Methods of Sputum and Mucus Assessment for Muco-Obstructive Lung Diseases in 2022: Time to “Unplug” from Our Daily Routine!
Abstract
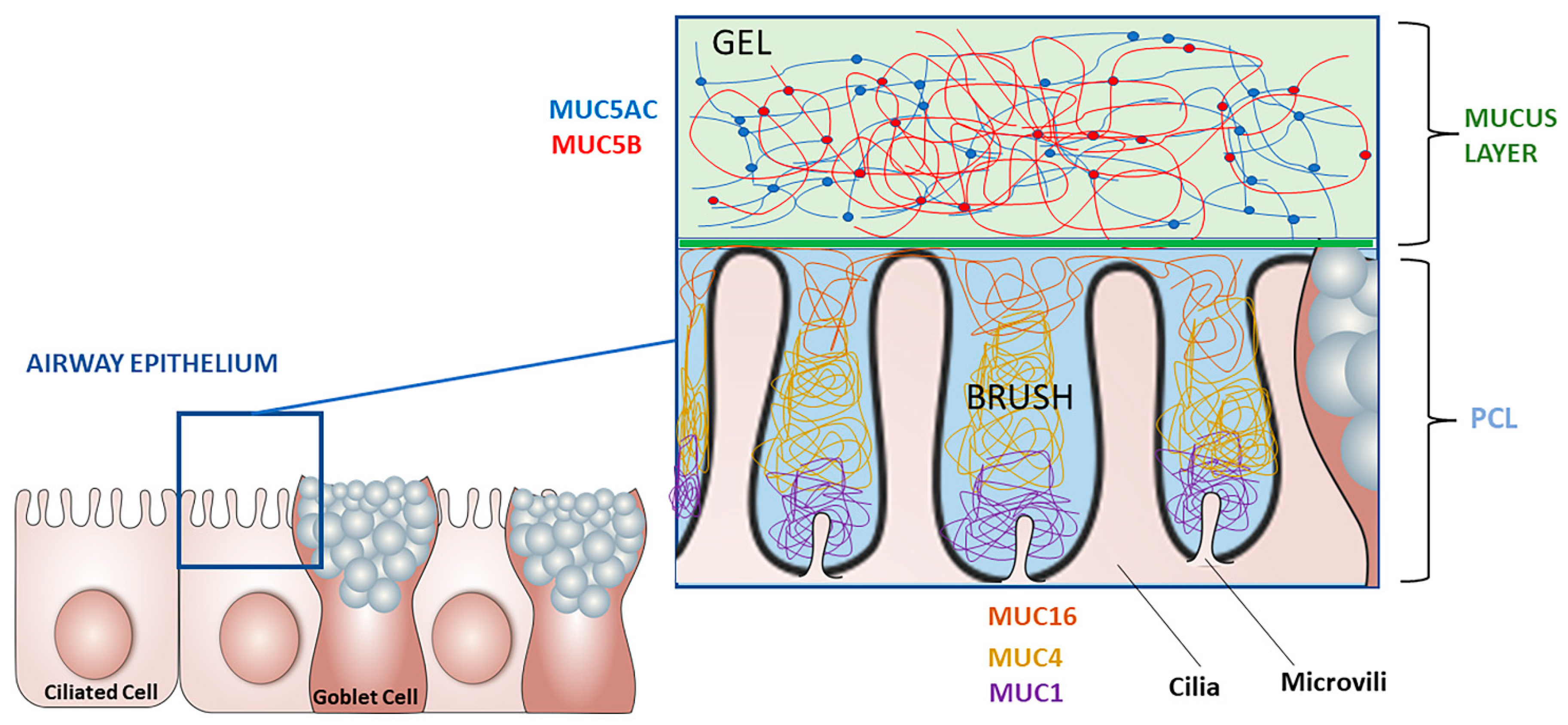
:1. Sputum, Mucus, and Mucins in Healthy Subjects
1.1. Role and Components
1.2. Regulation of Airway Mucins
2. Mucus and Mucins in Muco-Obstructive Lung Diseases
2.1. Chronic Obstructive Pulmonary Disease
2.2. Asthma
2.3. Non-Cystic Fibrosis Bronchiectasis
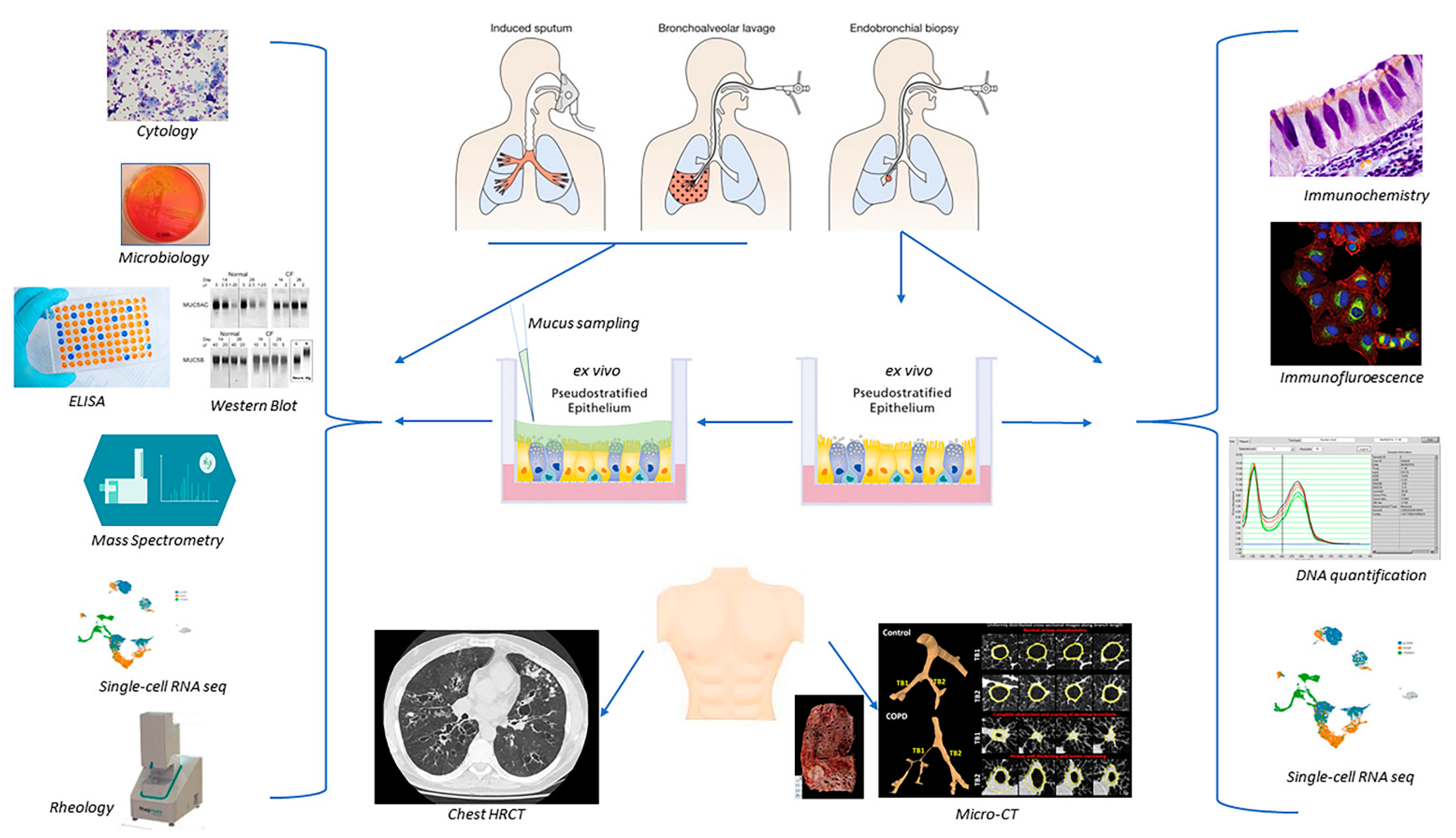
3. Sample Collection
3.1. Flexible Bronchoscopy
3.2. Sputum
4. Direct Assessment of Human Airway Mucus and Mucins
4.1. Macroscopic Studies of Sputum
4.2. Cytology
4.3. Microbiology
4.4. Histological Staining and Immunostaining
4.5. Molecular Assays
4.6. Semi-Quantitative and Quantitative Assessments of Mucin Proteins
4.7. Biophysical Properties and Rheology of Human Mucus
4.8. Ex Vivo Models
5. Indirect Assessment of Human Mucus Dysregulation
5.1. Respiratory Symptoms
5.2. Imaging: High-Resolution and Micro-Computed Tomography Scanning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB/PAS | Alcian blue/periodic acid-Schiff |
| ChIP | chromatin immunoprecipitation |
| COPD | chronic obstructive pulmonary disease |
| ELISA | enzyme-linked immunosorbent assay |
| FEV1 | forced expiratory volume in 1 s |
| FRAP | fluorescence recovery after photobleaching |
| HRCT | high-resolution computed tomography |
| IL | interleukin |
| MCC | mucociliary clearance |
| mRNA | messenger ribonucleic acid |
| MUC | human mucin protein (denoted with a number following MUC) |
| muc5AC | Mucin 5AC |
| muc5B | Mucin 5B |
| non-CF bronchiectasis | non-cystic fibrosis bronchiectasis |
| PCL | periciliary layer |
| RT-PCR | reverse transcription polymerase chain reaction |
References
- Meldrum, O.W.; Chotirmall, S.H. Mucus, Microbiomes and Pulmonary Disease. Biomedicines 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. npj Biofilms Microbiomes 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Button, B.; Cai, L.-H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. Periciliary Brush Promotes the Lung Health by Separating the Mucus Layer from Airway Epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef] [Green Version]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and Function of the Polymeric Mucins in Airways Mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2018, 199, 715–727. [Google Scholar] [CrossRef]
- Voynow, J.A. Mucins, Mucus, and Sputum. Chest J. 2009, 135, 505–512. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Nadel, J.A. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 2004, 59, 992–996. [Google Scholar] [CrossRef] [Green Version]
- Takeyama, K.; Jung, B.; Shim, J.J.; Burgel, P.-R.; Dao-Pick, T.; Ueki, I.F.; Protin, U.; Kroschel, P.; Nadel, J.A. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L165–L172. [Google Scholar] [CrossRef] [Green Version]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 Signaling in Allergic Airway Disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, Q.; Kolosov, V.P.; Perelman, J.M.; Zhou, X. Interleukin-13 Induces Mucin 5AC Production Involving STAT6/SPDEF in Human Airway Epithelial Cells. Cell Commun. Adhes. 2010, 17, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Reid, L. Measurement of the bronchial mucous gland layer: A diagnostic yardstick in chronic bronchitis. Thorax 1960, 15, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullol, J.; Baraniuk, J.N.; Logun, C.; Mérida, M.; Hausfeld, J.; Shelhamer, J.H.; Kaliner, M.A. M1 and M3 muscarinic antagonists inhibit human nasal glandular secretion in vitro. J. Appl. Physiol. 1992, 73, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Wine, J.J.; Joo, N.S. Submucosal Glands and Airway Defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [Green Version]
- Adler, K.; Tuvim, M.; Dickey, B. Regulated Mucin Secretion from Airway Epithelial Cells. Front. Endocrinol. 2013, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. [Google Scholar] [CrossRef]
- GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease–GOLD. 2021. Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 17 October 2021).
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, G.; Ceppe, A.; Ford, A.A.; Alexis, N.E.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Cooper, C.B.; Han, M.K.; Hansel, N.N.; et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2021, 9, 1241–1254. [Google Scholar] [CrossRef]
- Innes, A.L. Epithelial Mucin Stores Are Increased in the Large Airways of Smokers with Airflow Obstruction. Chest J. 2006, 130, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.X.G.; Ueki, I.F.; Nadel, J.A. Tumor necrosis factor–converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11618–11623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, M.X.G.; Nakanaga, T.; Nadel, J.A. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-α-converting enzyme in human airway epithelial (NCI-H292) cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L420–L427. [Google Scholar] [CrossRef] [PubMed]
- Clunes, L.A.; Davies, C.M.; Coakley, R.D.; Aleksandrov, A.A.; Henderson, A.G.; Zeman, K.L.; Worthington, E.N.; Gentzsch, M.; Kreda, S.M.; Cholon, D.; et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.H.; Coakley, R.D.; Button, B.; Henderson, A.G.; Zeman, K.L.; Alexis, N.E.; Peden, D.B.; Lazarowski, E.R.; Davis, C.W.; Bailey, S.; et al. The Relationship of Mucus Concentration (Hydration) to Mucus Osmotic Pressure and Transport in Chronic Bronchitis. Am. J. Respir. Crit. Care Med. 2015, 192, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Kreda, S.M.; Seminario-Vidal, L.; van Heusden, C.A.; O’Neal, W.; Jones, L.; Boucher, R.C.; Lazarowski, E.R. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J. Physiol. 2010, 588, 2255–2267. [Google Scholar] [CrossRef]
- Jeffries, J.L.; Jia, J.; Choi, W.; Choe, S.; Miao, J.; Xu, Y.; Powell, R.; Lin, J.; Kuang, Z.; Gaskins, H.R.; et al. Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewisx to increase binding to airway epithelial cells. Mucosal Immunol. 2016, 9, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, C.L.; Lalsiamthara, J.; Aballay, A. Host Mucin Is Exploited by Pseudomonas aeruginosa To Provide Monosaccharides Required for a Successful Infection. mBio 2020, 11, e00060-20. [Google Scholar] [CrossRef] [Green Version]
- Sibila, O.; Garcia-Bellmunt, L.; Giner, J.; Rodrigo-Troyano, A.; Suarez-Cuartin, G.; Torrego, A.; Castillo, D.; Solanes, I.; Mateus, E.F.; Vidal, S.; et al. Airway Mucin 2 Is Decreased in Patients with Severe Chronic Obstructive Pulmonary Disease with Bacterial Colonization. Ann. Am. Thorac. Soc. 2016, 13, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Perotin, J.-M.; Coraux, C.; Lagonotte, E.; Birembaut, P.; Delepine, G.; Polette, M.; Deslée, G.; Dormoy, V. Alteration of primary cilia in COPD. Eur. Respir. J. 2018, 52, 1800122. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, L.M.; Paré, P.D.; Hogg, J.C.; Lambert, R.K.; Ionescu, D.; Woods, R.; Bai, T.R. Characterization of airway plugging in fatal asthma. Am. J. Med. 2003, 115, 6–11. [Google Scholar] [CrossRef]
- Dunican, E.M.; Elicker, B.M.; Gierada, D.S.; Nagle, S.K.; Schiebler, M.L.; Newell, J.D.; Raymond, W.W.; Lachowicz-Scroggins, M.E.; Di Maio, S.; Hoffman, E.A.; et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Investig. 2018, 128, 997–1009. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Arron, J.R.; Koth, L.L.; Fahy, J.V. T-helper Type 2–driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef]
- Ordoñez, C.L.; Khashayar, R.; Wong, H.H.; Ferrando, R.; Wu, R.; Hyde, D.M.; Hotchkiss, J.A.; Zhang, Y.; Novikov, A.; Dolganov, G.; et al. Mild and Moderate Asthma Is Associated with Airway Goblet Cell Hyperplasia and Abnormalities in Mucin Gene Expression. Am. J. Respir. Crit. Care Med. 2001, 163, 517–523. [Google Scholar] [CrossRef]
- Bush, A. Pathophysiological Mechanisms of Asthma. Front. Pediatr. 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Chen, G.; Korfhagen, T.R.; Xu, Y.; Kitzmiller, J.; Wert, S.E.; Maeda, Y.; Gregorieff, A.; Clevers, H.; Whitsett, J.A. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Investig. 2009, 119, 2914–2924. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-S.; Korfhagen, T.R.; Bruno, M.D.; Kitzmiller, J.A.; Wan, H.; Wert, S.E.; Khurana Hershey, G.K.; Chen, G.; Whitsett, J.A. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Investig. 2007, 117, 978–988. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Yuan, S.; Kerr, S.C.; Dunican, E.M.; Yu, M.; Carrington, S.D.; Fahy, J.V. Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma. Am. J. Respir. Crit. Care Med. 2016, 194, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Kiwamoto, T.; Katoh, T.; Evans, C.M.; Janssen, W.J.; Brummet, M.E.; Hudson, S.A.; Zhu, Z.; Tiemeyer, M.; Bochner, B.S. Endogenous Airway Mucins Carry Glycans That Bind Siglec-F and Induce Eosinophil Apoptosis. J. Allergy Clin. Immunol. 2015, 135, 1329–1340.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonser, L.R.; Zlock, L.; Finkbeiner, W.; Erle, D.J. Epithelial Tethering of MUC5AC-Rich Mucus Impairs Mucociliary Transport in Asthma. J. Clin. Investig. 2016, 126, 2367–2371. [Google Scholar] [CrossRef]
- Grainge, C.L.; Lau, L.C.K.; Ward, J.A.; Dulay, V.; Lahiff, G.; Wilson, S.; Holgate, S.; Davies, D.E.; Howarth, P.H. Effect of bronchoconstriction on airway remodeling in asthma. N. Engl. J. Med. 2011, 364, 2006–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faiz, A.; Weckmann, M.; Tasena, H.; Vermeulen, C.J.; den Berge, M.V.; Hacken, N.H.T.; Halayko, A.J.; Ward, J.P.T.; Lee, T.H.; Tjin, G.; et al. Profiling of healthy and asthmatic airway smooth muscle cells following interleukin-1β treatment: A novel role for CCL20 in chronic mucus hypersecretion. Eur. Respir. J. 2018, 52, 1800310. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.G. Airway smooth muscle may drive mucus hypersecretion in asthma. Eur. Respir. J. 2018, 52, 1801166. [Google Scholar] [CrossRef] [Green Version]
- Araújo, D.; Shteinberg, M.; Aliberti, S.; Goeminne, P.C.; Hill, A.T.; Fardon, T.; Obradovic, D.; Dimakou, K.; Polverino, E.; Soyza, A.D.; et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur. Respir. J. 2017, 50, 1701289. [Google Scholar] [CrossRef] [Green Version]
- Whitwell, F. A Study of the Pathology and Pathogenesis of Bronchiectasis. Thorax 1952, 7, 213–239. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, K.A.; Chen, A.C.H.; Radicioni, G.; Lourie, R.; Martin, M.; Broomfield, A.; Sheng, Y.H.; Hasnain, S.Z.; Radford-Smith, G.; Simms, L.A.; et al. Airway Mucus Hyperconcentration in Non-Cystic Fibrosis Bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 201, 661–670. [Google Scholar] [CrossRef]
- Zheng, W.; Kuhlicke, J.; Jäckel, K.; Eltzschig, H.K.; Singh, A.; Sjöblom, M.; Riederer, B.; Weinhold, C.; Seidler, U.; Colgan, S.P.; et al. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, R.A.; Boucher, R.C.; Stutts, M.J. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L813–L819. [Google Scholar] [CrossRef] [Green Version]
- Ziedalski, T.M.; Kao, P.N.; Henig, N.R.; Jacobs, S.S.; Ruoss, S.J. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest 2006, 130, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.M.; Cárcamo-Oyarce, G.; Turner, B.S.; Dellos-Nolan, S.; Co, J.Y.; Lehoux, S.; Cummings, R.D.; Wozniak, D.J.; Ribbeck, K. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 2019, 4, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Ramphal, R.; Arora, S.K.; Ritchings, B.W. Recognition of mucin by the adhesin-flagellar system of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 1996, 154, S170–S174. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.H. Dilated bronchial mucous glands in chronic bronchitis, a neglected morphologic finding. Correlation of bronchoscopic and bronchographic appearance. Am. Rev. Respir. Dis. 1961, 83, 16–25. [Google Scholar] [CrossRef]
- Leaker, B.R.; Nicholson, G.C.; Ali, F.Y.; Daudi, N.; O’Connor, B.J.; Barnes, P.J. Bronchoabsorption; a novel bronchoscopic technique to improve biomarker sampling of the airway. Respir. Res. 2015, 16, 102. [Google Scholar] [CrossRef]
- Bush, A.; Pohunek, P. Brush Biopsy and Mucosal Biopsy. Am. J. Respir. Crit. Care Med. 2000, 162, S18–S22. [Google Scholar] [CrossRef]
- Gosselink, R.; Gayan-Ramirez, G.; Houtmeyers, E.; de Paepe, K.; Decramer, M. High-dose lidocaine reduces airway mucus transport velocity in intubated anesthetized dogs. Respir. Med. 2006, 100, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Serra, M.F.; Anjos-Valotta, E.A.; Olsen, P.C.; Couto, G.C.; Jurgilas, P.B.; Cotias, A.C.; Pão, C.R.; Ferreira, T.P.T.; Arantes, A.C.S.; Pires, A.L.A.; et al. Nebulized lidocaine prevents airway inflammation, peribronchial fibrosis, and mucus production in a murine model of asthma. Anesthesiology 2012, 117, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-H.; Hyun Song, M.; Eun Ahn, Y.; Lee, J.-G.; Yoon, J.-H. Effect of hypo-, iso- and hypertonic saline irrigation on secretory mucins and morphology of cultured human nasal epithelial cells. Acta Otolaryngol. 2005, 125, 1296–1300. [Google Scholar] [CrossRef]
- Ramnarine, S.I.; Haddad, E.B.; Khawaja, A.M.; Mak, J.C.; Rogers, D.F. On muscarinic control of neurogenic mucus secretion in ferret trachea. J. Physiol. 1996, 494, 577–586. [Google Scholar] [CrossRef]
- Ledowski, T.; Paech, M.J.; Patel, B.; Schug, S.A. Bronchial Mucus Transport Velocity in Patients Receiving Propofol and Remifentanil Versus Sevoflurane and Remifentanil Anesthesia. Anesth. Analg. 2006, 102, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.M.; Popov, T.A.; Efthimiadis, A.; Hussack, P.; Evans, S.; Pizzichini, E.; Dolovich, J.; Hargreave, F.E. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 1996, 154, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Weiszhar, Z.; Horvath, I. Induced sputum analysis: Step by step. Breathe 2013, 9, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.R.; Wickström, C.; Thornton, D.J. Gel-forming and cell-associated mucins: Preparation for structural and functional studies. Methods Mol. Biol. 2012, 842, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.M. Sputum analysis and culture. Ann. Emerg. Med. 1986, 15, 325–328. [Google Scholar] [CrossRef]
- Bhowmik, A.; Seemungal, T.A.R.; Sapsford, R.J.; Devalia, J.L.; Wedzicha, J.A. Comparison of spontaneous and induced sputum for investigation of airway inflammation in chronic obstructive pulmonary disease. Thorax 1998, 53, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.G.; Fuller, F.; Anderson, W.H.; Alexis, N.E.; Lazarowski, E.R.; Kesimer, M.; Bordonali, E.; Qaqish, B.; Boucher, R.C. Differences Between Spontaneous and Induced Sputum in Chronic Obstructive Pulmonary Disease (COPD). In B38. TALKING ABOUT COPD BIOMARKERS; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2015; p. A2914. [Google Scholar]
- Patarin, J.; Ghiringhelli, É.; Darsy, G.; Obamba, M.; Bochu, P.; Camara, B.; Quétant, S.; Cracowski, J.-L.; Cracowski, C.; Robert de Saint Vincent, M. Rheological analysis of sputum from patients with chronic bronchial diseases. Sci. Rep. 2020, 10, 15685. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Dasgupta, B.; Tomkiewicz, R.P.; Brown, N.E. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. Am. J. Respir. Crit. Care Med. 1997, 156, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.; Hershfield, E.S.; Harding, G.K.; Nelson, N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef]
- Daniels, J.M.A.; Graaff, C.S.D.; Vlaspolder, F.; Snijders, D.; Jansen, H.M.; Boersma, W.G. Sputum colour reported by patients is not a reliable marker of the presence of bacteria in acute exacerbations of chronic obstructive pulmonary disease. Clin. Microbiol. Infect. 2010, 16, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Allegra, L.; Blasi, F.; Diano, P.; Cosentini, R.; Tarsia, P.; Confalonieri, M.; Dimakou, K.; Valenti, V. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Kruesmann, F.; Haverstock, D.; Perroncel, R.; Choudhri, S.H.; Arvis, P. Sputum colour and bacteria in chronic bronchitis exacerbations: A pooled analysis. Eur. Respir. J. 2012, 39, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Pabreja, K.; Gibson, P.; Lochrin, A.J.; Wood, L.; Baines, K.J.; Simpson, J.L. Sputum colour can identify patients with neutrophilic inflammation in asthma. BMJ Open Respir. Res. 2017, 4, e000236. [Google Scholar] [CrossRef]
- Berlyne, G.S.; Efthimiadis, A.; Hussack, P.; Groves, D.; Dolovich, J.; Hargreave, F.E. Sputum in asthma: Color versus cell counts. J. Allergy Clin. Immunol. 2000, 105, 182–183. [Google Scholar] [CrossRef]
- Hamid, Q.; Kelly, M.M.; Linden, M.; Louis, R.; Pizzichini, M.M.M.; Pizzichini, E.; Ronchi, C.; Overveld, F.V.; Djukanović, R. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur. Respir. J. 2002, 20, 19S–23S. [Google Scholar] [CrossRef] [Green Version]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, M.X.; Nadel, J.A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J. Immunol. 2005, 175, 4009–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, E.K.; Verstraete, K.; Heyndrickx, I.; Gevaert, E.; Aegerter, H.; Percier, J.-M.; Deswarte, K.; Verschueren, K.H.G.; Dansercoer, A.; Gras, D.; et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 2019, 364, eaaw4295. [Google Scholar] [CrossRef]
- Shen, F.; Sergi, C. Sputum Analysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ranzani, O.T.; Senussi, T.; Idone, F.; Ceccato, A.; Li Bassi, G.; Ferrer, M.; Torres, A. Invasive and non-invasive diagnostic approaches for microbiological diagnosis of hospital-acquired pneumonia. Crit. Care 2019, 23, 51. [Google Scholar] [CrossRef] [Green Version]
- Naidus, E.L.; Lasalvia, M.T.; Marcantonio, E.R.; Herzig, S.J. The Diagnostic Yield of Noninvasive Microbiologic Sputum Sampling in a Cohort of Patients with Clinically Diagnosed Hospital-Acquired Pneumonia. J. Hosp. Med. 2018, 13, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Hanson, K.E.; Azar, M.M.; Banerjee, R.; Chou, A.; Colgrove, R.C.; Ginocchio, C.C.; Hayden, M.K.; Holodiny, M.; Jain, S.; Koo, S.; et al. Molecular Testing for Acute Respiratory Tract Infections: Clinical and Diagnostic Recommendations from the IDSA’s Diagnostics Committee. Clin. Infect. Dis. 2020, 71, 2744–2751. [Google Scholar] [CrossRef]
- Kurai, D.; Saraya, T.; Ishii, H.; Takizawa, H. Virus-induced exacerbations in asthma and COPD. Front. Microbiol. 2013, 4, 293. [Google Scholar] [CrossRef] [Green Version]
- Monard, C.; Pehlivan, J.; Auger, G.; Alviset, S.; Tran Dinh, A.; Duquaire, P.; Gastli, N.; d’Humières, C.; Maamar, A.; Boibieux, A.; et al. Multicenter evaluation of a syndromic rapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit. Care 2020, 24, 434. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Moninger, T.O.; McMenimen, J.D.; Sawin, N.M.; Parker, C.P.; Thornell, I.M.; Powers, L.S.; Gansemer, N.D.; Bouzek, D.C.; Cook, D.P.; et al. Gel-forming mucins form distinct morphologic structures in airways. Proc. Natl. Acad. Sci. USA 2017, 114, 6842–6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuckin, M.A.; Thornton, D.J. Detection and quantitation of mucins using chemical, lectin, and antibody methods. Methods Mol. Biol. 2000, 125, 45–55. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Reznikov, L.R. Strategies for measuring airway mucus and mucins. Respir. Res. 2019, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Khelloufi, M.-K.; Loiseau, E.; Jaeger, M.; Molinari, N.; Chanez, P.; Gras, D.; Viallat, A. Spatiotemporal organization of cilia drives multiscale mucus swirls in model human bronchial epithelium. Sci. Rep. 2018, 8, 2447. [Google Scholar] [CrossRef]
- Requena, S.; Ponomarchuk, O.; Castillo, M.; Rebik, J.; Brochiero, E.; Borejdo, J.; Gryczynski, I.; Dzyuba, S.V.; Gryczynski, Z.; Grygorczyk, R.; et al. Imaging viscosity of intragranular mucin matrix in cystic fibrosis cells. Sci. Rep. 2017, 7, 16761. [Google Scholar] [CrossRef]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.C. Accuracy and Precision in Quantitative Fluorescence Microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef] [Green Version]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Takeyama, K.; Dabbagh, K.; Lee, H.-M.; Agustí, C.; Lausier, J.A.; Ueki, I.F.; Grattan, K.M.; Nadel, J.A. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. USA 1999, 96, 3081–3086. [Google Scholar] [CrossRef] [Green Version]
- Amatngalim, G.D.; Schrumpf, J.A.; Dishchekenian, F.; Mertens, T.C.J.; Ninaber, D.K.; van der Linden, A.C.; Pilette, C.; Taube, C.; Hiemstra, P.S.; Does, A.M. van der Aberrant epithelial differentiation by cigarette smoke dysregulates respiratory host defence. Eur. Respir. J. 2018, 51, 1701009. [Google Scholar] [CrossRef]
- Thornton, D.J.; Gray, T.; Nettesheim, P.; Howard, M.; Koo, J.S.; Sheehan, J.K. Characterization of mucins from cultured normal human tracheobronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1118–L1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gras, D.; Chanez, P.; Vachier, I.; Petit, A.; Bourdin, A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol. Ther. 2013, 140, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Gras, D.; Bourdin, A.; Vachier, I.; de Senneville, L.; Bonnans, C.; Chanez, P. An ex vivo model of severe asthma using reconstituted human bronchial epithelium. J. Allergy Clin. Immunol. 2012, 129, 1259–1266.e1. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Luo, D.; Wang, X.; Zhang, Y.; Liu, Z.; Zhong, N.; Wu, M.; Li, G. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci. Rep. 2017, 7, 42675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Ribeiro, C.M.P.; Sun, L.; Okuda, K.; Kato, T.; Gilmore, R.C.; Martino, M.B.; Dang, H.; Abzhanova, A.; Lin, J.M.; et al. XBP1S Regulates MUC5B in a Promoter Variant–Dependent Pathway in Idiopathic Pulmonary Fibrosis Airway Epithelia. Am. J. Respir. Crit. Care Med. 2019, 200, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.D.; Everman, J.L.; Chioccioli, M.; Feriani, L.; Goldfarbmuren, K.C.; Sajuthi, S.P.; Rios, C.L.; Powell, R.; Armstrong, M.; Gomez, J.; et al. Single-Cell and Population Transcriptomics Reveal Pan-epithelial Remodeling in Type 2-High Asthma. Cell Rep. 2020, 32, 107872. [Google Scholar] [CrossRef] [PubMed]
- Ruiz García, S.; Deprez, M.; Lebrigand, K.; Cavard, A.; Paquet, A.; Arguel, M.-J.; Magnone, V.; Truchi, M.; Caballero, I.; Leroy, S.; et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 2019, 146, dev177428. [Google Scholar] [CrossRef] [Green Version]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Sibila, O.; Suarez-Cuartin, G.; Rodrigo-Troyano, A.; Fardon, T.C.; Finch, S.; Mateus, E.F.; Garcia-Bellmunt, L.; Castillo, D.; Vidal, S.; Sanchez-Reus, F.; et al. Secreted mucins and airway bacterial colonization in non-CF bronchiectasis: Mucins in bronchiectasis. Respirology 2015, 20, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, L.H.; Wolber, C.; Kesimer, M.; Sheehan, J.K.; Davis, C.W. Studying Mucin Secretion from Human Bronchial Epithelial Cell Primary Cultures. In Mucins: Methods and Protocols; McGuckin, M.A., Thornton, D.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 259–277. ISBN 978-1-61779-513-8. [Google Scholar]
- THORNTON, D.J.; CARLSTEDT, I.; HOWARD, M.; DEVINE, P.L.; PRICE, M.R.; SHEEHAN, J.K. Respiratory mucins: Identification of core proteins and glycoforms. Biochem. J. 1996, 316, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, A.G.; Ehre, C.; Button, B.; Abdullah, L.H.; Cai, L.-H.; Leigh, M.W.; DeMaria, G.C.; Matsui, H.; Donaldson, S.H.; Davis, C.W.; et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Investig. 2014, 124, 3047–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesimer, M.; Sheehan, J.K. Mass Spectrometric Analysis of Mucin Core Proteins. In Mucins: Methods and Protocols; McGuckin, M.A., Thornton, D.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 67–79. ISBN 978-1-61779-513-8. [Google Scholar]
- Nicholas, B.; Skipp, P.; Mould, R.; Rennard, S.; Davies, D.E.; O’Connor, C.D.; Djukanović, R. Shotgun proteomic analysis of human-induced sputum. Proteomics 2006, 6, 4390–4401. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, G.; Cao, R.; Carpenter, J.; Ford, A.A.; Wang, T.T.; Li, Y.; Kesimer, M. The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: The mucin interactome. Mucosal Immunol. 2016, 9, 1442–1454. [Google Scholar] [CrossRef]
- Hill, D.B.; Vasquez, P.A.; Mellnik, J.; McKinley, S.A.; Vose, A.; Mu, F.; Henderson, A.G.; Donaldson, S.H.; Alexis, N.E.; Boucher, R.C.; et al. A Biophysical Basis for Mucus Solids Concentration as a Candidate Biomarker for Airways Disease. PLoS ONE 2014, 9, e87681. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, Y.; Liu, S.; Liu, G.; Li, Z.; Dong, W. Comparison of the sensitivity of Western blotting between PVDF and NC membranes. Sci. Rep. 2021, 11, 12022. [Google Scholar] [CrossRef]
- Thornton, D.J.; Howard, M.; Khan, N.; Sheehan, J.K. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J. Biol. Chem. 1997, 272, 9561–9566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdugo, P. Supramolecular Dynamics of Mucus. Cold Spring Harb. Perspect. Med. 2012, 2, a009597. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.K.; Wang, Y.-Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.B.; Button, B. Establishment of respiratory air-liquid interface cultures and their use in studying mucin production, secretion, and function. Methods Mol. Biol. 2012, 842, 245–258. [Google Scholar] [CrossRef]
- Hill, D.B.; Long, R.F.; Kissner, W.J.; Atieh, E.; Garbarine, I.C.; Markovetz, M.R.; Fontana, N.C.; Christy, M.; Habibpour, M.; Tarran, R.; et al. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur. Respir. J. 2018, 52, 1801297. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, O.W.; Yakubov, G.E.; Bonilla, M.R.; Deshmukh, O.; McGuckin, M.A.; Gidley, M.J. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+-mediated links, and hydrogen bonding. Sci. Rep. 2018, 8, 5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jory, M.; Bellouma, K.; Blanc, C.; Casanellas, L.; Petit, A.; Reynaud, P.; Vernisse, C.; Vachier, I.; Bourdin, A.; Massiera, G. Mucus Microrheology Measured on Human Bronchial Epithelium Culture. Front. Phys. 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Budai-Szűcs, M.; Berkó, S.; Kovács, A.; Jaikumpun, P.; Ambrus, R.; Halász, A.; Szabó-Révész, P.; Csányi, E.; Zsembery, Á. Rheological effects of hypertonic saline and sodium bicarbonate solutions on cystic fibrosis sputum in vitro. BMC Pulm. Med. 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Goodell, H.P.; Atieh, E.; Chen, Y.-C.; Williams, R.; Shenoy, S.; Lackey, E.; Shenkute, N.T.; Cai, L.-H.; Dennis, R.G.; et al. Roles of mucus adhesion and cohesion in cough clearance. Proc. Natl. Acad. Sci. USA 2018, 115, 12501–12506. [Google Scholar] [CrossRef] [Green Version]
- Knabe, L.; Petit, A.; Vernisse, C.; Charriot, J.; Pugnière, M.; Henriquet, C.; Sasorith, S.; Molinari, N.; Chanez, P.; Berthet, J.-P.; et al. CCSP counterbalances airway epithelial-driven neutrophilic chemotaxis. Eur. Respir. J. 2019, 54, 1802408. [Google Scholar] [CrossRef]
- Gamez, A.S.; Gras, D.; Petit, A.; Knabe, L.; Molinari, N.; Vachier, I.; Chanez, P.; Bourdin, A. SUpplementing defect in club cell secretory protein attenuates airway inflammation in copd. Chest 2014, 147, 1467–1476. [Google Scholar] [CrossRef]
- Singanayagam, A.; Glanville, N.; Girkin, J.L.; Ching, Y.M.; Marcellini, A.; Porter, J.D.; Toussaint, M.; Walton, R.P.; Finney, L.J.; Aniscenko, J.; et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat. Commun. 2018, 9, 2229. [Google Scholar] [CrossRef]
- Gras, D.; Petit, A.; Charriot, J.; Knabe, L.; Alagha, K.; Gamez, A.S.; Garulli, C.; Bourdin, A.; Chanez, P.; Molinari, N.; et al. Epithelial ciliated beating cells essential for ex vivo ALI culture growth. BMC Pulm. Med. 2017, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, M.J.; Jang, J.H.; Panariti, A.; Bedrat, A.; Ijpma, G.; Lemos, B.; Park, J.A.; Lauzon, A.M.; Martin, J.G. Airway Epithelial Cells Drive Airway Smooth Muscle Cell Phenotype Switching to the Proliferative and Pro-inflammatory Phenotype. Front. Physiol. 2021, 12, 754. [Google Scholar] [CrossRef]
- Van der Vaart, J.; Clevers, H. Airway organoids as models of human disease. J. Intern. Med. 2021, 289, 604–613. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Sachs, N.; Chiu, M.C.; Wong, B.H.-Y.; Chu, H.; Poon, V.K.-M.; Wang, D.; Zhao, X.; Wen, L.; et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc. Natl. Acad. Sci. USA 2018, 115, 6822–6827. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, C.; Vernisse, C.; Mianné, J.; Fieldès, M.; Ahmed, E.; Petit, A.; Vachier, I.; Bertrand, T.L.; Assou, S.; Bourdin, A.; et al. Lung organoids. Med. Sci. 2020, 36, 382–388. [Google Scholar] [CrossRef]
- De Oca, M.M.; Halbert, R.J.; Lopez, M.V.; Perez-Padilla, R.; Tálamo, C.; Moreno, D.; Muiño, A.; Jardim, J.R.B.; Valdivia, G.; Pertuzé, J.; et al. The chronic bronchitis phenotype in subjects with and without COPD: The PLATINO study. Eur. Respir. J. 2012, 40, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgel, P.-R.; Nesme-Meyer, P.; Chanez, P.; Caillaud, D.; Carré, P.; Perez, T.; Roche, N. Initiatives Bronchopneumopathie Chronique Obstructive (BPCO) Scientific Committee Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 2009, 135, 975–982. [Google Scholar] [CrossRef]
- Lahousse, L.; Seys, L.J.M.; Joos, G.F.; Franco, O.H.; Stricker, B.H.; Brusselle, G.G. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur. Respir. J. 2017, 50, 1602470. [Google Scholar] [CrossRef] [PubMed]
- Alagha, K.; Bourdin, A.; Vernisse, C.; Garulli, C.; Tummino, C.; Charriot, J.; Vachier, I.; Suehs, C.; Chanez, P.; Gras, D. Goblet cell hyperplasia as a feature of neutrophilic asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019, 49, 781–788. [Google Scholar] [CrossRef]
- Deslee, G.; Burgel, P.-R.; Escamilla, R.; Chanez, P.; Court-Fortune, I.; Nesme-Meyer, P.; Brinchault-Rabin, G.; Perez, T.; Jebrak, G.; Caillaud, D.; et al. Impact of current cough on health-related quality of life in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Monz, B.U.; Sachs, P.; McDonald, J.; Crawford, B.; Nivens, M.C.; Tetzlaff, K. Responsiveness of the cough and sputum assessment questionnaire in exacerbations of COPD and chronic bronchitis. Respir. Med. 2010, 104, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Bommart, S.; Marin, G.; Bourdin, A.; Molinari, N.; Klein, F.; Hayot, M.; Vachier, I.; Chanez, P.; Mercier, J.; Vernhet-Kovacsik, H. Relationship between CT air trapping criteria and lung function in small airway impairment quantification. BMC Pulm. Med. 2014, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Okajima, Y.; Come, C.; Nardelli, P.; Sonavane, S.; Yen, A.; Hrudaya, N.; Terry, N.; Grumley, S.; Ahmed, A.; Jacobs, K.; et al. Mucus plugging on CT and mortality in smokers. Eur. Respir. J. 2019, 54, OA1917. [Google Scholar] [CrossRef]
- Gono, H.; Fujimoto, K.; Kawakami, S.; Kubo, K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur. Respir. J. 2003, 22, 965–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telenga, E.D.; Oudkerk, M.; van Ooijen, P.M.A.; Vliegenthart, R.; ten Hacken, N.H.T.; Postma, D.S.; van den Berge, M. Airway wall thickness on HRCT scans decreases with age and increases with smoking. BMC Pulm. Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.E.; Leung, A.N.; Moss, R.B.; Blankenberg, F.G.; Bloch, D.A.; Oehlert, J.W.; Al-Dabbagh, H.; Hubli, S.; Northway, W.H. Spirometer-Triggered High Resolution Computed Tomography (HRCT) of the Chest, Clinical Score, and Pulmonary Function Measurements in Cystic Fibrosis (CF) Patients before and after Treatment for a Pulmonary Exacerbation. Pediatr. Res. 1999, 45, 355. [Google Scholar] [CrossRef] [Green Version]
- King, G.G.; Carroll, J.D.; Müller, N.L.; Whittall, K.P.; Gao, M.; Nakano, Y.; Paré, P.D. Heterogeneity of narrowing in normal and asthmatic airways measured by HRCT. Eur. Respir. J. 2004, 24, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, G.; Gamez, A.S.; Molinari, N.; Kacimi, D.; Vachier, I.; Paganin, F.; Chanez, P.; Bourdin, A. Distal airway impairment in obese normoreactive women. BioMed Res. Int. 2013, 2013, 707856. [Google Scholar] [CrossRef]
- Dunican, E.M.; Elicker, B.M.; Henry, T.; Gierada, D.S.; Schiebler, M.L.; Anderson, W.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Boucher, R.C.; et al. Mucus Plugs and Emphysema in the Pathophysiology of Airflow Obstruction and Hypoxemia in Smokers. Am. J. Respir. Crit. Care Med. 2021, 203, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; McDonough, J.E.; Sanchez, P.G.; Cooper, J.D.; Coxson, H.O.; Elliott, W.M.; Naiman, D.; Pochettino, M.; Horng, D.; Gefter, W.B.; et al. Micro-computed tomography measurements of peripheral lung pathology in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Kirby, M.; Tanabe, N.; Vasilescu, D.M.; Cooper, J.D.; McDonough, J.E.; Verleden, S.E.; Vanaudenaerde, B.M.; Sin, D.D.; Tan, W.C.; Coxson, H.O.; et al. Computed Tomography Total Airway Count Is Associated with the Number of Micro–Computed Tomography Terminal Bronchioles. Am. J. Respir. Crit. Care Med. 2020, 201, 613–615. [Google Scholar] [CrossRef]
- Ortiz, J.L.; Ortiz, A.; Milara, J.; Armengot, M.; Sanz, C.; Compañ, D.; Morcillo, E.; Cortijo, J. Evaluation of Mucociliary Clearance by Three Dimension Micro-CT-SPECT in Guinea Pig: Role of Bitter Taste Agonists. PLoS ONE 2016, 11, e0164399. [Google Scholar] [CrossRef] [Green Version]
- Uzun, S.; Djamin, R.S.; Kluytmans, J.A.J.W.; Mulder, P.G.H.; van’t Veer, N.E.; Ermens, A.A.M.; Pelle, A.J.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Eerden, M.M. van der Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 361–368. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Boersma, W.; Lonergan, M.; Jayaram, L.; Crichton, M.L.; Karalus, N.; Taylor, S.L.; Martin, M.L.; Burr, L.D.; Wong, C.; et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: An individual participant data meta-analysis. Lancet Respir. Med. 2019, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, T.D.Y.; Saris, A.; Schultz, M.J.; Poll, T. van der Immunomodulation by macrolides: Therapeutic potential for critical care. Lancet Respir. Med. 2020, 8, 619–630. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.; Wenzel, S.E.; Bleecker, E.R.; Pizzichini, E.; Kuna, P.; Busse, W.W.; Gossage, D.L.; Ward, C.K.; Wu, Y.; Wang, B.; et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: A phase 2b randomised dose-ranging study. Lancet Respir. Med. 2014, 2, 879–890. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]

| Production | Secretion | Biophysicial Behaviour | Strengths | Limitations | |
|---|---|---|---|---|---|
| Total cell count (sputum/BAL fluid) | +/− | − | + (indirect link) | Simple, unexpensive, performed routinely in health facilities | Information provided about mucus is limited |
| Microbiology | +/− (indirect link) | +/− (Indirect link) | +/− (indirect link) | Simple, unexpensive, performed routinely in health facilities | No clear direct correlation in vivo bacterial load and mucus production/secretion |
| ELISA (mucins) | ++ (cell lysates) | ++ | − | Quantitative assay, can be used in vivo Rapid and simple measurement of intra- or extra-cellular mucins | Caution needed with sample processing and epitope integrity, or homologous regions |
| Western Blot (mucins) | ++ (cell lysates) | ++ | − | Rapid and simple measurement of intra- or extra-cellular mucins | Semi-quantitative Caution needed with sample processing and verification of specificity Requires denaturation of mucins for agarose gel electrophoresis |
| Immunohistochemistry and Immunofluorescence (secretory cells, mucus) | + (intracellular mucins) | + | − | Spatial localization of mucins in airway and/or in secretory cells, co-localization with other components | Qualitative or semi-quantitative assessment. Time-consuming Scoring system needs blinded individuals |
| Mass Spectrometry | ++ | ++ | − | Accurate quantitative assay Very high specificity | Not routinely performed expensive, Time-consuming |
| Quantitative RT-PCR (mucins mRNA) | +++ | − | − | Simple, unexpensive Specific quantitative information on mucin expression at the mRNA level | No detection of post-transcriptional modifications |
| Single-cell RNA seq | + (intracellular mechanisms) | + (intracellular mechanisms) | − | Dynamic overview of the intracellular mucus-secreting/producing machinery | No quantitative assessment of mucin production and secretion Expensive and time-consuming |
| Rheology | − | +/− (indirect link) | +++ (viscoelastic properties) | Can be easily performed with in vivo samples Relationship with clinical phenotyping | The yield of sputum collection is variable Caution needed with sample processing and quality check (salivary contamination) |
| Ex vivo models | ++ | ++ | + | Chronic airway disease phenotype is maintened Measurement of exposure to drugs or toxins is feasible | High expertise needed for cell culture, mucus sampling can be difficult (PBS washes) Time consuming |
| HRCT | − | + (indirect scoring) | − | Routinely performed, unexpensive In vivo endotyping of chronic airway disease | No specific information on mucus production or secretion |
| Micro-CT | − | + (indirect observation) | − | High-quality ex vivo imaging of small airway diseases | expensive Invasive method (surgical lung biopsy or lung explant) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charriot, J.; Volpato, M.; Petit, A.; Vachier, I.; Bourdin, A. Methods of Sputum and Mucus Assessment for Muco-Obstructive Lung Diseases in 2022: Time to “Unplug” from Our Daily Routine! Cells 2022, 11, 812. https://doi.org/10.3390/cells11050812
Charriot J, Volpato M, Petit A, Vachier I, Bourdin A. Methods of Sputum and Mucus Assessment for Muco-Obstructive Lung Diseases in 2022: Time to “Unplug” from Our Daily Routine! Cells. 2022; 11(5):812. https://doi.org/10.3390/cells11050812
Chicago/Turabian StyleCharriot, Jeremy, Mathilde Volpato, Aurélie Petit, Isabelle Vachier, and Arnaud Bourdin. 2022. "Methods of Sputum and Mucus Assessment for Muco-Obstructive Lung Diseases in 2022: Time to “Unplug” from Our Daily Routine!" Cells 11, no. 5: 812. https://doi.org/10.3390/cells11050812
APA StyleCharriot, J., Volpato, M., Petit, A., Vachier, I., & Bourdin, A. (2022). Methods of Sputum and Mucus Assessment for Muco-Obstructive Lung Diseases in 2022: Time to “Unplug” from Our Daily Routine! Cells, 11(5), 812. https://doi.org/10.3390/cells11050812






