Accelerated Wound Healing and Keratinocyte Proliferation through PI3K/Akt/pS6 and VEGFR2 Signaling by Topical Use of Pleural Fluid
Abstract
:1. Introduction
2. Materials and Methods
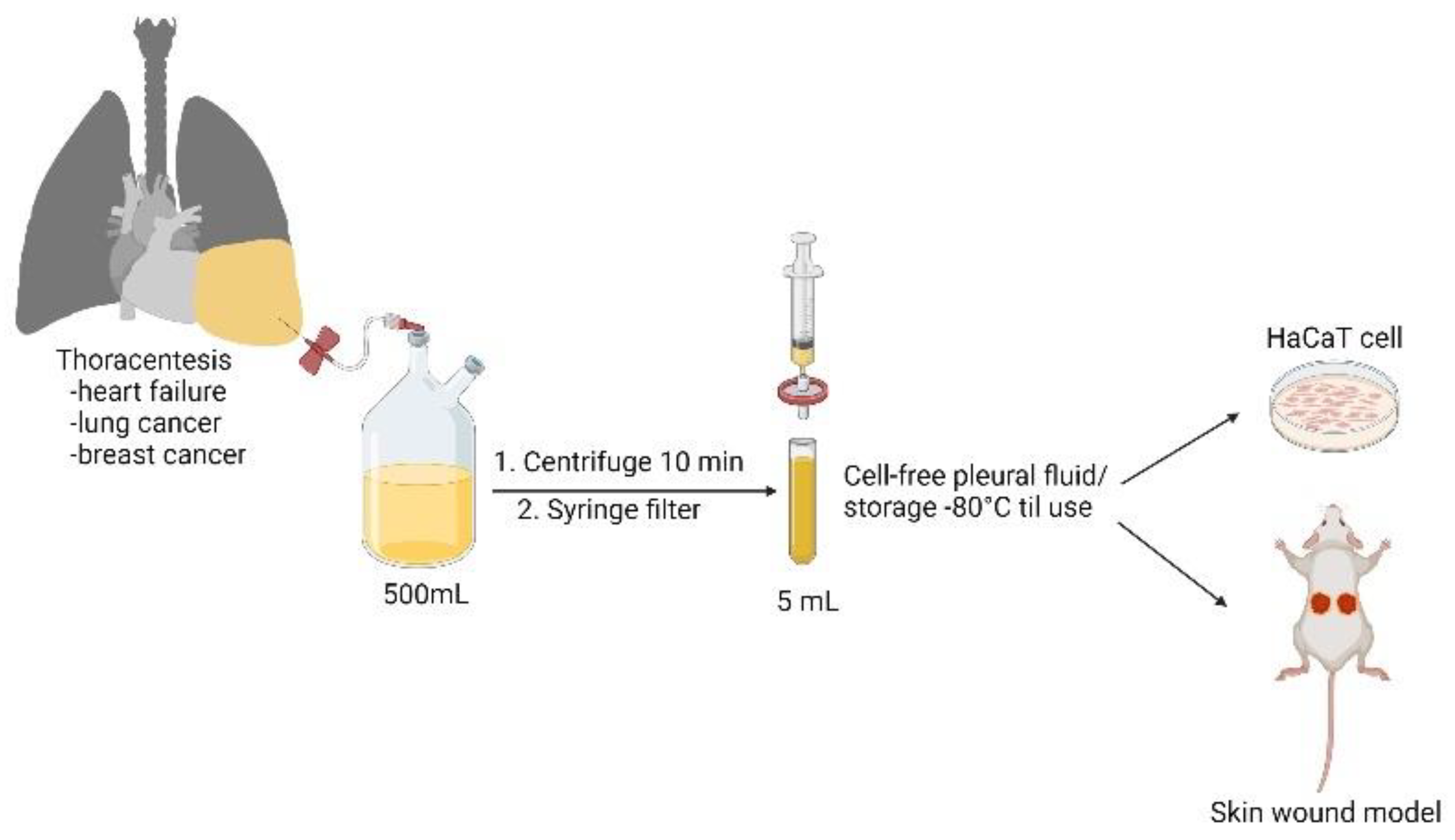
2.1. Patient Characteristics and Collection of Pleural Fluid Samples
2.2. Culture of Keratinocytes
2.3. Drugs and Reagents
2.4. Cell Survival Assay
2.5. Flow Cytometric Analysis
2.6. Migration and Transwell Assays
2.7. Western Blotting
2.8. Immunofluorescence Staining
2.9. Mouse Full-Thickness Wound Model and Daily Change of Wound Dressings
2.10. Hematoxylin and Eosin (HE) Staining, Masson’s Trichrome Staining, and Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. Effect of Cell-Free Pleural Fluid on Keratinocyte Cell Viability, Motility, and Cell Cycle Progression
3.2. HFPF and LCPF Upregulates p-PI3K/Akt and VEGFR2 Signaling in Keratinocytes
3.3. VEGFR2 Inhibitor, But Not p-PI3K Inhibitor, Attenuates LCPF-Induced Cell Viability and MMP2 Expression
3.4. Application of LCPF-Based Wet Dressing Improves Early Cutaneous Wound Closure in Mice through Increased Re-Epithelization and Collagen Deposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Aaling. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changchien, C.Y.; Chen, Y.; Chang, H.H.; Chang, S.Y.; Tsai, W.C.; Tsai, H.C.; Wang, C.Y.; Lee, H.S.; Tsai, C.L. Effect of malignant-associated pleural effusion on endothelial viability, motility and angiogenesis in lung cancer. Cancer Sci. 2020, 111, 3747. [Google Scholar] [CrossRef]
- Changchien, C.Y.; Chang, H.H.; Dai, M.S.; Tsai, W.C.; Tsai, H.C.; Wang, C.Y.; Shen, M.S.; Cheng, L.T.; Lee, H.S.; Chen, Y.; et al. Distinct JNK/VEGFR signaling on angiogenesis of breast cancer-associated pleural fluid based on hormone receptor status. Cancer Sci. 2021, 112, 781–791. [Google Scholar] [CrossRef]
- Velnar, T.; Gradisnik, L. Tissue augmentation in wound healing: The role of endothelial and epithelial cells. Med. Arch. 2018, 72, 444. [Google Scholar] [CrossRef]
- Miserocchi, G. Physiology and pathophysiology of pleural fluid turnover. Eur. Respir. J. 1997, 10, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Feller-Kopman, D.; Light, R. Pleural disease. N. Engl. J. Med. 2018, 378, 740–751. [Google Scholar] [CrossRef]
- Light, R.W. Pleural effusion. N. Engl. J. Med. 2002, 346, 1971–1977. [Google Scholar] [CrossRef]
- Jany, B.; Welte, T. Pleural effusion in adults—Etiology, diagnosis, and treatment. Dtsch. Ärzteblatt Int. 2019, 116, 377. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.; Yu, E.Z.; Hennessey, P.; Ko, F.; Robson, M.C. Determination of endogenous cytokines in chronic wounds. Ann. Surg. 1994, 219, 688. [Google Scholar] [CrossRef]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Han, C.-M.; Cheng, B.; Wu, P. Clinical guideline on topical growth factors for skin wounds. Burn. Trauma 2020, 8, 8. [Google Scholar] [CrossRef]
- Ackermann, M.; Wolloscheck, T.; Wellmann, A.; Li, V.W.; Li, W.W.; Konerding, M.A. Priming with a combination of proangiogenic growth factors improves wound healing in normoglycemic mice. Int. J. Mol. Med. 2011, 27, 647–653. [Google Scholar]
- Cheng, D.-S.; Rodriguez, R.M.; Perkett, E.A.; Rogers, J.; Bienvenu, G.; Lappalainen, U.; Light, R.W. Vascular endothelial growth factor in pleural fluid. Chest 1999, 116, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.-H.; Hsu, P.-C.; Liao, T.-L.; Feng, A.-C.; Chu, N.-M.; Kao, S.-H. Pleural fluid osteopontin, vascular endothelial growth factor, and urokinase-type plasminogen activator levels as predictors of pleurodesis outcome and prognosticators in patients with malignant pleural effusion: A prospective cohort study. BMC Cancer 2016, 16, 463. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef]
- Calautti, E.; Li, J.; Saoncella, S.; Brissette, J.L.; Goetinck, P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005, 280, 32856–32865. [Google Scholar] [CrossRef] [Green Version]
- Castilho, R.M.; Squarize, C.H.; Gutkind, J.S. Exploiting PI 3 K/m TOR signaling to accelerate epithelial wound healing. Oral Dis. 2013, 19, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Dobrokhotov, O.; Sokabe, M. Coordination between Cell Motility and Cell Cycle Progression in Keratinocyte Sheets via Cell-Cell Adhesion and Rac1. IScience 2020, 23, 101729. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, U.; Hoffmann, M.; Zhao, X.; Chan, K.; Hui, D.; Gosse, H.; Engelmann, L.; Schauer, J.; Emmrich, F.; Hoheisel, G. Vascular endothelial growth factor in pleural effusions of different origin. Eur. Respir. J. 2005, 25, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Sabino, F.; Auf dem Keller, U. Matrix metalloproteinases in impaired wound healing. Met. Med. 2015, 2, 1–8. [Google Scholar]
- Patruno, A.; Pesce, M.; Grilli, A.; Speranza, L.; Franceschelli, S.; De Lutiis, M.A.; Vianale, G.; Costantini, E.; Amerio, P.; Muraro, R. mTOR activation by PI3K/Akt and ERK signaling in short ELF-EMF exposed human keratinocytes. PLoS ONE 2015, 10, e0139644. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Wei, J.; Yang, W.; Li, W.; Liu, F.; Yan, X.; Yan, X.; Hu, N.; Li, J. MicroRNA-26a inhibits wound healing through decreased keratinocytes migration by regulating ITGA5 through PI3K/AKT signaling pathway. Biosci. Rep. 2020, 40, BSR20201361. [Google Scholar] [CrossRef]
- Yang, R.; Liu, F.; Wang, J.; Chen, X.; Xie, J.; Xiong, K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Sangai, T.; Akcakanat, A.; Chen, H.; Wei, C.; Meric-Bernstam, F. Vertical inhibition of the PI3K/Akt/mTOR pathway is synergistic in breast cancer. Oncogenesis 2017, 6, e385. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Krieg, T. Molecular mechanisms of VEGF—A action during tissue repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Hsu, I.-L.; Su, W.-C.; Yan, J.-J.; Chang, J.-M.; Lai, W.-W. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: Correlations with patient survival and pleural effusion control. Lung Cancer 2009, 65, 371–376. [Google Scholar] [CrossRef]
- Fafliora, E.; Hatzoglou, C.; Gourgoulianis, K.I.; Zarogiannis, S.G. Systematic review and meta-analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol. Rep. 2016, 4, e12978. [Google Scholar] [CrossRef]
- Safi, A.; Sadmi, M.; Martinet, N.; Menard, O.; Vaillant, P.; Gallati, H.; Hosang, M.; Martinet, Y. Presence of elevated levels of platelet-derived growth factor (PDGF) in lung adenocarcinoma pleural effusions. Chest 1992, 102, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, O.; Saijo, Y.; Narumi, K.; Kimura, Y.; Ebina, M.; Matsubara, N.; Asou, N.; Nakai, Y.; Nukiwa, T. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology 2002, 63, 70–75. [Google Scholar] [CrossRef]
- Rossiter, H.; Barresi, C.; Pammer, J.; Rendl, M.; Haigh, J.; Wagner, E.F.; Tschachler, E. Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res. 2004, 64, 3508–3516. [Google Scholar] [CrossRef] [Green Version]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef] [Green Version]
- Saaristo, A.; Tammela, T.; Fārkkilā, A.; Kärkkäinen, M.; Suominen, E.; Yla-Herttuala, S.; Alitalo, K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am. J. Pathol. 2006, 169, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1. [Google Scholar]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J. Biomed. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Etulain, J.; Mena, H.A.; Meiss, R.P.; Frechtel, G.; Gutt, S.; Negrotto, S.; Schattner, M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci. Rep. 2018, 8, 1513. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet rich plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Oreffo, R.O.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Karim, R.B.; Brito, B.L.; Dutrieux, R.P.; Lassance, F.P.; Hage, J.J. MMP-2 assessment as an indicator of wound healing: A feasibility study. Adv. Skin Wound Care 2006, 19, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, H.F. Tumors: Wounds that do not heal—A historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin. Thromb. Hemost. 2019, 45, 576–592. [Google Scholar] [CrossRef]
- Wojczakowski, W.; Kobylarek, D.; Lindner, J.; Limphaibool, N.; Kaczmarek, M. MicroRNAs–Novel biomarkers for malignant pleural effusions. Contemp. Oncol. 2019, 23, 133. [Google Scholar] [CrossRef] [Green Version]
- Sorolla, M.A.; Sorolla, A.; Parisi, E.; Salud, A.; Porcel, J.M. Diving into the Pleural Fluid: Liquid Biopsy for Metastatic Malignant Pleural Effusions. Cancers 2021, 13, 2798. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-L.; Changchien, C.-Y.; Chen, Y.; Chang, H.-H.; Tsai, W.-C.; Wang, Y.-W.; Chou, K.-C.; Chiang, M.-H.; Tsai, Y.-L.; Tsai, H.-C.; et al. Accelerated Wound Healing and Keratinocyte Proliferation through PI3K/Akt/pS6 and VEGFR2 Signaling by Topical Use of Pleural Fluid. Cells 2022, 11, 817. https://doi.org/10.3390/cells11050817
Tsai C-L, Changchien C-Y, Chen Y, Chang H-H, Tsai W-C, Wang Y-W, Chou K-C, Chiang M-H, Tsai Y-L, Tsai H-C, et al. Accelerated Wound Healing and Keratinocyte Proliferation through PI3K/Akt/pS6 and VEGFR2 Signaling by Topical Use of Pleural Fluid. Cells. 2022; 11(5):817. https://doi.org/10.3390/cells11050817
Chicago/Turabian StyleTsai, Chen-Liang, Chih-Ying Changchien, Ying Chen, Hsin-Han Chang, Wen-Chiuan Tsai, Yi-Wen Wang, Kai-Chieh Chou, Ming-Hsien Chiang, Yu-Ling Tsai, Hao-Chung Tsai, and et al. 2022. "Accelerated Wound Healing and Keratinocyte Proliferation through PI3K/Akt/pS6 and VEGFR2 Signaling by Topical Use of Pleural Fluid" Cells 11, no. 5: 817. https://doi.org/10.3390/cells11050817








