CRISPR/Cas9-Mediated Models of Retinitis Pigmentosa Reveal Differential Proliferative Response of Müller Cells between Xenopus laevis and Xenopus tropicalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. CRISPR sgRNA Design and RNA Synthesis
2.2. Animals and Micro-Injections
2.3. Genotyping of Embryos
2.4. BrdU Labelling and Tissue Preparation
2.5. Immunofluorescence, H&E Staining
2.6. Microscopy, Quantification, and Statistical Analysis
2.7. Ethics Statement
3. Results
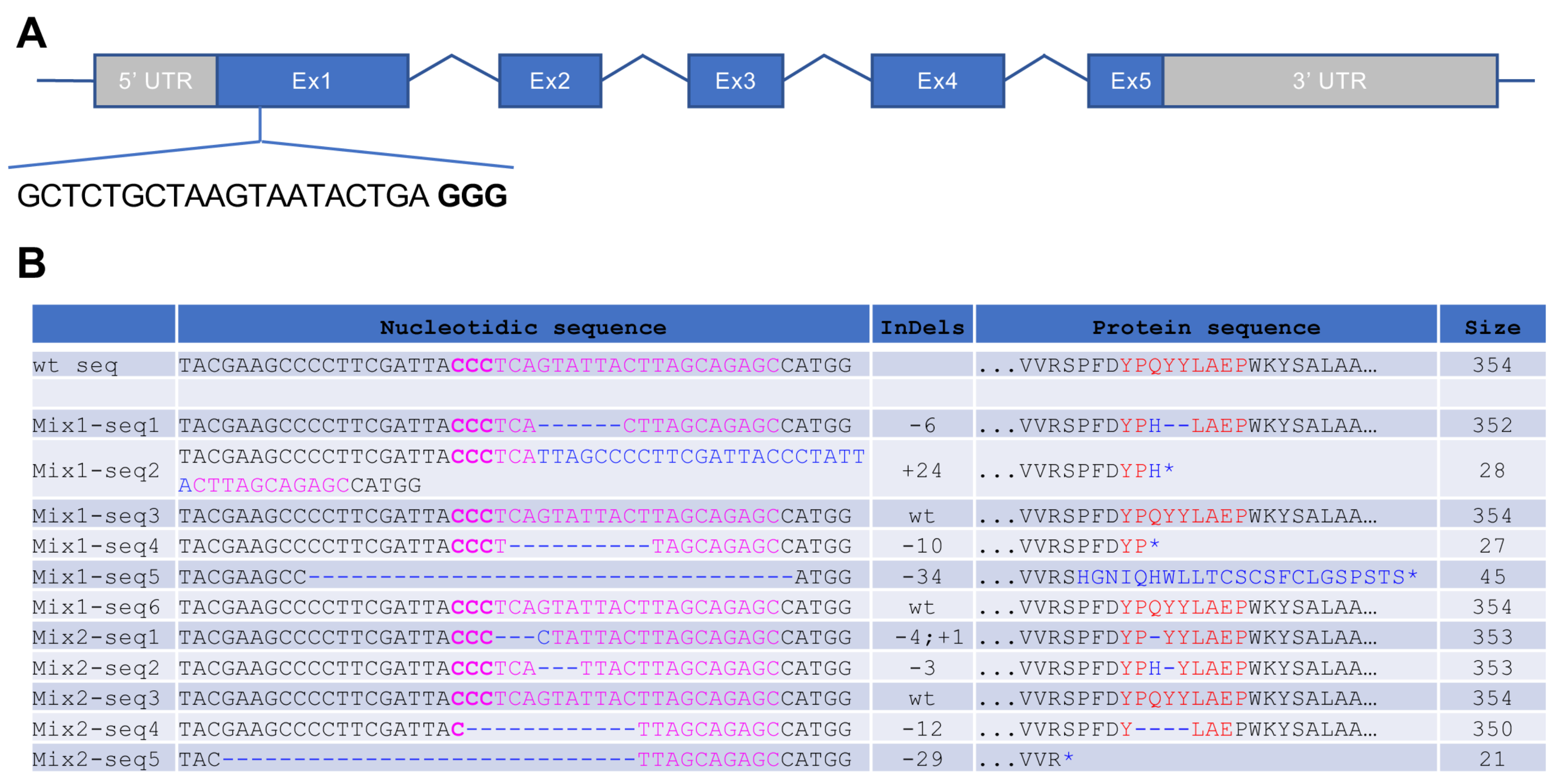
3.1. Generation of CRISPR/Cas9-Induced Rhodopsin Mutations in X. tropicalis
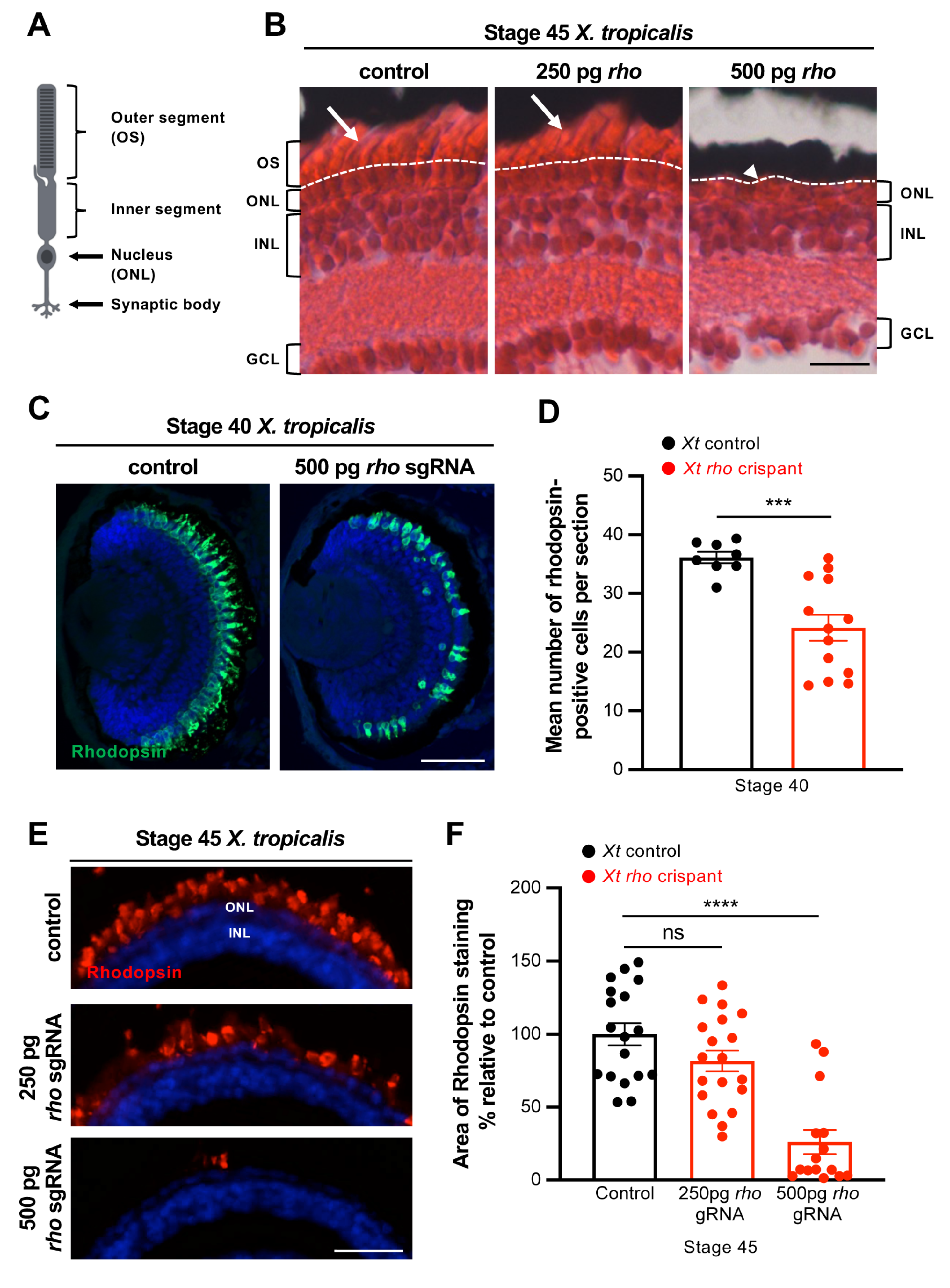
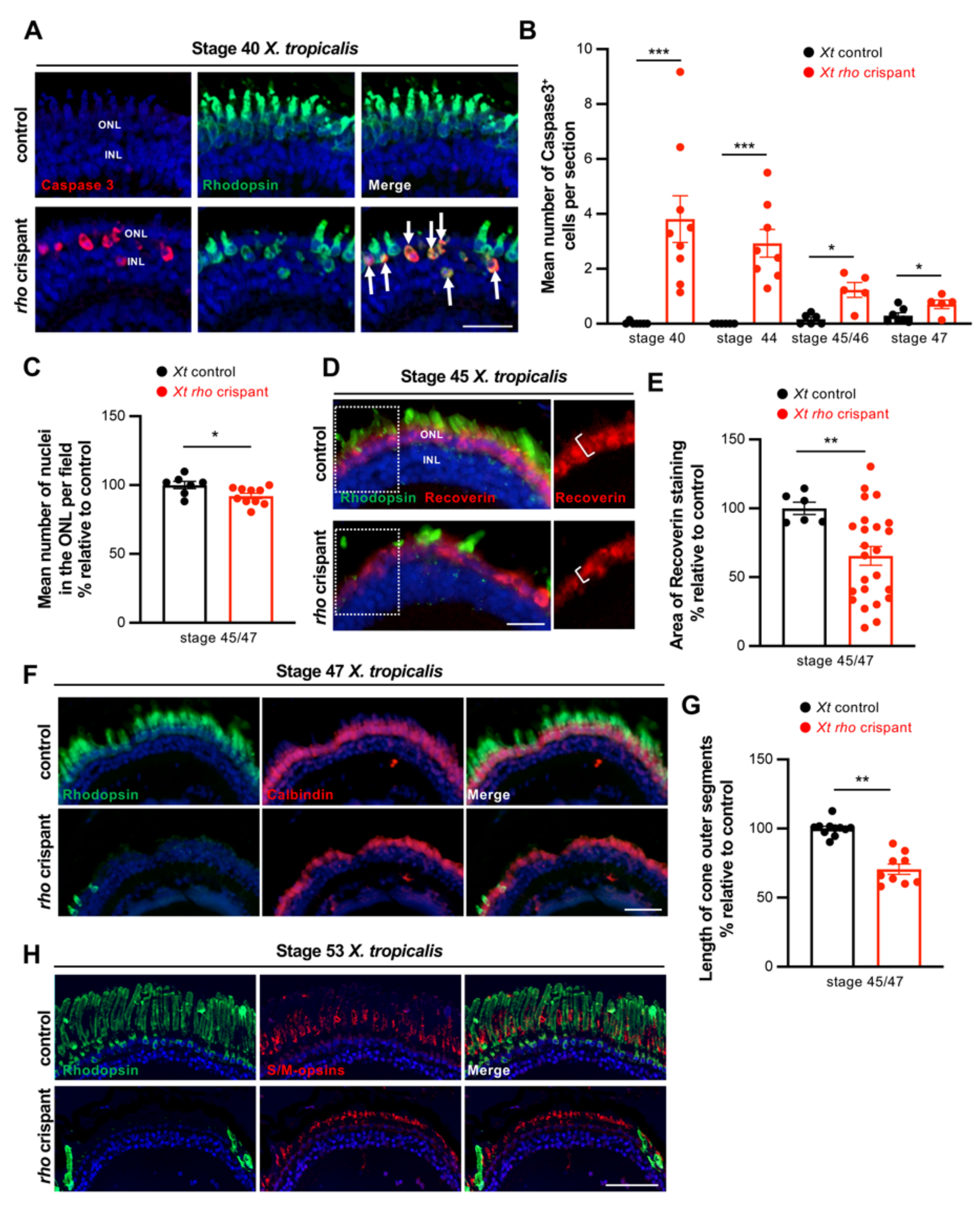
3.2. Characterization of Photoreceptor Degeneration in X. tropicalis rho Crispants
3.3. Characterization of Indels and Associated Phenotypes in F1 X. tropicalis rho Mutant Tadpoles
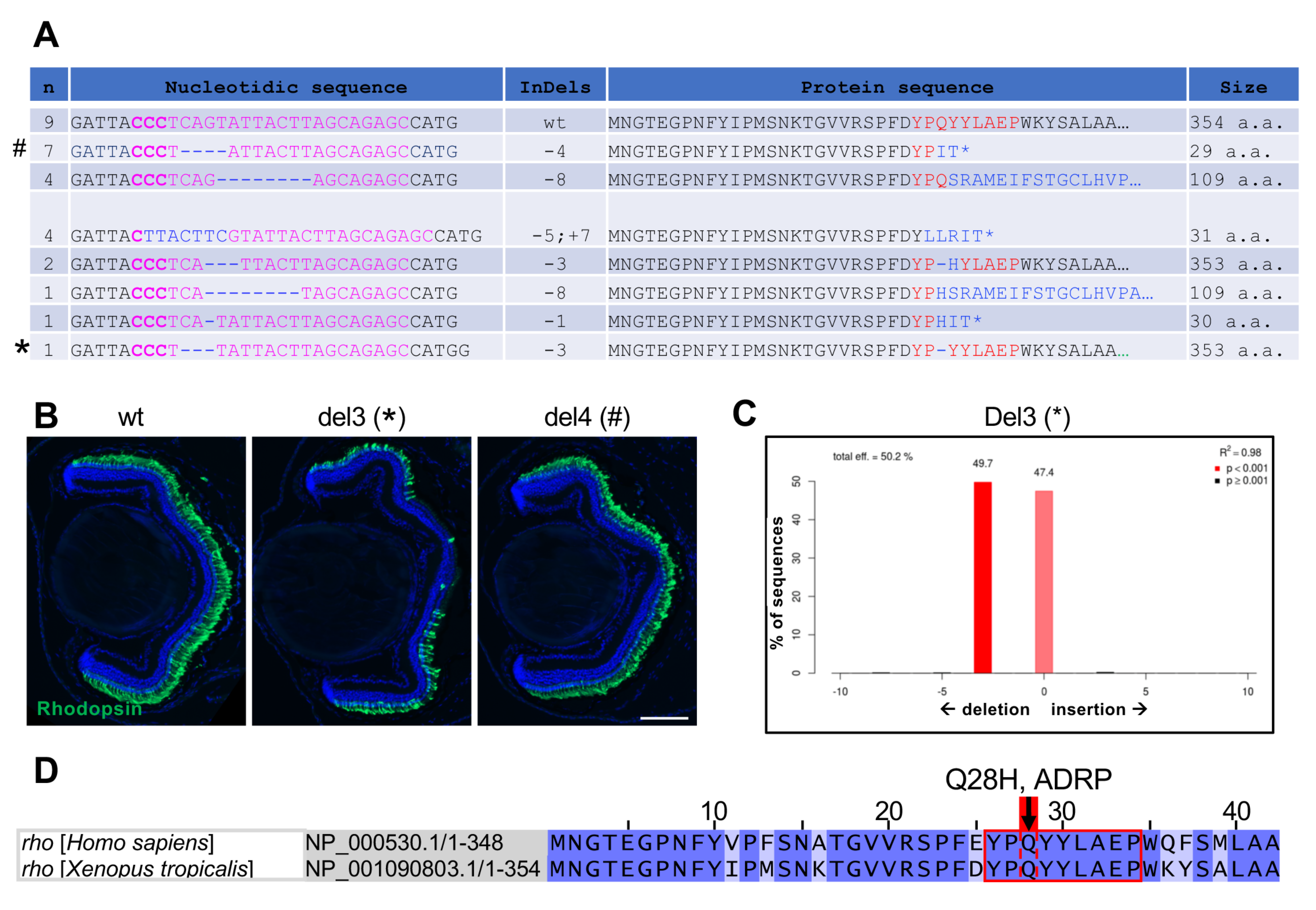
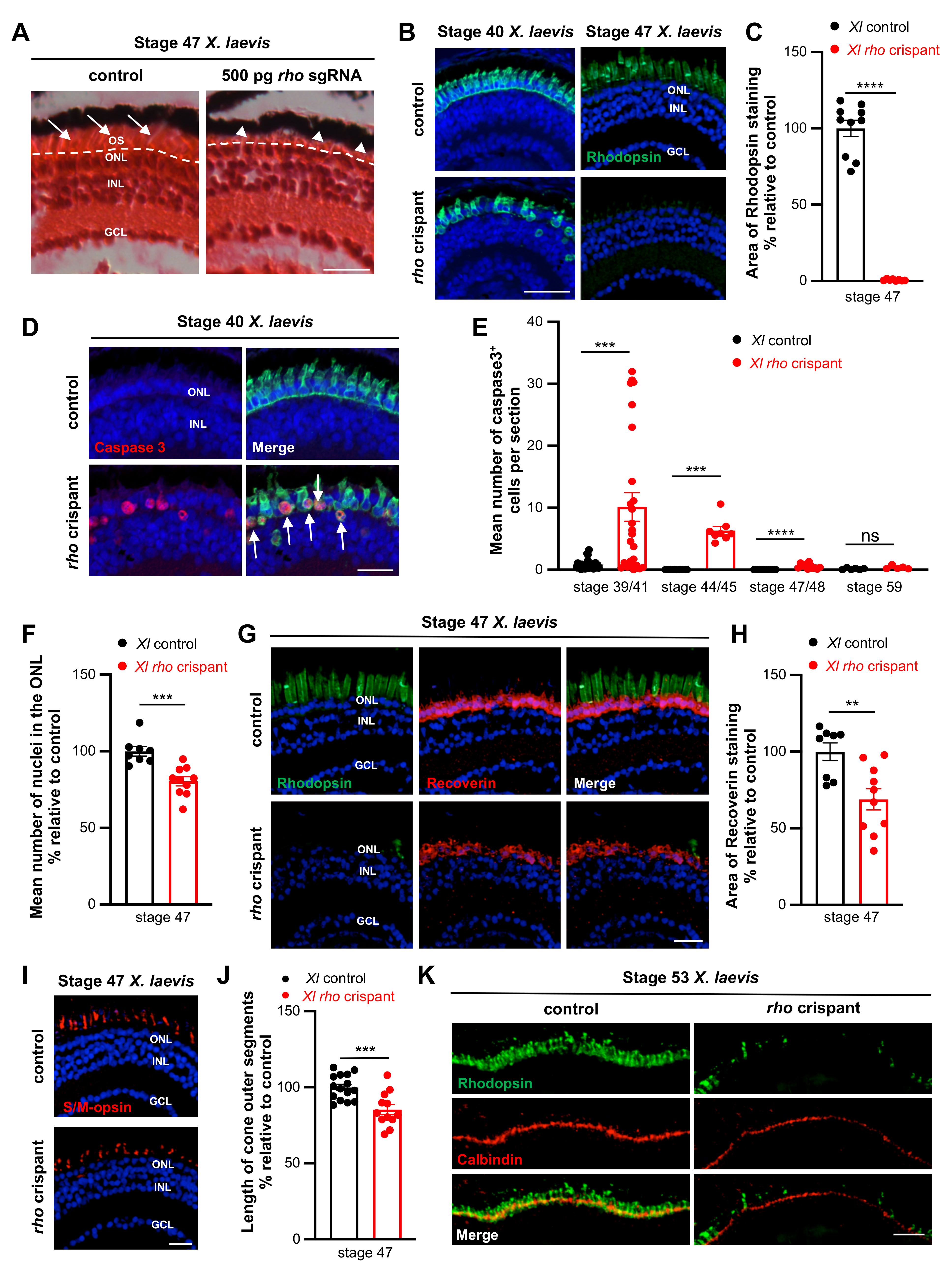
3.4. CRISPR/Cas9-Mediated Rhodopsin Editing in X. laevis
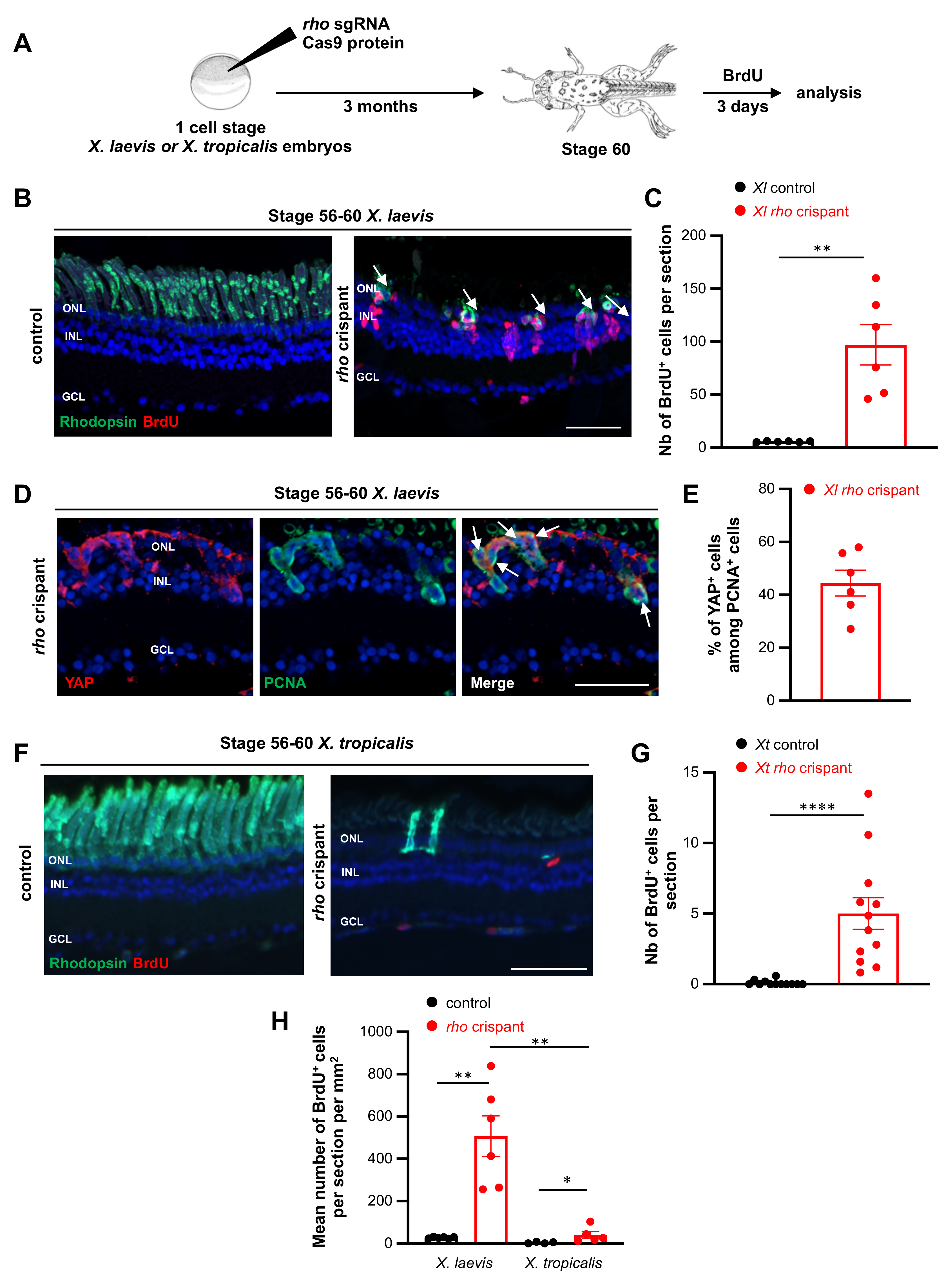
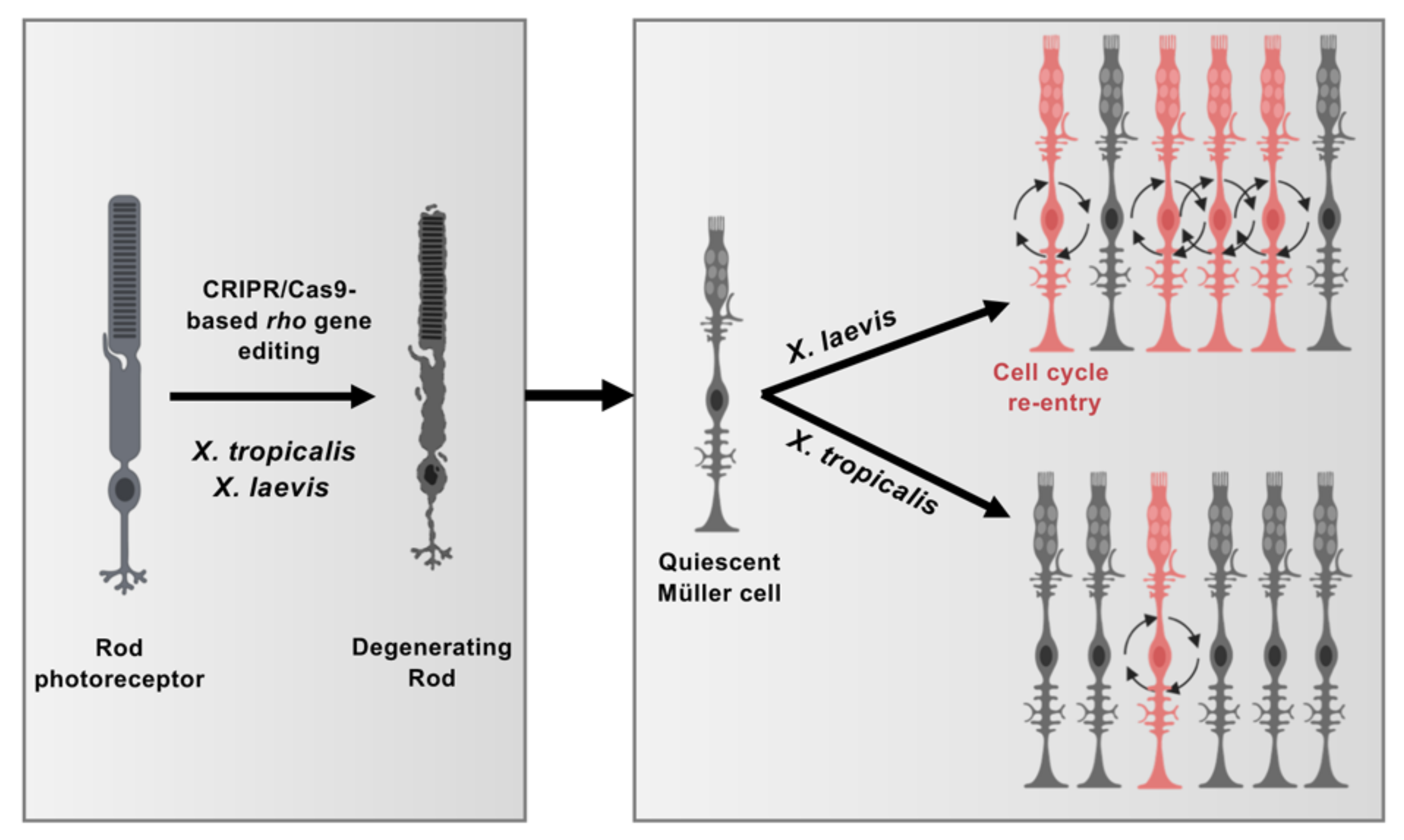
3.5. Comparison of X. laevis and X. tropicalis Müller Cell Response to Rod Degeneration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Goldman, D. Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 2016, 40, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Langhe, R.; Chesneau, A.; Colozza, G.; Hidalgo, M.; Ail, D.; Locker, M.; Perron, M. Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia 2017, 65, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; McClements, M.E.; MacLaren, R.E. Insights on the Regeneration Potential of Müller Glia in the Mammalian Retina. Cells 2021, 10, 1957. [Google Scholar] [CrossRef]
- García-García, D.; Locker, M.; Perron, M. Update on Müller glia regenerative potential for retinal repair. Curr. Opin. Genet. Dev. 2020, 64, 52–59. [Google Scholar] [CrossRef]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef]
- Wilken, M.S.; Reh, T.A. Retinal regeneration in birds and mice. Curr. Opin. Genet. Dev. 2016, 40, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, C.; Ueda, Y.; Okamoto, M.; Araki, M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007, 303, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Miyake, A.; Araki, M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: A study of retinal regeneration in a novel animal model. Dev. Neurobiol. 2014, 74, 739–756. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feehan, J.M.; Chiu, C.N.; Stanar, P.; Tam, B.M.; Ahmed, S.N.; Moritz, O.L. Modeling Dominant and Recessive Forms of Retinitis Pigmentosa by Editing Three Rhodopsin-Encoding Genes in Xenopus Laevis Using Crispr/Cas9. Sci. Rep. 2017, 7, 6920. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, T.; Hu, Z.; Zhang, Y.; Shi, Z.; Wang, Q.; Cui, Y.; Wang, F.; Zhao, H.; Chen, Y. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development 2014, 141, 707–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwkoop, P.; Faber, J. Normal Table of Xenopus laevis; Garland Pub: New York, NY, USA, 1994. [Google Scholar]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]
- Zelinka, C.P.; Sotolongo-Lopez, M.; Fadool, J.M. Targeted disruption of the endogenous zebrafish rhodopsin locus as models of rapid rod photoreceptor degeneration. Mol. Vis. 2018, 24, 587. [Google Scholar] [PubMed]
- Shigeta, M.; Sakane, Y.; Iida, M.; Suzuki, M.; Kashiwagi, K.; Kashiwagi, A.; Fujii, S.; Yamamoto, T.; Suzuki, K.I.T. Rapid and efficient analysis of gene function using CRISPR-Cas9 in Xenopus tropicalis founders. Genes Cells 2016, 21, 755–771. [Google Scholar] [CrossRef] [Green Version]
- Wiechmann, A.F.; Martin, T.A.; Horb, M.E. CRISPR/Cas9 mediated mutation of the mtnr1a melatonin receptor gene causes rod photoreceptor degeneration in developing Xenopus tropicalis. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Mehravar, M.; Shirazi, A.; Nazari, M.; Banan, M. Mosaicism in CRISPR/Cas9-mediated genome editing. Dev. Biol. 2019, 445, 156–162. [Google Scholar] [CrossRef]
- Chang, W.S.; Harris, W.A. Sequential genesis and determination of cone and rod photoreceptors in Xenopus. J. Neurobiol. 1998, 35, 227–244. [Google Scholar] [CrossRef]
- Opefi, C.A.; South, K.; Reynolds, C.A.; Smith, S.O.; Reeves, P.J. Retinitis pigmentosa mutants provide insight into the role of the N-terminal cap in rhodopsin folding, structure, and function. J. Biol. Chem. 2013, 288, 33912–33926. [Google Scholar] [CrossRef] [Green Version]
- Hamon, A.; Masson, C.; Bitard, J.; Gieser, L.; Roger, J.E.; Perron, M. Retinal Degeneration Triggers the Activation of YAP/TEAD in Reactive Müller Cells. Investig. Opthalmology Vis. Sci. 2017, 58, 1941. [Google Scholar] [CrossRef]
- Poché, R.A.; Furuta, Y.; Chaboissier, M.-C.; Schedl, A.; Behringer, R.R. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Müller glial cell development. J. Comp. Neurol. 2008, 510, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Naert, T.; Vleminckx, K. CRISPR/Cas9 disease models in zebrafish and Xenopus: The genetic renaissance of fish and frogs. Drug Discov. Today Technol. 2018, 28, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.M.; Rancourt, D.; Farrar, G.J.; Kenna, P.; Hazel, M.; Bush, R.A.; Sieving, P.A.; Sheils, D.M.; McNally, N.; Creighton, P.; et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 1997, 15, 216–219. [Google Scholar] [CrossRef]
- Humphries, M.M.; Kiang, S.; McNally, N.; Donovan, M.A.; Sieving, P.A.; Bush, R.A.; Machida, S.; Cotter, T.; Hobson, A.; Farrar, J.; et al. Comparative structural and functional analysis of photoreceptor neurons of Rho-/- mice reveal increased survival on C57BL/6J in comparison to 129Sv genetic background. Vis. Neurosci. 2001, 18, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Araki, M. A novel mode of retinal regeneration: The merit of a new Xenopus model. Neural Regen. Res. 2014, 9, 2125–2127. [Google Scholar] [CrossRef]
- Chiba, C. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp. Eye Res. 2014, 123, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lust, K.; Wittbrodt, J. Activating the regenerative potential of Müller glia cells in a regeneration-deficient retina. Elife 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.C.; Patel, J.H.; Kakebeen, A.D.; Wills, A.E. Nutrient availability contributes to a graded refractory period for regeneration in Xenopus tropicalis. Dev. Biol. 2021, 473, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C.; Scholz, T.L.; Brockerhoff, S.E.; Fadool, J.M. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev. Neurobiol. 2008, 68, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Santhanam, A.; Shihabeddin, E.; Atkinson, J.A.; Nguyen, D.; Lin, Y.-P.; O’Brien, J. A Zebrafish Model of Retinitis Pigmentosa Shows Continuous Degeneration and Regeneration of Rod Photoreceptors. Cells 2020, 9, 2242. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parain, K.; Lourdel, S.; Donval, A.; Chesneau, A.; Borday, C.; Bronchain, O.; Locker, M.; Perron, M. CRISPR/Cas9-Mediated Models of Retinitis Pigmentosa Reveal Differential Proliferative Response of Müller Cells between Xenopus laevis and Xenopus tropicalis. Cells 2022, 11, 807. https://doi.org/10.3390/cells11050807
Parain K, Lourdel S, Donval A, Chesneau A, Borday C, Bronchain O, Locker M, Perron M. CRISPR/Cas9-Mediated Models of Retinitis Pigmentosa Reveal Differential Proliferative Response of Müller Cells between Xenopus laevis and Xenopus tropicalis. Cells. 2022; 11(5):807. https://doi.org/10.3390/cells11050807
Chicago/Turabian StyleParain, Karine, Sophie Lourdel, Alicia Donval, Albert Chesneau, Caroline Borday, Odile Bronchain, Morgane Locker, and Muriel Perron. 2022. "CRISPR/Cas9-Mediated Models of Retinitis Pigmentosa Reveal Differential Proliferative Response of Müller Cells between Xenopus laevis and Xenopus tropicalis" Cells 11, no. 5: 807. https://doi.org/10.3390/cells11050807
APA StyleParain, K., Lourdel, S., Donval, A., Chesneau, A., Borday, C., Bronchain, O., Locker, M., & Perron, M. (2022). CRISPR/Cas9-Mediated Models of Retinitis Pigmentosa Reveal Differential Proliferative Response of Müller Cells between Xenopus laevis and Xenopus tropicalis. Cells, 11(5), 807. https://doi.org/10.3390/cells11050807






