Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements
Abstract
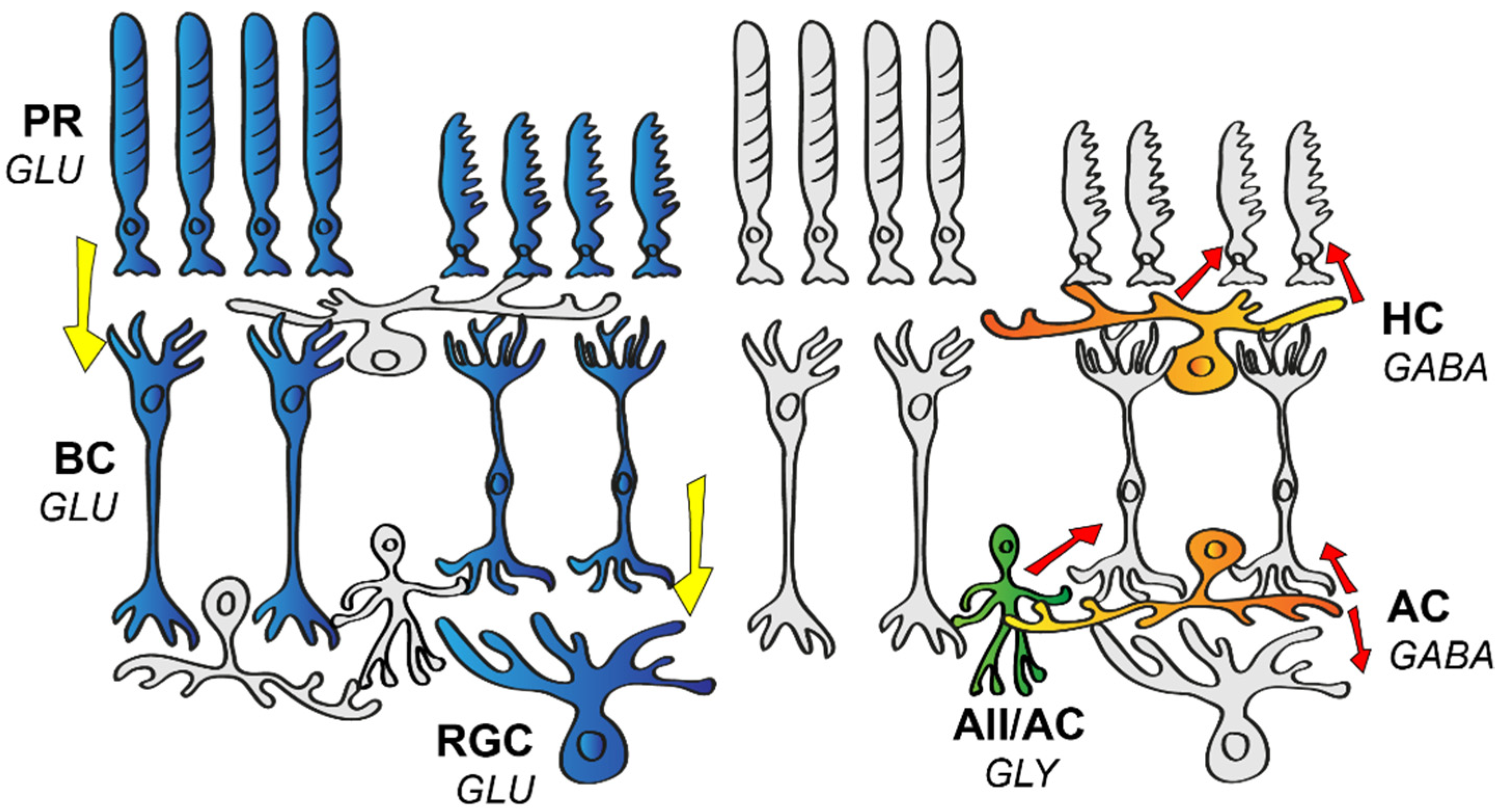
:1. The Wiring of the Mammalian Retina
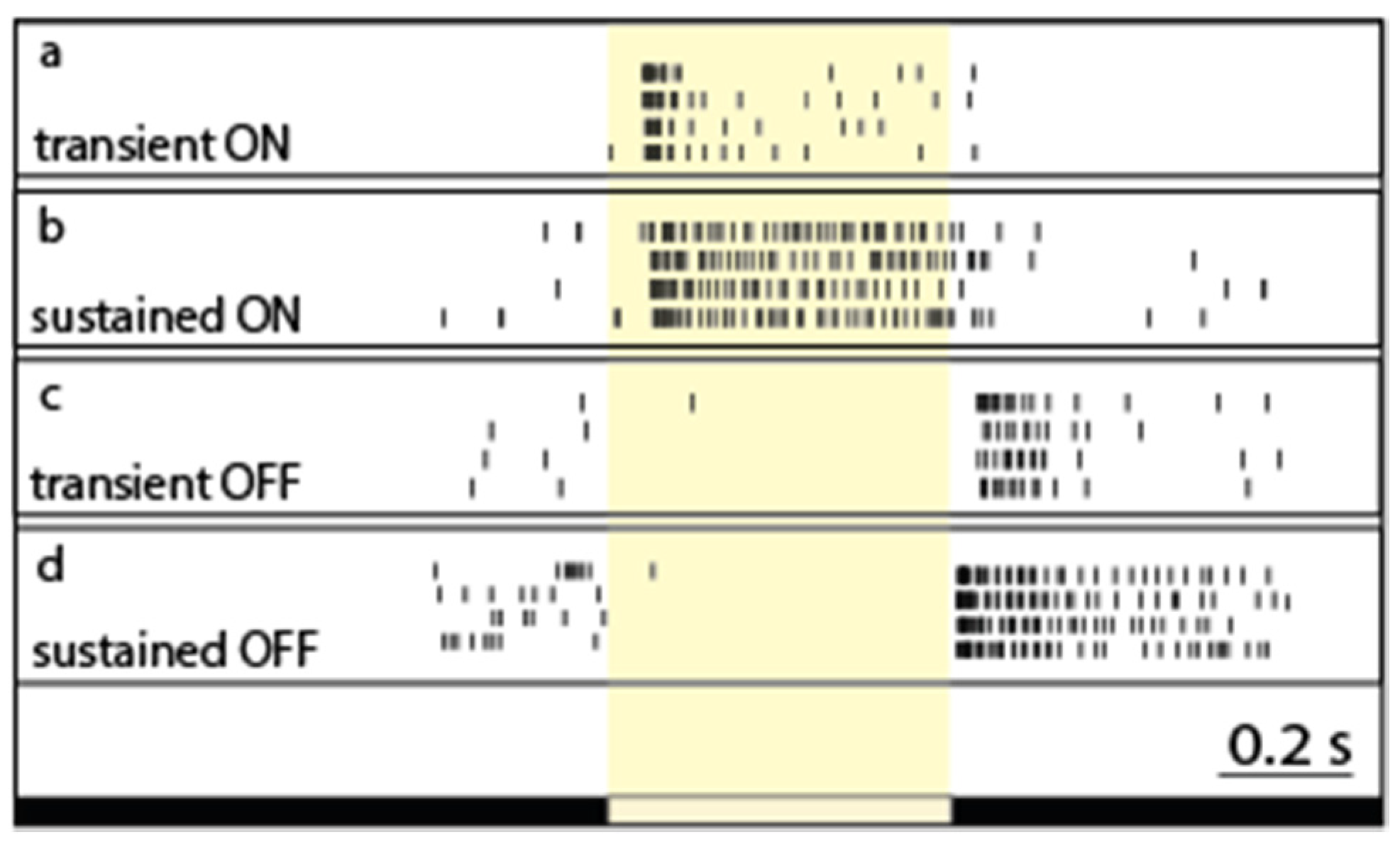
2. RGC Response Transience and Possible Visual Functions
3. Circuit Elements That Contribute to Response Transience
3.1. The Photoreceptor to Bipolar Cell Synapses in the Outer Retina
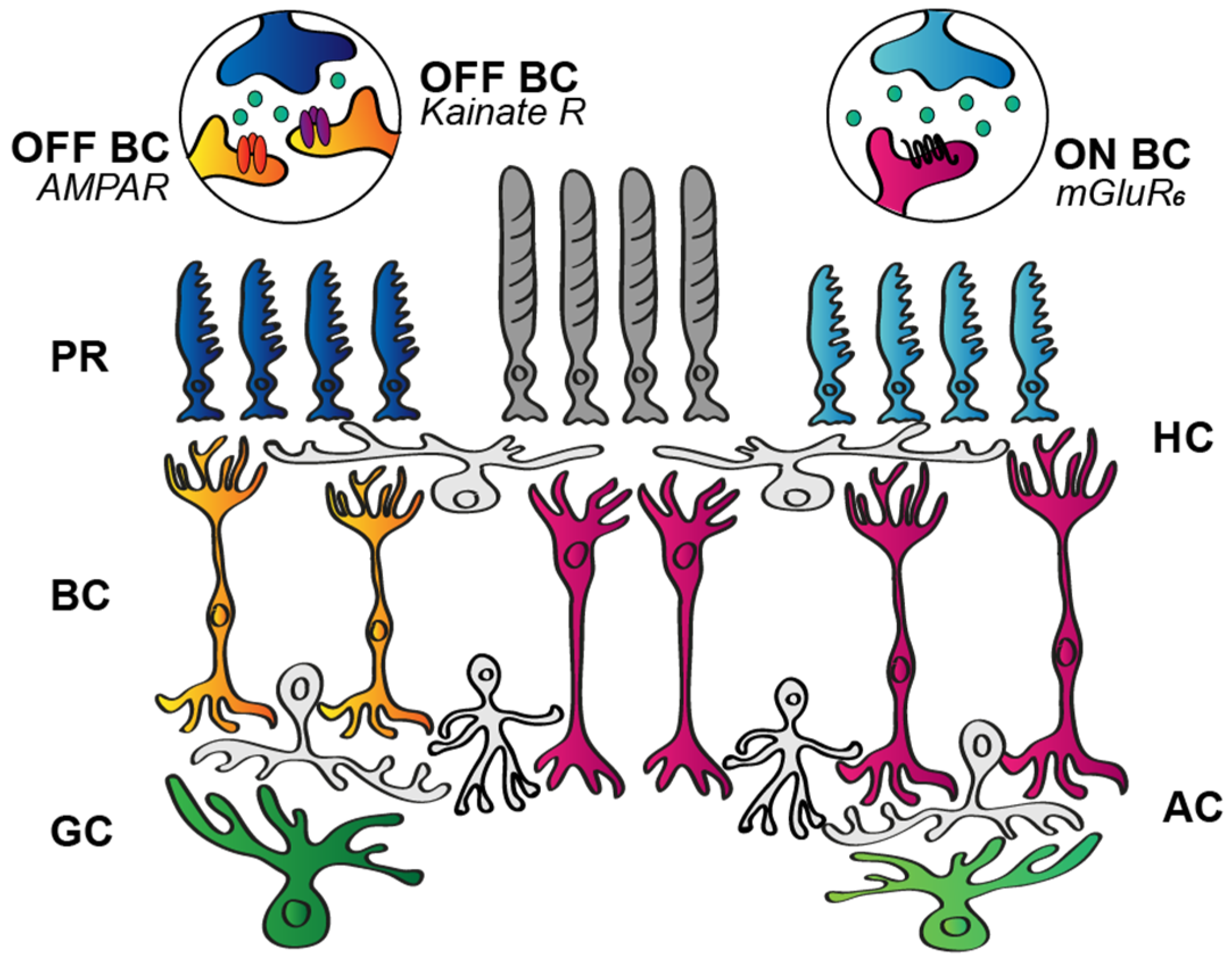
3.1.1. Postsynaptic BC Glutamate Receptors
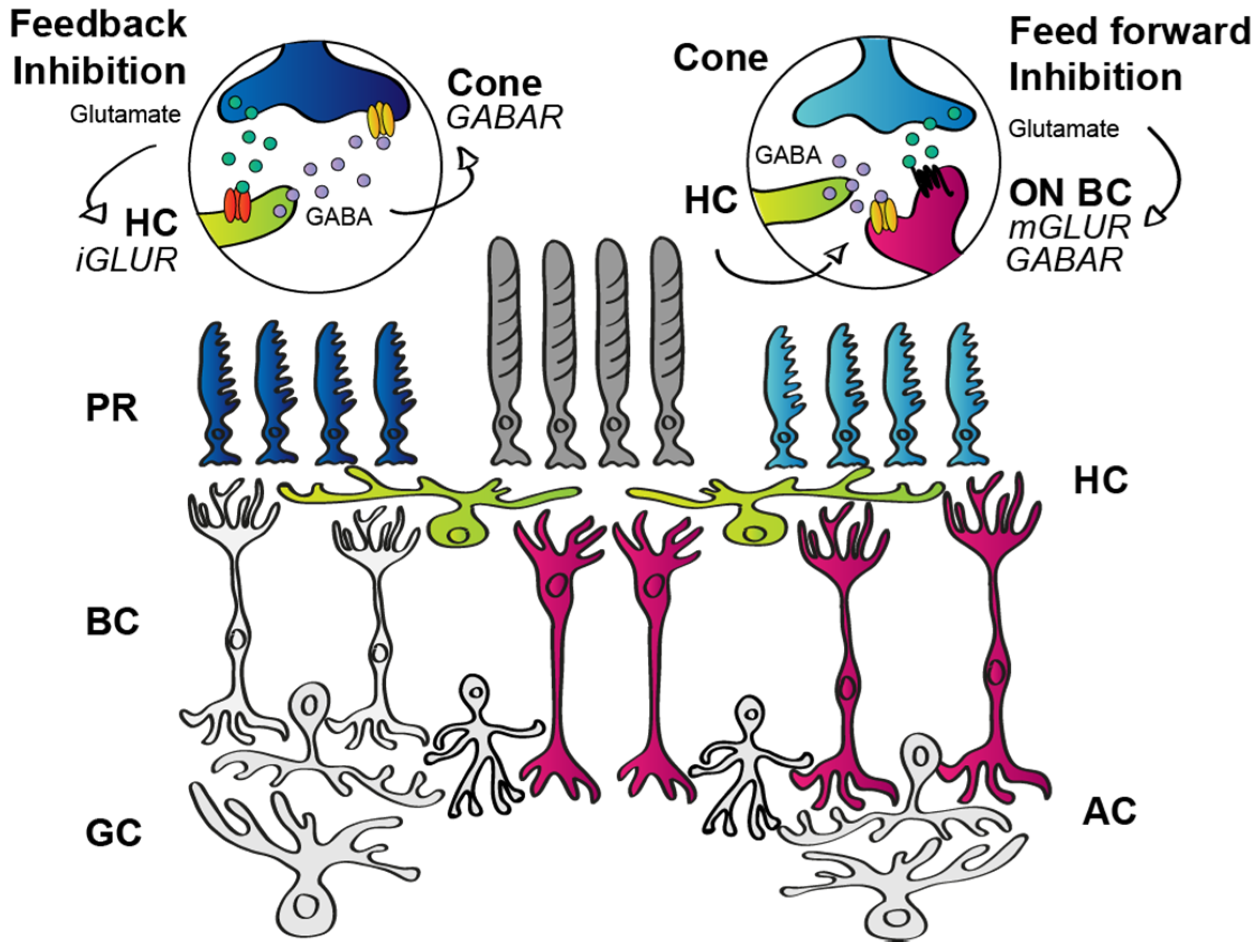
3.1.2. Outer Retinal Inhibition
3.2. Bipolar Cell Characteristics
3.2.1. Active Membrane Properties of BCs
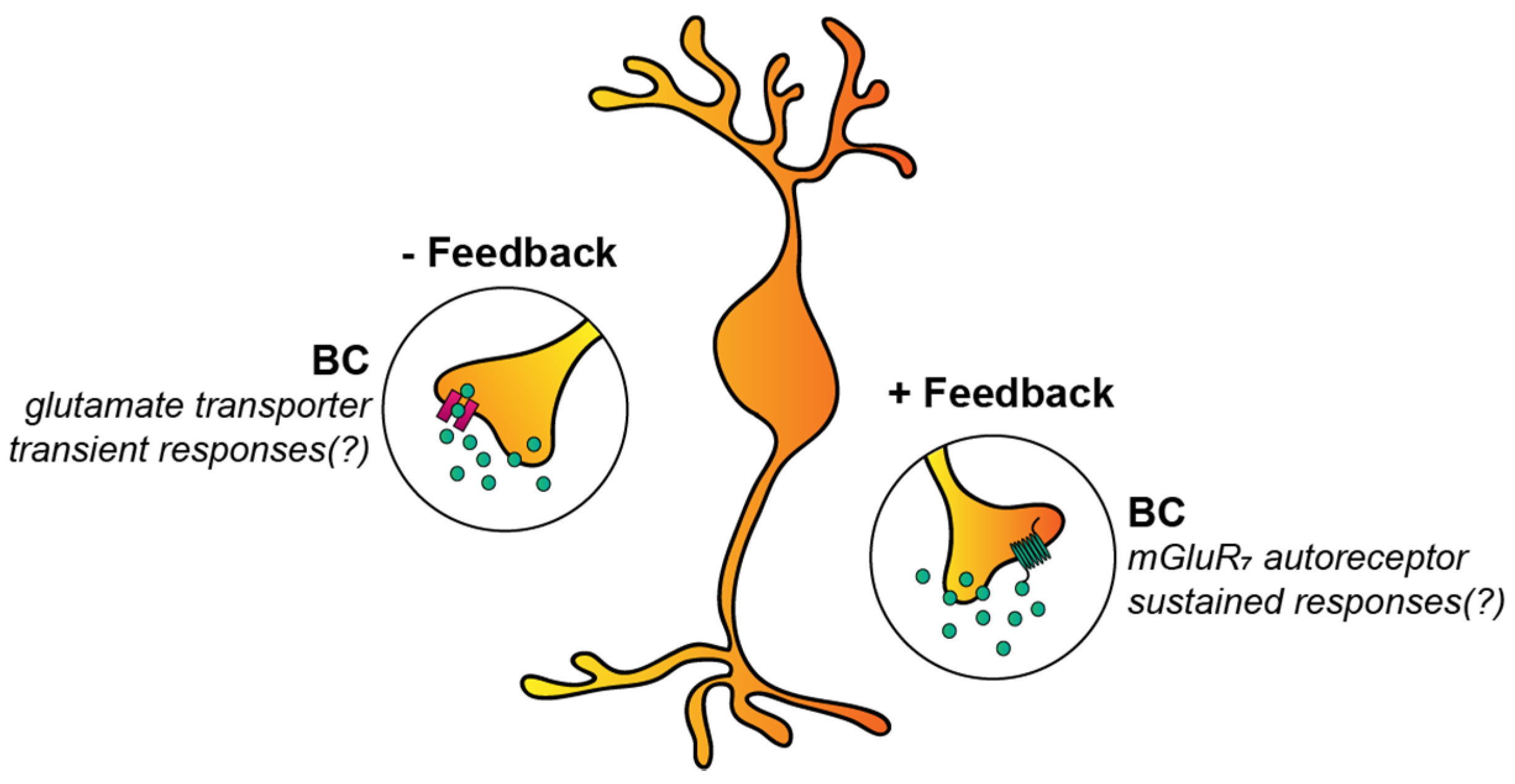
3.2.2. Autoreceptors and Glutamate Transporters of BCs
3.3. Inner Retinal Contribution
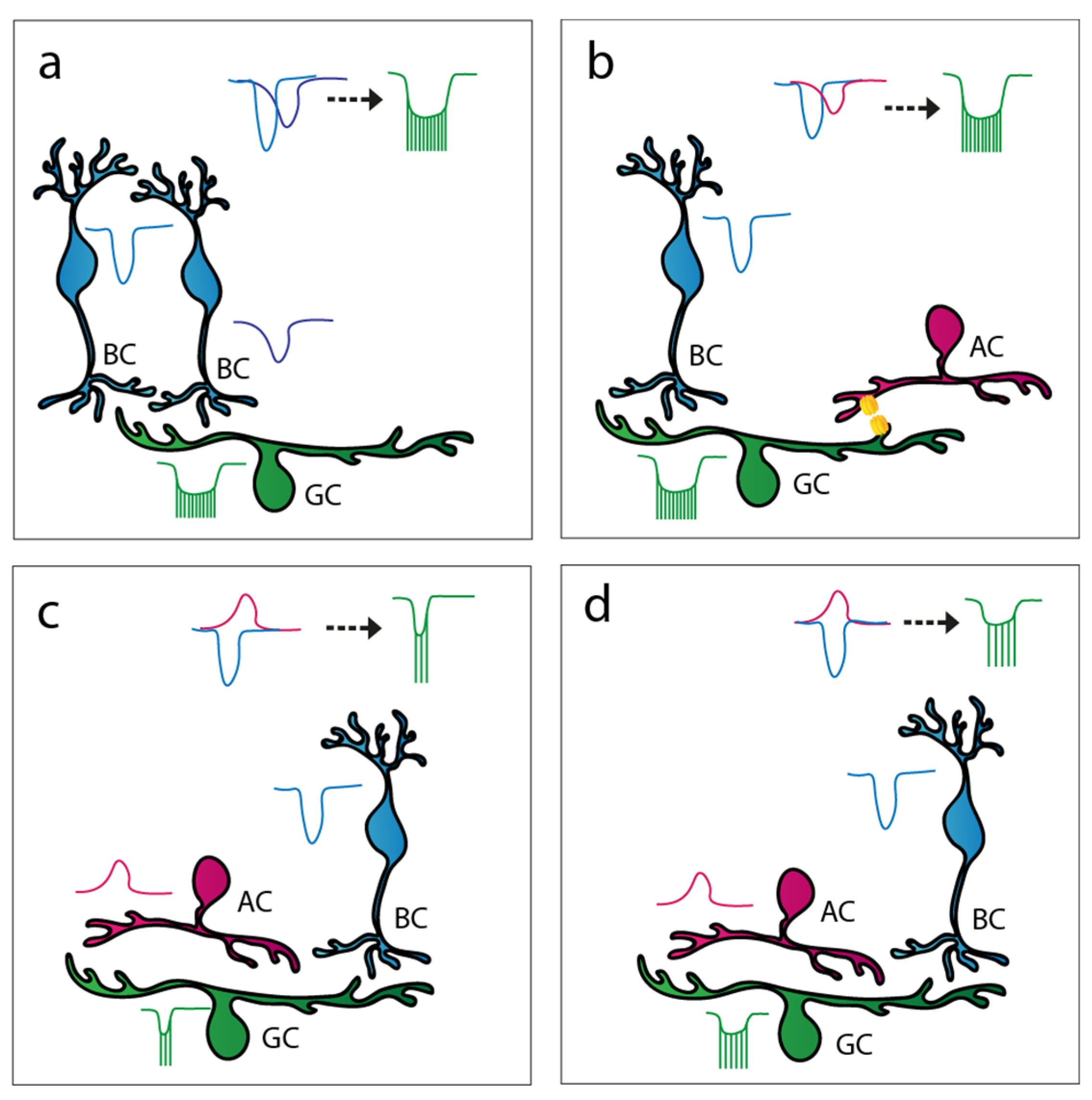
3.3.1. AC to BC Feedback Inhibition
3.3.2. AC to RGC Feedforward Inhibition
3.3.3. Crossover Inhibition
3.3.4. AC to AC Inhibition
3.4. Summation of Signals in the Inner Retina
4. The Visual Function of RGC Transience and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, F.A.; Wong, R.O.L.; Sidhu, S.K.; Weavil, J.C.; Venturelli, M.; Garten, R.S.; Rossman, M.J.; Richardson, R.S.; Gmelch, B.S.; Morgan, D.E.; et al. Wiring patterns in the mouse retina: Collecting evidence across the connectome, physiology and light microscopy. J. Physiol. 2014, 592, 4809–4823. [Google Scholar] [CrossRef] [PubMed]
- Baden, T.; Schubert, T.; Chang, L.; Wei, T.; Zaichuk, M.; Wissinger, B.; Euler, T. A Tale of Two Retinal Domains: Near-Optimal Sampling of Achromatic Contrasts in Natural Scenes through Asymmetric Photoreceptor Distribution. Neuron 2013, 80, 1206–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peichl, L.; González-Soriano, J. Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 1994, 11, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, K.; Lapan, S.W.; Whitney, I.E.; Tran, N.M.; Macosko, E.Z.; Kowalczyk, M.; Adiconis, X.; Levin, J.; Nemesh, J.; Goldman, M.; et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 2016, 166, 1308–1323.e30. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar] [CrossRef]
- Baylor, D. How photons start vision. Proc. Natl. Acad. Sci. USA 1996, 93, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.R. Imaging single cells in the living retina. Vis. Res. 2011, 51, 1379–1396. [Google Scholar] [CrossRef] [Green Version]
- Wässle, H.; Puller, C.; Müller, F.; Haverkamp, S.; Mueller, F. Cone Contacts, Mosaics, and Territories of Bipolar Cells in the Mouse Retina. J. Neurosci. 2009, 29, 106–117. [Google Scholar] [CrossRef]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthussery, T.; Percival, K.A.; Venkataramani, S.; Gayet-Primo, J.; Grünert, U.; Taylor, W. Kainate Receptors Mediate Synaptic Input to Transient and Sustained OFF Visual Pathways in Primate Retina. J. Neurosci. 2014, 34, 7611–7621. [Google Scholar] [CrossRef] [Green Version]
- Behrens, C.; Yadav, S.C.; Korympidou, M.M.; Zhang, Y.; Haverkamp, S.; Irsen, S.; Schaedler, A.; Lu, X.; Liu, Z.; Lause, J.; et al. Retinal horizontal cells use different synaptic sites for global feedforward and local feedback signaling. Curr. Biol. 2021, 32, 545–558.e5. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B.; Mangel, S.C. Lateral interactions in the outer retina. Prog. Retin. Eye Res. 2012, 31, 407–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, E.D.; Lukasiewicz, P.D. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis. Neurosci. 2011, 28, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; McCall, M.A. Receptor targets of amacrine cells. Vis. Neurosci. 2012, 29, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Baccus, S.A.; Ölveczky, B.P.; Manu, M.; Meister, M. A Retinal Circuit That Computes Object Motion. J. Neurosci. 2008, 28, 6807–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völgyi, B.; Deans, M.R.; Paul, D.L.; Bloomfield, S.A. Convergence and Segregation of the Multiple Rod Pathways in Mammalian Retina. J. Neurosci. 2004, 24, 11182–11192. [Google Scholar] [CrossRef] [Green Version]
- Pahlberg, J.; Sampath, A.P. Visual threshold is set by linear and nonlinear mechanisms in the retina that mitigate noise. BioEssays 2011, 33, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.; Esposti, F.; Lagnado, L. Color Vision: Retinal Blues. Curr. Biol. 2012, 22, R637–R639. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Chen, L.; Chen, M.; Ye, M.; Seal, R.P.; Zhou, Z.J. An Unconventional Glutamatergic Circuit in the Retina Formed by vGluT3 Amacrine Cells. Neuron 2014, 84, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [Green Version]
- Gollisch, T.; Meister, M. Rapid Neural Coding in the Retina with Relative Spike Latencies. Science 2008, 319, 1108–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, H.; Wright, M. Receptive field organization of ‘sustained’ and ‘transient’ retinal ganglion cells which subserve different functional roles. J. Physiol. 1972, 227, 769–800. [Google Scholar] [CrossRef]
- Tengölics, J.; Szarka, G.; Ganczer, A.; Szabó-Meleg, E.; Nyitrai, M.; Kovács-Öller, T.; Völgyi, B. Response Latency Tuning by Retinal Circuits Modulates Signal Efficiency. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bonaventure, N.; Wioland, N.; Roussel, G. Effects of some amino acids (GABA, glycine, taurine) and of their antagonists (picrotoxin, strychnine) on spatial and temporal features of frog retinal ganglion cell responses. Pflügers Arch. 1980, 385, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.H.; Daw, N.W. New properties of rabbit retinal ganglion cells. J. Physiol. 1978, 276, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Cleland, B.G.; Dubin, M.W.; Levick, W.R. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J. Physiol. 1971, 217, 473–496. [Google Scholar] [CrossRef]
- Gouras, P. Identification of cone mechanisms in monkey ganglion cells. J. Physiol. 1968, 199, 533–547. [Google Scholar] [CrossRef] [Green Version]
- Granda, A.M.; Fulbrook, J.E. Classification of turtle retinal ganglion cells. J. Neurophysiol. 1989, 62, 723–737. [Google Scholar] [CrossRef]
- Jones, I.L.; Russell, T.L.; Farrow, K.; Efiscella, M.; Franke, F.; Emüller, J.; Ejäckel, D.; Hierlemann, A. A method for electrophysiological characterization of hamster retinal ganglion cells using a high-density CMOS microelectrode array. Front. Neurosci. 2015, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.D.; Petry, H.M. Temporal modulation sensitivity of tree shrew retinal ganglion cells. Vis. Neurosci. 2003, 20, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirenberg, S.; Meister, M. The Light Response of Retinal Ganglion Cells Is Truncated by a Displaced Amacrine Circuit. Neuron 1997, 18, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Vallerga, S.; Usai, C. Relation between light responses and dendritic branching in the salamander ganglion cells. Exp. Biol. 1986, 45, 81–90. [Google Scholar] [PubMed]
- Werblin, F.S.; Dowling, J.E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 1969, 32, 339–355. [Google Scholar] [CrossRef]
- Wong, K.Y.; Dunn, F.A.; Graham, D.; Berson, D.M. Synaptic influences on rat ganglion-cell photoreceptors. J. Physiol. 2007, 582, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Schnapf, J.L.; Nunn, B.J.; Meister, M.; Baylor, D.A. Visual transduction in cones of the monkey Macaca fascicularis. J. Physiol. 1990, 427, 681–713. [Google Scholar] [CrossRef]
- Hartveit, E. Functional Organization of Cone Bipolar Cells in the Rat Retina. J. Neurophysiol. 1997, 77, 1716–1730. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, Y.; Omi, N. Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front. Neuroanat. 2017, 11, 92. [Google Scholar] [CrossRef]
- Ikeda, H.; Sheardown, M. Aspartate may be an excitatory transmitter mediating visual excitation of “sustained” but not “transient” cells in the cat retina: Iontophoretic studies in vivo. Neuroscience 1982, 7, 25–36. [Google Scholar] [CrossRef]
- Awatramani, G.B.; Slaughter, M.M. Origin of Transient and Sustained Responses in Ganglion Cells of the Retina. J. Neurosci. 2000, 20, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.H. Bipolar Cells Use Kainate and AMPA Receptors to Filter Visual Information into Separate Channels. Neuron 2000, 28, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, T.; Hellmer, C.B. Differential signalling and glutamate receptor compositions in the OFF bipolar cell types in the mouse retina. J. Physiol. 2015, 594, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, M.; Miller, R. The role of excitatory amino acid transmitters in the mudpuppy retina: An analysis with kainic acid and N-methyl aspartate. J. Neurosci. 1983, 3, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.H.; Li, W.; Saszik, S. Parallel Processing in Two Transmitter Microenvironments at the Cone Photoreceptor Synapse. Neuron 2006, 50, 735–748. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, S.H.; Ryan, D.G.; Shi, J.; Devries, S.H. Kainate receptor subunit diversity underlying response diversity in retinal off bipolar cells. J. Physiol. 2014, 592, 1457–1477. [Google Scholar] [CrossRef] [Green Version]
- Baden, T.; Berens, P.; Bethge, M.; Euler, T. Spikes in Mammalian Bipolar Cells Support Temporal Layering of the Inner Retina. Curr. Biol. 2013, 23, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Borghuis, B.G.; Looger, L.L.; Tomita, S.; Demb, J.B. Kainate receptors mediate signaling in both transient and sustained OFF bipolar cell pathways in mouse retina. J. Neurosci. 2014, 34, 6128–6139. [Google Scholar] [CrossRef] [Green Version]
- Euler, T.; Masland, R.H. Light-evoked responses of bipolar cells in a mammalian retina. J. Neurophysiol. 2000, 83, 1817–1829. [Google Scholar] [CrossRef]
- Puller, C.; Ivanova, E.; Euler, T.; Haverkamp, S.; Schubert, T. OFF bipolar cells express distinct types of dendritic glutamate receptors in the mouse retina. Neuroscience 2013, 243, 136–148. [Google Scholar] [CrossRef]
- Qin, P.; Pourcho, R.G. AMPA-selective glutamate receptor subunits GluR2 and GluR4 in the cat retina: An immunocytochemical study. Vis. Neurosci. 1999, 16, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Masu, M.; Iwakabe, H.; Tagawa, Y.; Miyoshi, T.; Yamashita, M.; Fukuda, Y.; Sasaki, H.; Hiroi, K.; Nakamura, Y.; Shigemoto, R.; et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGIuR6 gene. Cell 1995, 80, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Song, H.; Okawa, H.; Sampath, A.P.; Sokolov, M.; Martemyanov, K.A. Targeting of RGS7/G 5 to the Dendritic Tips of ON-Bipolar Cells Is Independent of Its Association with Membrane Anchor R7BP. J. Neurosci. 2008, 28, 10443–10449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Masuho, I.; Okawa, H.; Xie, K.; Asami, J.; Kammermeier, P.J.; Maddox, D.M.; Furukawa, T.; Inoue, T.; Sampath, A.P.; et al. Retina-Specific GTPase Accelerator RGS11/G 5S/R9AP Is a Constitutive Heterotrimer Selectively Targeted to mGluR6 in ON-Bipolar Neurons. J. Neurosci. 2009, 29, 9301–9313. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Pahlberg, J.; Sarria, I.; Kamasawa, N.; Sampath, A.P.; Martemyanov, K.A. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 7905–7910. [Google Scholar] [CrossRef] [Green Version]
- Pearring, J.N.; Bojang, P.; Shen, Y.; Koike, C.; Furukawa, T.; Nawy, S.; Gregg, R.G. A Role for Nyctalopin, a Small Leucine-Rich Repeat Protein, in Localizing the TRP Melastatin 1 Channel to Retinal Depolarizing Bipolar Cell Dendrites. J. Neurosci. 2011, 31, 10060–10066. [Google Scholar] [CrossRef]
- Dong, C.-J.; Werblin, F.S. Temporal Contrast Enhancement via GABAC Feedback at Bipolar Terminals in the Tiger Salamander Retina. J. Neurophysiol. 1998, 79, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Reifler, A.N.; Schroeder, M.M.; Jaeckel, E.R.; Chervenak, A.P.; Wong, K.Y. Mechanisms creating transient and sustained photoresponses in mammalian retinal ganglion cells. J. Gen. Physiol. 2017, 149, 335–353. [Google Scholar] [CrossRef] [Green Version]
- Kamermans, M.; Fahrenfort, I. Ephaptic interactions within a chemical synapse: Hemichannel-mediated ephaptic inhibition in the retina. Curr. Opin. Neurobiol. 2004, 14, 531–541. [Google Scholar] [CrossRef]
- Kamermans, M.; Fahrenfort, I.; Schultz, K.; Janssen-Bienhold, U.; Sjoerdsma, T.; Weiler, R. Hemichannel-Mediated Inhibition in the Outer Retina. Science 2001, 292, 1178–1180. [Google Scholar] [CrossRef]
- Kemmler, R.; Schultz, K.; Dedek, K.; Euler, T.; Schubert, T. Differential Regulation of Cone Calcium Signals by Different Horizontal Cell Feedback Mechanisms in the Mouse Retina. J. Neurosci. 2014, 34, 11826–11843. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.H.; Davenport, C.M. Lateral Inhibition in the Vertebrate Retina: The Case of the Missing Neurotransmitter. PLOS Biol. 2015, 13, e1002322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapot, C.A.; Euler, T.; Schubert, T. How do horizontal cells ‘talk’ to cone photoreceptors? Different levels of complexity at the cone-horizontal cell synapse. J. Physiol. 2017, 595, 5495–5506. [Google Scholar] [CrossRef]
- Drinnenberg, A.; Franke, F.; Morikawa, R.K.; Jüttner, J.; Hillier, D.; Hantz, P.; Hierlemann, A.; da Silveira, R.A.; Roska, B. How Diverse Retinal Functions Arise from Feedback at the First Visual Synapse. Neuron 2018, 99, 117–134.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, S.L.; Babai, N.; Chambers, J.; Thoreson, W.B.; Kramer, R.H. A Positive Feedback Synapse from Retinal Horizontal Cells to Cone Photoreceptors. PLoS Biol. 2011, 9, e1001057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ströh, S.; Puller, C.; Swirski, S.; Hölzel, M.-B.; Van Der Linde, L.I.S.; Segelken, J.; Schultz, K.; Block, C.; Monyer, H.; Willecke, K.; et al. Eliminating Glutamatergic Input onto Horizontal Cells Changes the Dynamic Range and Receptive Field Organization of Mouse Retinal Ganglion Cells. J. Neurosci. 2018, 38, 2015–2028. [Google Scholar] [CrossRef]
- Behrens, J.R.; Kraft, A.; Irlbacher, K.; Gerhardt, H.; Olma, M.C.; Brandt, S.A. Long-Lasting Enhancement of Visual Perception with Repetitive Noninvasive Transcranial Direct Current Stimulation. Front. Cell. Neurosci. 2017, 11, 238. [Google Scholar] [CrossRef] [Green Version]
- Dowling, J.E.; Brown, J.E.; Major, D. Synapses of Horizontal Cells in Rabbit and Cat Retinas. Science 1966, 153, 1639–1641. [Google Scholar] [CrossRef]
- Duebel, J.; Haverkamp, S.; Schleich, W.; Feng, G.; Augustine, G.J.; Kuner, T.; Euler, T. Two-Photon Imaging Reveals Somatodendritic Chloride Gradient in Retinal ON-Type Bipolar Cells Expressing the Biosensor Clomeleon. Neuron 2006, 49, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Marchiafava, P.L. Horizontal cells influence membrane potential of bipolar cells in the retina of the turtle. Nature 1978, 275, 141–142. [Google Scholar] [CrossRef]
- Yang, X.L.; Wu, S.M. Feedforward lateral inhibition in retinal bipolar cells: Input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc. Natl. Acad. Sci. USA 1991, 88, 3310–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaya, T.; Matsumoto, A.; Sugita, Y.; Watanabe, S.; Kuwahara, R.; Tachibana, M.; Furukawa, T. Versatile functional roles of horizontal cells in the retinal circuit. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, K.; Berens, P.; Schubert, T.; Bethge, M.; Euler, T.; Baden, T. Inhibition decorrelates visual feature representations in the inner retina. Nature 2017, 542, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, A.; Pinto, L.H.; Tachibana, M. Transient calcium current of retinal bipolar cells of the mouse. J. Physiol. 1989, 410, 613–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, A.; Suzuki, S.; Pinto, L.; Tachibana, M. Membrane currents and pharmacology of retinal bipolar cells: A comparative study on goldfish and mouse. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 98, 115–127. [Google Scholar] [CrossRef]
- Heidelberger, R.; Matthews, G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J. Physiol. 1992, 447, 235–256. [Google Scholar] [CrossRef]
- Connaughton, V.P.; Maguire, G. Differential expression of voltage-gated K+ and Ca2+ currents in bipolar cells in the zebrafish retinal slice. Eur. J. Neurosci. 1998, 10, 1350–1362. [Google Scholar] [CrossRef]
- Maguire, G.; Maple, B.; Lukasiewicz, P.; Werblin, F. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc. Natl. Acad. Sci. USA 1989, 86, 10144–10147. [Google Scholar] [CrossRef] [Green Version]
- Dreosti, E.; Esposti, F.; Baden, T.; Lagnado, L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat. Neurosci. 2011, 14, 951–952. [Google Scholar] [CrossRef] [Green Version]
- Baden, T.; Esposti, F.; Nikolaev, A.; Lagnado, L. Spikes in Retinal Bipolar Cells Phase-Lock to Visual Stimuli with Millisecond Precision. Curr. Biol. 2011, 21, 1859–1869. [Google Scholar] [CrossRef] [Green Version]
- Burrone, J.; Lagnado, L. Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. J. Physiol. 1997, 505, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Protti, D.A.; Flores-Herr, N.; von Gersdorff, H. Light Evokes Ca 2+ Spikes in the Axon Terminal of a Retinal Bipolar Cell. Neuron 2000, 25, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Pan, Z.-H. Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis. Neurosci. 2008, 25, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puthussery, T.; Venkataramani, S.; Gayet-Primo, J.; Smith, R.G.; Taylor, W.R. NaV1.1 Channels in Axon Initial Segments of Bipolar Cells Augment Input to Magnocellular Visual Pathways in the Primate Retina. J. Neurosci. 2013, 33, 16045–16059. [Google Scholar] [CrossRef]
- Saszik, S.; Devries, S.H. A Mammalian Retinal Bipolar Cell Uses Both Graded Changes in Membrane Voltage and All-or-Nothing Na+ Spikes to Encode Light. J. Neurosci. 2012, 32, 297–307. [Google Scholar] [CrossRef]
- Kaneko, A.; Tachibana, M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J. Physiol. 1985, 358, 131–152. [Google Scholar] [CrossRef] [Green Version]
- Lasansky, A. Properties of depolarizing bipolar cell responses to central illumination in salamander retinal slices. Brain Res. 1992, 576, 181–196. [Google Scholar] [CrossRef]
- Lasater, E.M. Membrane currents of retinal bipolar cells in culture. J. Neurophysiol. 1988, 60, 1460–1480. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Attwell, D.; Mobbs, P.; Wilson, M. Membrane currents in retinal bipolar cells of the axolotl. J. Gen. Physiol. 1988, 91, 49–72. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-J.; Pan, Z.-H. Differential expression of K+ currents in mammalian retinal bipolar cells. Vis. Neurosci. 2002, 19, 163–173. [Google Scholar] [CrossRef]
- Müller, B.; Butz, E.; Peichl, L.; Haverkamp, S. The Rod Pathway of the Microbat Retina Has Bistratified Rod Bipolar Cells and Tristratified AII Amacrine Cells. J. Neurosci. 2013, 33, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Horiguchi, H.; Tachibana, M. Ca2+-dependent Cl− current at the presynaptic terminals of goldfish retinal bipolar cells. Neurosci. Res. 1995, 23, 297–303. [Google Scholar] [CrossRef]
- Brandstätter, J.H.; Koulen, P.; Kuhn, R.; Van Der Putten, H.; Wässle, H. Compartmental Localization of a Metabotropic Glutamate Receptor (mGluR7): Two Different Active Sites at a Retinal Synapse. J. Neurosci. 1996, 16, 4749–4756. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Souza, E.; Calaza, K. Selective activation of group III metabotropic glutamate receptor subtypes produces different patterns of γ-aminobutyric acid immunoreactivity and glutamate release in the retina. J. Neurosci. Res. 2012, 90, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Higgs, M.H.; Lukasiewicz, P.D. Glutamate Uptake Limits Synaptic Excitation of Retinal Ganglion Cells. J. Neurosci. 1999, 19, 3691–3700. [Google Scholar] [CrossRef]
- Matsui, K.; Hosoi, N.; Tachibana, M. Active Role of Glutamate Uptake in the Synaptic Transmission from Retinal Nonspiking Neurons. J. Neurosci. 1999, 19, 6755–6766. [Google Scholar] [CrossRef]
- Asari, H.; Meister, M. Divergence of visual channels in the inner retina. Nat. Neurosci. 2012, 15, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Asari, H.; Meister, M. The Projective Field of Retinal Bipolar Cells and Its Modulation by Visual Context. Neuron 2014, 81, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Amthor, F.R.; Takahashi, E.S.; Oyster, C.W. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J. Comp. Neurol. 1989, 280, 72–96. [Google Scholar] [CrossRef]
- Ariel, M.; Daw, N.W. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J. Physiol. 1982, 324, 161–185. [Google Scholar] [CrossRef] [Green Version]
- Dacheux, R.F.; Miller, R.F. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I. Receptors, horizontal, and bipolar cells. J. Comp. Neurol. 1981, 198, 307–326. [Google Scholar] [CrossRef]
- Marchiafava, P.L. An “antagonistic” surround facilitates central responses by retinal ganglion cells. Vis. Res. 1983, 23, 1097–1099. [Google Scholar] [CrossRef]
- Masland, R.H.; Mills, J.W.; Cassidy, C. The functions of acetylcholine in the rabbit retina. Proc. R. Soc. London. Ser. B Boil. Sci. 1984, 223, 121–139. [Google Scholar] [CrossRef]
- Masland, R.H. Amacrine cells. Trends Neurosci. 1988, 11, 405–410. [Google Scholar] [CrossRef]
- Nelson, R.; Kolb, H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vis. Res. 1983, 23, 1183–1195. [Google Scholar] [CrossRef]
- Werblin, F.S. Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J. Physiol. 1977, 264, 767–785. [Google Scholar] [CrossRef] [Green Version]
- Chun, M.-H.; Han, S.-H.; Chung, J.-W.; Wässle, H. Electron microscopic analysis of the rod pathway of the rat retina. J. Comp. Neurol. 1993, 332, 421–432. [Google Scholar] [CrossRef]
- Dacheux, R.F.; Raviola, E. The rod pathway in the rabbit retina: A depolarizing bipolar and amacrine cell. J. Neurosci. 1986, 6, 331–345. [Google Scholar] [CrossRef]
- Grimes, W.N.; Zhang, J.; Graydon, C.W.; Kachar, B.; Diamond, J.S. Retinal Parallel Processors: More than 100 Independent Microcircuits Operate within a Single Interneuron. Neuron 2010, 65, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Grunert, U.; Martin, P.R. Rod bipolar cells in the macaque monkey retina: Immunoreactivity and connectivity. J. Neurosci. 1991, 11, 2742–2758. [Google Scholar] [CrossRef] [Green Version]
- Hartveit, E. Reciprocal Synaptic Interactions Between Rod Bipolar Cells and Amacrine Cells in the Rat Retina. J. Neurophysiol. 1999, 81, 2923–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.; Kolb, H. A17: A broad-field amacrine cell in the rod system of the cat retina. J. Neurophysiol. 1985, 54, 592–614. [Google Scholar] [CrossRef] [PubMed]
- Raviola, E.; Dacheux, R.F. Excitatory dyad synapse in rabbit retina. Proc. Natl. Acad. Sci. USA 1987, 84, 7324–7328. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, Y.; Morigiwa, K.; Ueda, M.; Sterling, P. Microcircuits for Night Vision in Mouse Retina. J. Neurosci. 2001, 21, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Pourcho, R.G.; Goebel, D.J. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: A combined Golgi and autoradiographic study. J. Comp. Neurol. 1983, 219, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, B.; Xin, D.; Bloomfield, S.A. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J. Physiol. 2002, 539, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.; Hare, W.A. Temporal Modulation of Scotopic Visual Signals by A17 Amacrine Cells in Mammalian Retina In Vivo. J. Neurophysiol. 2003, 89, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Koulen, P. GABAA and GABAC receptors on mammalian rod bipolar cells. J. Comp. Neurol. 1998, 396, 351–365. [Google Scholar] [CrossRef]
- Karschin, A.; Wässle, H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J. Neurophysiol. 1990, 63, 860–876. [Google Scholar] [CrossRef]
- McCall, M.A.; Lukasiewicz, P.D.; Gregg, R.G.; Peachey, N.S. Elimination of the ρ1 Subunit Abolishes GABACReceptor Expression and Alters Visual Processing in the Mouse Retina. J. Neurosci. 2002, 22, 4163–4174. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.R.; Tran, M.N.; Wong, R.O.L.; Lukasiewicz, P.D. Distinct Ionotropic GABA Receptors Mediate Presynaptic and Postsynaptic Inhibition in Retinal Bipolar Cells. J. Neurosci. 2000, 20, 2673–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, J.H.; Diamond, J.S. Sustained Ca2+Entry Elicits Transient Postsynaptic Currents at a Retinal Ribbon Synapse. J. Neurosci. 2003, 23, 10923–10933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez, A.E.; Singer, J.H.; Diamond, J.S. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 2006, 443, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.; Werblin, F. Inhibitory Feedback Shapes Bipolar Cell Responses in the Rabbit Retina. J. Neurophysiol. 2007, 98, 3423–3435. [Google Scholar] [CrossRef] [Green Version]
- Nagy, J.; Ebbinghaus, B.; Hoon, M.; Sinha, R. GABAA presynaptic inhibition regulates the gain and kinetics of retinal output neurons. eLife 2021, 10, e60994. [Google Scholar] [CrossRef]
- Chen, X.; Hsueh, H.-A.; Greenberg, K.; Werblin, F.S. Three Forms of Spatial Temporal Feedforward Inhibition Are Common to Different Ganglion Cell Types in Rabbit Retina. J. Neurophysiol. 2010, 103, 2618–2632. [Google Scholar] [CrossRef] [Green Version]
- Casini, G.; Brecha, N.C. Vasoactive intestinal polypeptide-containing cells in the rabbit retina: Immunohistochemical localization and quantitative analysis. J. Comp. Neurol. 1991, 305, 313–327. [Google Scholar] [CrossRef]
- Casini, G.; Brecha, N.C. Colocalization of vasoactive intestinal polypeptide and GABA immunoreactivities in a population of wide-field amacrine cells in the rabbit retina. Vis. Neurosci. 1992, 8, 373–378. [Google Scholar] [CrossRef]
- Lammerding-Köppel, M.; Thier, P.; Koehler, W. Morphology and mosaics of VIP-like immunoreactive neurons in the retina of the rhesus monkey. J. Comp. Neurol. 1991, 312, 251–263. [Google Scholar] [CrossRef]
- Lee, C.W.; Eglen, S.J.; Wong, R.O.L. Segregation of on and offRetinogeniculate Connectivity Directed by Patterned Spontaneous Activity. J. Neurophysiol. 2002, 88, 2311–2321. [Google Scholar] [CrossRef]
- Park, D.J.; Senok, S.S.; Goo, Y.S. Degeneration stage-specific response pattern of retinal ganglion cell spikes in rd10 mouse retina. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3351–3354. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, J.; Rieke, F. Noise correlations improve response fidelity and stimulus encoding. Nature 2010, 468, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Arman, A.C.; Sampath, A.P. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J. Neurophysiol. 2012, 107, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Wässle, H.; Voigt, T. Pharmacological modulation of the rod pathway in the cat retina. J. Neurophysiol. 1988, 59, 1657–1672. [Google Scholar] [CrossRef]
- Famiglietti, E.; Kolb, H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975, 84, 293–300. [Google Scholar] [CrossRef]
- Kolb, H.; Famigilietti, E.V. Rod and Cone Pathways in the Inner Plexiform Layer of Cat Retina. Science 1974, 186, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.; Nelson, R. Off-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. J. Comp. Neurol. 1993, 329, 85–110. [Google Scholar] [CrossRef]
- Marc, R.E.; Anderson, J.R.; Jones, B.W.; Sigulinsky, C.L.; Lauritzen, J.S. The AII amacrine cell connectome: A dense network hub. Front. Neural Circuits 2014, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Strettoi, E.; Raviola, E.; Dacheux, R.F. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J. Comp. Neurol. 1992, 325, 152–168. [Google Scholar] [CrossRef]
- Molnar, A.; Hsueh, H.-A.; Roska, B.; Werblin, F.S. Crossover inhibition in the retina: Circuitry that compensates for nonlinear rectifying synaptic transmission. J. Comput. Neurosci. 2009, 27, 569–590. [Google Scholar] [CrossRef] [Green Version]
- Rosa, J.M.; Ruehle, S.; Ding, H.; Lagnado, L. Crossover Inhibition Generates Sustained Visual Responses in the Inner Retina. Neuron 2016, 90, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Anderson, J.R.; Sigulinsky, C.; Lauritzen, S. Retinal connectomics: Towards complete, accurate networks. Prog. Retin. Eye Res. 2013, 37, 141–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, H.-A.; Molnar, A.; Werblin, F.S. Amacrine-to-Amacrine Cell Inhibition in the Rabbit Retina. J. Neurophysiol. 2008, 100, 2077–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukasiewicz, P.D.; Lawrence, J.E.; Valentino, T.L. Desensitizing glutamate receptors shape excitatory synaptic inputs to tiger salamander retinal ganglion cells. J. Neurosci. 1995, 15, 6189–6199. [Google Scholar] [CrossRef] [Green Version]
- Mobbs, P.; Everett, K.; Cook, A. Signal shaping by voltage-gated currents in retinal ganglion cells. Brain Res. 1992, 574, 217–223. [Google Scholar] [CrossRef]
- Ganczer, A.; Balogh, M.; Albert, L.; Debertin, G.; Kovács-Öller, T.; Völgyi, B. Transiency of retinal ganglion cell action potential responses determined by PSTH time constant. PLoS ONE 2017, 12, e0183436. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.W.; Okawa, H.; Dunn, F.A.; Morgan, J.L.; Kerschensteiner, D.; O Wong, R.; Rieke, F. The spatial structure of a nonlinear receptive field. Nat. Neurosci. 2012, 15, 1572–1580. [Google Scholar] [CrossRef]
- Della Santina, L.; Kuo, S.P.; Yoshimatsu, T.; Okawa, H.; Suzuki, S.C.; Hoon, M.; Tsuboyama, K.; Rieke, F.; Wong, R.O. Glutamatergic Monopolar Interneurons Provide a Novel Pathway of Excitation in the Mouse Retina. Curr. Biol. 2016, 26, 2070–2077. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.-Q.; El-Danaf, R.N.; Okawa, H.; Pacholec, J.M.; Matti, U.; Schwarz, K.; Odermatt, B.; Dunn, F.A.; Lagnado, L.; Schmitz, F.; et al. Synaptic Convergence Patterns onto Retinal Ganglion Cells Are Preserved despite Topographic Variation in Pre- and Postsynaptic Territories. Cell Rep. 2018, 25, 2017–2026.e3. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, T.; Fyk-Kolodziej, B.; Cohn, J. Roles of ON Cone Bipolar Cell Subtypes in Temporal Coding in the Mouse Retina. J. Neurosci. 2014, 34, 8761–8771. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, J.R.; Jacobs, A.L.; Nirenberg, S. Selective Ablation of a Class of Amacrine Cells Alters Spatial Processing in the Retina. J. Neurosci. 2004, 24, 1459–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oesch, N.W.; Diamond, J.S. Synaptic inhibition tunes contrast computation in the retina. Vis. Neurosci. 2019, 36, E006. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, T.; Puller, C.; Haverkamp, S.; Euler, T. Chromatic Bipolar Cell Pathways in the Mouse Retina. J. Neurosci. 2011, 31, 6504–6517. [Google Scholar] [CrossRef] [PubMed]
- Baden, T.; Berens, P.; Franke, K.J.; Rosón, M.R.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Münch, T.; Da Silveira, R.A.; Siegert, S.; Viney, T.; Awatramani, G.B.; Roska, B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat. Neurosci. 2009, 12, 1308–1316. [Google Scholar] [CrossRef]
- Ölveczky, B.P.; Baccus, S.A.; Meister, M. Segregation of object and background motion in the retina. Nature 2003, 423, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Roska, B.; Werblin, F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat. Neurosci. 2003, 6, 600–608. [Google Scholar] [CrossRef]
- Barlow, H.B.; Hill, R.M. Selective Sensitivity to Direction of Movement in Ganglion Cells of the Rabbit Retina. Science 1963, 139, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Barlow, H.B.; Levick, W.R. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. 1965, 178, 477–504. [Google Scholar] [CrossRef] [Green Version]
- Fried, S.I.; Mu, T.A.; Werblin, F.S. Directional Selectivity Is Formed at Multiple Levels by Laterally Offset Inhibition in the Rabbit Retina. Neuron 2005, 46, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.; Vaney, D.I. New directions in retinal research. Trends Neurosci. 2003, 26, 379–385. [Google Scholar] [CrossRef]
- Johnson, K.P.; Zhao, L.; Kerschensteiner, D. A Pixel-Encoder Retinal Ganglion Cell with Spatially Offset Excitatory and Inhibitory Receptive Fields. Cell Rep. 2018, 22, 1462–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacey, D.M.; Lee, B.B. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 1994, 367, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Schwartz, G.W. Electrical synapses convey orientation selectivity in the mouse retina. Nat. Commun. 2017, 8, 2025. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.; Ahn, D.; Greschner, M.; Chichilnisky, E.J.; Field, G.D. Pathway-Specific Asymmetries between ON and OFF Visual Signals. J. Neurosci. 2018, 38, 9728–9740. [Google Scholar] [CrossRef] [Green Version]
- Hart, W.M. Acquired dyschromatopsias. Surv. Ophthalmol. 1987, 32, 10–31. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganczer, A.; Szarka, G.; Balogh, M.; Hoffmann, G.; Tengölics, Á.J.; Kenyon, G.; Kovács-Öller, T.; Völgyi, B. Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements. Cells 2022, 11, 810. https://doi.org/10.3390/cells11050810
Ganczer A, Szarka G, Balogh M, Hoffmann G, Tengölics ÁJ, Kenyon G, Kovács-Öller T, Völgyi B. Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements. Cells. 2022; 11(5):810. https://doi.org/10.3390/cells11050810
Chicago/Turabian StyleGanczer, Alma, Gergely Szarka, Márton Balogh, Gyula Hoffmann, Ádám Jonatán Tengölics, Garrett Kenyon, Tamás Kovács-Öller, and Béla Völgyi. 2022. "Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements" Cells 11, no. 5: 810. https://doi.org/10.3390/cells11050810
APA StyleGanczer, A., Szarka, G., Balogh, M., Hoffmann, G., Tengölics, Á. J., Kenyon, G., Kovács-Öller, T., & Völgyi, B. (2022). Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements. Cells, 11(5), 810. https://doi.org/10.3390/cells11050810






