damen et al., 2021 | TE | 355 | - | •/• | • | • | • |
| Lee et al., 2019 | B | 39 | • | - | - | - | - |
| TE | 998 | • | - | • | - | • | |
| De Munk et al., 2021 | B | 112 | • | - | - | - | • |
| TE | 112 | • | - | - | - | • | |
| Diez-Juan et al., 2015 | B | 205 | - | • | • | - | - |
| TE | 65 | - | • | • | - | • | |
| Arnanz et al., 2020 | TE | 504 | • | • | - | - | - |
| Boynukalin et al., 2020 | TE | 707 | - | - | - | • | - |
| Du et al., 2021 | TE | 246 | • | • | • | - | - |
| Wang et al., 2021 | TE | 769 | - | • | • | • | • |
| Klimczak et al., 2018 | TE | 1510 | - | • | • | - | • |
| de Los Santos et al., 2018 | TE | 465 | • | • | - | - | • |
| Victor et al., 2017 | TE | 1396 | • | - | • | - | • |
| Fragouli et al., 2015 | B | 39 | - | - | - | - | • |
| TE | 340 | • | - | • | - | • | |
| Fragouli et al., 2017 | TE | 199 | - | - | - | - | • |
| Ravichandran et al., 2017 | TE | 1505 | - | • | • | - | • |
| Treff et al., 2017 | TE | 374 | - | • | • | - | • |
| Shang et al., 2018 | B | 149 | • | • | • | - | • |
| TE | 250 | ||||||
| Podolak et al., 2022 | B | 314 | • | • | • | • | • |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolak, A.; Woclawek-Potocka, I.; Lukaszuk, K. The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA? Cells 2022, 11, 797. https://doi.org/10.3390/cells11050797
Podolak A, Woclawek-Potocka I, Lukaszuk K. The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA? Cells. 2022; 11(5):797. https://doi.org/10.3390/cells11050797
Chicago/Turabian StylePodolak, Amira, Izabela Woclawek-Potocka, and Krzysztof Lukaszuk. 2022. "The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA?" Cells 11, no. 5: 797. https://doi.org/10.3390/cells11050797
APA StylePodolak, A., Woclawek-Potocka, I., & Lukaszuk, K. (2022). The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA? Cells, 11(5), 797. https://doi.org/10.3390/cells11050797






