Altered Plasma Fatty Acid Abundance Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
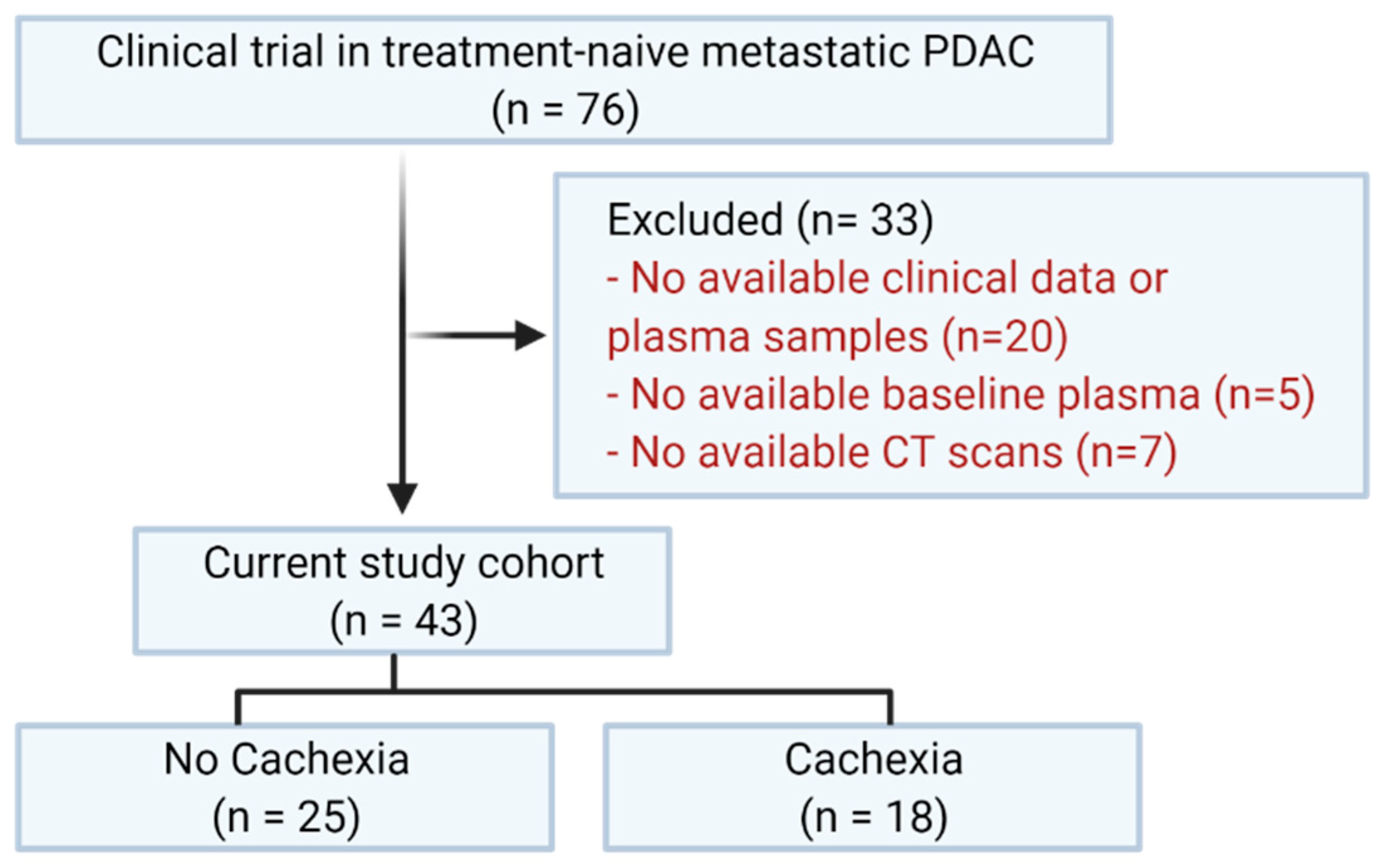
2.1. Study Population
2.2. Cachexia Classification
2.3. Blood Collection and Processing
2.4. Fatty Acid Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
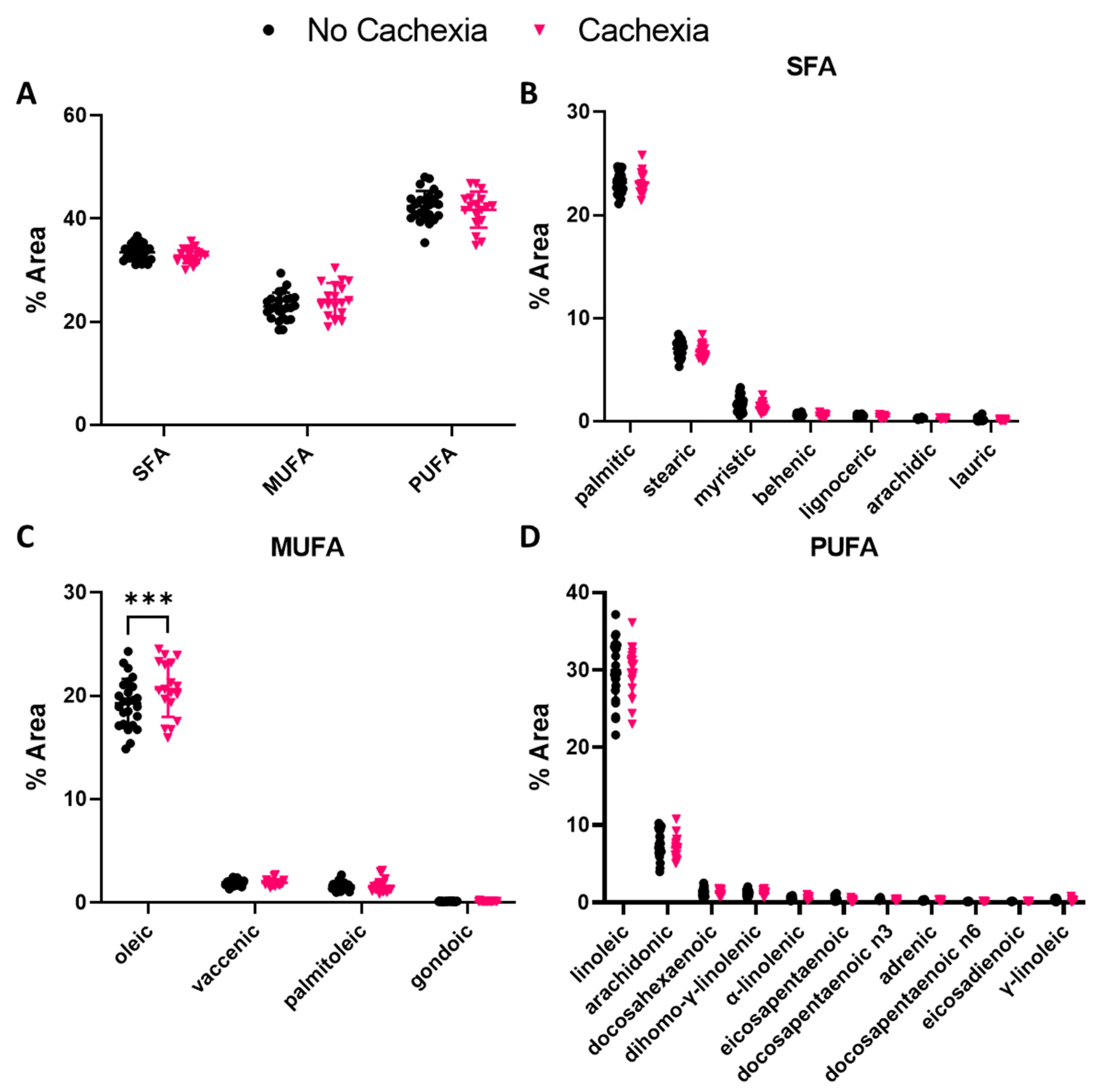
3.2. Oleic Acid Abundance Is Elevated in Subjects with Cachexia
3.3. Lauric and Eicosapentaenoic Acid Abundance Correlate with Age in Subjects with Cachexia
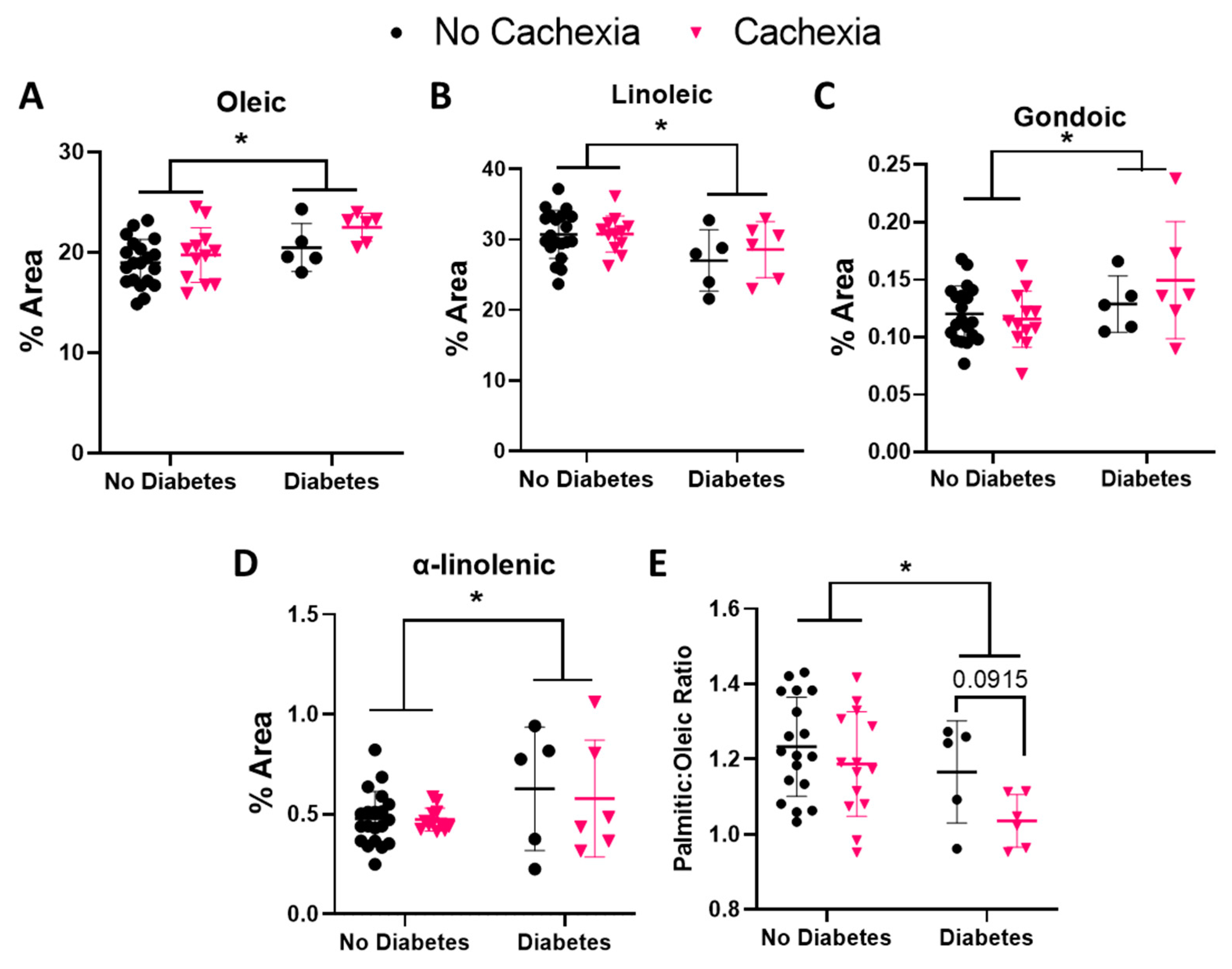
3.4. BMI and Diabetes Alter Oleic and Linoleic Acid Levels in Subjects with Cachexia
3.5. Some FAs Correlate with Other Indicators of Cachexia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef]
- Sun, L.; Quan, X.-Q.; Yu, S. An Epidemiological Survey of Cachexia in Advanced Cancer Patients and Analysis on Its Diagnostic and Treatment Status. Nutr. Cancer 2015, 67, 1056–1062. [Google Scholar] [CrossRef]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.; O′Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachexia Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, K.; Fakelman, F.; Hartmann, D.; Giese, N.A.; Gaida, M.M.; Schnölzer, M.; Flad, T.; Büchler, M.W.; Werner, J. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 2010, 88, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Macášek, J.; Burda, M.; Tvrzická, E.; Vecka, M.; Krechler, T.; Staňková, B.; Hrabák, P.; Žák, A. Chronic pancreatitis and the composition of plasma phosphatidylcholine fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2016, 108, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.-M.; Broch, M.; Vendrell, J.; Ricart, W. Insulin Resistance, Inflammation, and Serum Fatty Acid Composition. Diabetes Care 2003, 26, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Klein-Platat, C.; Drai, J.; Oujaa, M.; Schlienger, J.-L.; Simon, C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am. J. Clin. Nutr. 2005, 82, 1178–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, D.M.D.R.; Das Neves, R.X.; De Matos-Neto, E.M.; Camargo, R.G.; Lima, J.D.C.C.; Radloff, K.; Alves, M.J.; Costa, R.G.F.; Tokeshi, F.; Otoch, J.P.; et al. Plasma Lipid Profile and Systemic Inflammation in Patients with Cancer Cachexia. Front. Nutr. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Lu, C.; Meng, Q.; Halim, A.; Yean, T.J.; Wu, G. Plasma concentration of interleukin-6 was upregulated in cancer cachexia patients and was positively correlated with plasma free fatty acid in female patients. Nutr. Metab. 2019, 16, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Naito, T. Emerging Treatment Options for Cancer-Associated Cachexia: A Literature Review. Ther. Clin. Risk Manag. 2019, 15, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J. Gastrointest. Surg. 2018, 10, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Gorjão, R.; dos Santos, C.M.M.; Serdan, T.D.A.; Diniz, V.L.S.; Alba-Loureiro, T.C.; Cury-Boaventura, M.F.; Hatanaka, E.; Levada-Pires, A.C.; Sato, F.T.; Pithon-Curi, T.C.; et al. New insights on the regulation of cancer cachexia by N-3 polyunsaturated fatty acids. Pharmacol. Ther. 2018, 196, 117–134. [Google Scholar] [CrossRef]
- Garcia, J.M.; Shamliyan, T.A. Omega-3 Fatty Acids in Patients with Anorexia-Cachexia Syndrome Associated with Malignancy and Its Treatments. Am. J. Med. 2017, 130, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Wijler, L.A.; Raats, D.A.; Elias, S.G.; Dijk, F.J.; Quirindongo, H.; May, A.M.; Furber, M.J.; Dorresteijn, B.; van Dijk, M.; Kranenburg, O. Specialized nutrition improves muscle function and physical activity without affecting chemotherapy efficacy in C26 tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2021, 12, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007, 2007, CD004597. [Google Scholar] [CrossRef] [PubMed]
- Patursson, P.; Møller, G.; Muhic, A.; Andersen, J.R. N-3 fatty acid EPA supplementation in cancer patients receiving abdominal radiotherapy—A randomised controlled trial. Clin. Nutr. ESPEN 2021, 43, 130–136. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Smith, R.; Aulet, M.; Bensen, B.; Lichtman, S.; Wang, J.; Pierson, R.N. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 1990, 52, 214–218. [Google Scholar] [CrossRef]
- Dennis, R.A.; Long, D.E.; Landes, R.D.; Padala, K.P.; Padala, P.; Garner, K.K.; Wise, J.N.; Peterson, C.A.; Sullivan, D.H. Tutorial for using SliceOmatic to calculate thigh area and composition from computed tomography images from older adults. PLoS ONE 2018, 13, e0204529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Stoffel, W.; Chu, F.; Ahrens, E.H. Analysis of Long-Chain Fatty Acids by Gas-Liquid Chromatography–Micromethod for Preparation of Methyl Esters. Anal. Chem. 1959, 31, 307–308. [Google Scholar] [CrossRef]
- Yee, L.D.; Lester, J.L.; Cole, R.M.; Richardson, J.R.; Hsu, J.C.; Li, Y.; Lehman, A.; A Belury, M.; Clinton, S.K. ω-3 Fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 2010, 91, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton-Smith, C.; Woodward, M.; Tavendale, R. Evidence for age-related differences in the fatty acid composition of human adipose tissue, independent of diet. Eur. J. Clin. Nutr. 1997, 51, 619–624. [Google Scholar] [CrossRef] [Green Version]
- de Groot, R.H.M.; van Boxtel, M.P.J.; Schiepers, O.J.G.; Hornstra, G.; Jolles, J. Age dependence of plasma phospholipid fatty acid levels: Potential role of linoleic acid in the age-associated increase in docosahexaenoic acid and eicosapentaenoic acid concentrations. Br. J. Nutr. 2009, 102, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, J.L.; Andersen, D.K.; Borrebaeck, C.A.; Chari, S.T.; Costello, E.; Cruz-Monserrate, Z.; Eibl, G.; Engleman, E.G.; Fisher, W.E.; Habtezion, A.; et al. The Interface of Pancreatic Cancer with Diabetes, Obesity, and Inflammation: Research Gaps and Opportunities. Pancreas 2018, 47, 516–525. [Google Scholar] [CrossRef]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.-M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and insulin resistance in the obese may develop as part of a homeostatic response to elevated free fatty acids: A mechanistic case-control and a population-based cohort study. eBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Poynter, M.E.; Stevens, R.; Bain, J.; Ikayeva, O.; Fukagawa, N.K.; Champagne, C.M.; Crain, K.I.; Koves, T.R.; et al. A Lipidomics Analysis of the Relationship Between Dietary Fatty Acid Composition and Insulin Sensitivity in Young Adults. Diabetes 2013, 62, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Schwarz, S.; Prokopchuk, O.; Esefeld, K.; Gröschel, S.; Bachmann, J.; Lorenzen, S.; Friess, H.; Halle, M.; Martignoni, M.E. The clinical picture of cachexia: A mosaic of different parameters (experience of 503 patients). BMC Cancer 2017, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- van der Vusse, G.J. Albumin as Fatty Acid Transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Bakke, S.S.; Feng, Y.Z.; Nikolić, N.; Kase, E.T.; Moro, C.; Stensrud, C.; Damlien, L.; Ludahl, M.O.; Sandbu, R.; Solheim, B.M.; et al. Myotubes from Severely Obese Type 2 Diabetic Subjects Accumulate Less Lipids and Show Higher Lipolytic Rate than Myotubes from Severely Obese Non-Diabetic Subjects. PLoS ONE 2015, 10, e0119556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, R.D.S.; Muradás, T.C.; Dagnino, A.P.A.; Rost, F.L.; Costa, K.M.; Venturin, G.T.; Greggio, S.; Da Costa, J.C.; Campos, M.M. Targeting FFA1 and FFA4 receptors in cancer-induced cachexia. Am. J. Physiol. Metab. 2020, 319, E877–E892. [Google Scholar] [CrossRef]
- Werner, K.; De Gaudry, D.K.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil-a randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, K.C.; Barber, M.D.; Moses, A.G.; Ahmedzai, S.; Taylor, G.S.; Tisdale, M.J.; Murray, G. Double-Blind, Placebo-Controlled, Randomized Study of Eicosapentaenoic Acid Diester in Patients with Cancer Cachexia. J. Clin. Oncol. 2006, 24, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Rowland, K.; Loprinzi, C.L.; Sloan, J.A.; Dakhil, S.R.; Macdonald, N.; Gagnon, B.; Novotny, P.J.; Mailliard, J.A.; Bushey, T.I.; et al. An Eicosapentaenoic Acid Supplement Versus Megestrol Acetate Versus Both for Patients with Cancer-Associated Wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada Collaborative Effort. J. Clin. Oncol. 2004, 22, 2469–2476. [Google Scholar] [CrossRef]
- Sands, S.A.; Reid, K.J.; Windsor, S.L.; Harris, W.S. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 2005, 40, 343–347. [Google Scholar] [CrossRef]
- Walker, C.G.; Browning, L.M.; Mander, A.P.; Madden, J.; West, A.L.; Calder, P.C.; Jebb, S.A. Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br. J. Nutr. 2013, 111, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 30–33. [Google Scholar] [CrossRef] [PubMed]
- E Norris, L.; Collene, A.L.; Asp, M.L.; Hsu, J.C.; Liu, L.-F.; Richardson, J.R.; Li, D.; Bell, D.; Osei, K.; Jackson, R.D.; et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009, 90, 468–476. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2018, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Angelotti, A.; Belury, M. The Impact of Consuming Healthy Cookies Enriched with Linoleic Acid for Two Weeks on Blood Fatty Acid Profiles (P08-099-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Sailaja, Y.; Baskar, R.; Rao, C.S.; Saralakumari, D. Membrane lipids and protein-bound carbohydrates status during the maturation of reticulocytes to erythrocytes in type 2 diabetics. Clin. Chim. Acta 2004, 341, 185–192. [Google Scholar] [CrossRef] [PubMed]
| No Cachexia (n = 25) | Cachexia (n = 18) | p-Value | |
|---|---|---|---|
| Mean age (years) | 62.9 ± 11.1 | 60.5 ± 10.5 | 0.480 |
| Male sex, n (%) | 18 (72.0) | 10 (55.6) | 0.338 |
| % Weight loss | 4.3 ± 7.5 | 11.1 ± 8.5 | 0.002 * |
| Skeletal muscle index (SMI) | 49.6 ± 9.3 | 40.9 ± 5.9 | 0.0002 * |
| Body mass index (BMI) (kg/m2) 18.5–24.9 (% Normal) 25–29.9 (% Overweight) ≥ 30 (% Obese) | 7 (28.0) 11 (44.0) 7 (28.0) | 5 (27.8) 8 (44.4) 5 (27.8) | 0.999 |
| Diabetes present, n (%) | 5 (20) | 6 (33.3) | 0.481 |
| Hemoglobin (g/dL) | 13.5 ± 1.8 | 13.5 ± 1.1 | 0.953 |
| Albumin (g/dL) | 3.9 ± 0.6 | 3.9 ± 0.4 | 0.652 |
| Fatty Acid | No Cachexia R | Cachexia R |
|---|---|---|
| Lauric | 0.364 | 0.592 ** |
| Myristic | 0.032 | 0.307 |
| Palmitic | 0.281 | −0.030 |
| Palmitoleic | −0.300 | −0.115 |
| Stearic | −0.071 | 0.381 |
| Oleic | −0.187 | −0.083 |
| Vaccenic | −0.037 | −0.204 |
| Linoleic | −0.048 | −0.306 |
| γ-Linolenic | −0.055 | 0.271 |
| α-Linolenic | −0.002 | 0.137 |
| Arachidic | 0.116 | 0.107 |
| Gondoic | 0.029 | −0.169 |
| Eicosadienoic | −0.170 | −0.058 |
| Dihomo-γ-Linolenic | −0.109 | 0.213 |
| Arachidonic | 0.087 | 0.349 |
| Eicosapentaenoic | 0.364 | 0.564 * |
| Behenic | −0.086 | 0.094 |
| Adrenic | −0.053 | 0.151 |
| Docosapentaenoic n6 | 0.059 | 0.130 |
| Docosapentaneoic n3 | 0.323 | 0.378 |
| Lignoceric | 0.114 | 0.083 |
| Docosahexaneoic | 0.355 | 0.318 |
| Fatty Acid | No Cachexia R | Cachexia R |
|---|---|---|
| Lauric | −0.250 | −0.287 |
| Myristic | 0.199 | −0.281 |
| Palmitic | −0.045 | 0.162 |
| Palmitoleic | −0.99 | 0.291 |
| Stearic | −0.099 | −0.101 |
| Oleic | 0.070 | 0.426 |
| Vaccenic | −0.014 | 0.483 * |
| Linoleic | 0.116 | −0.258 |
| γ-Linolenic | −0.166 | −0.293 |
| α-Linolenic | 0.386 | −0.052 |
| Arachidic | −0.187 | 0.094 |
| Gondoic | −0.026 | 0.304 |
| Eicosadienoic | 0.013 | −0.207 |
| Dihomo-γ-Linolenic | −0.255 | −0.204 |
| Arachidonic | −0.152 | −0.169 |
| Eicosapentaenoic | 0.009 | −0.420 |
| Behenic | −0.301 | −0.201 |
| Adrenic | −0.37 | −0.067 |
| Docosapentaenoic n6 | −0.550 * | −0.135 |
| Docosapentaneoic n3 | −0.342 | −0.334 |
| Lignoceric | −0.258 | −0.433 |
| Docosahexaneoic | 0.074 | −0.111 |
| Fatty Acid | No Cachexia R | Cachexia R |
|---|---|---|
| Lauric | −0.423 * | 0.143 |
| Myristic | −0.274 | −0.123 |
| Palmitic | −0.187 | −0.489 * |
| Palmitoleic | −0.270 | −0.102 |
| Stearic | −0.089 | 0.003 |
| Oleic | −0.004 | −0.419 |
| Vaccenic | −0.130 | 0.048 |
| Linoleic | 0.232 | 0.519 * |
| γ-Linolenic | 0.023 | −0.121 |
| α-Linolenic | −0.212 | 0.346 |
| Arachidic | −0.024 | −0.379 |
| Gondoic | 0.406 * | −0.070 |
| Eicosadienoic | 0.248 | 0.229 |
| Dihomo-γ-Linolenic | −0.127 | 0.186 |
| Arachidonic | −0.109 | 0.014 |
| Eicosapentaenoic | 0.074 | −0.145 |
| Behenic | 0.304 | −0.052 |
| Adrenic | −0.182 | −0.364 |
| Docosapentaenoic n6 | −0.392 | −0.182 |
| Docosapentaneoic n3 | −0.209 | 0.050 |
| Lignoceric | 0.178 | 0.139 |
| Docosahexaneoic | 0.021 | −0.007 |
| Fatty Acid | No Cachexia R | Cachexia R |
|---|---|---|
| Lauric | −0.533 ** | 0.006 |
| Myristic | −0.010 | 0.010 |
| Palmitic | −0.181 | −0.225 |
| Palmitoleic | 0.049 | −0.276 |
| Stearic | −0.171 | −0.058 |
| Oleic | −0.099 | −0.361 |
| Vaccenic | −0.560 ** | −0.646 ** |
| Linoleic | 0.116 | 0.577 * |
| γ-Linolenic | 0.464 * | −0.432 |
| α-Linolenic | 0.367 | 0.014 |
| Arachidic | −0.184 | −0.293 |
| Gondoic | −0.026 | −0.025 |
| Eicosadienoic | −0.056 | −0.278 |
| Dihomo-γ-Linolenic | 0.098 | −0.217 |
| Arachidonic | 0.167 | −0.018 |
| Eicosapentaenoic | 0.102 | −0.388 |
| Behenic | 0.021 | 0.175 |
| Adrenic | 0.221 | −0.346 |
| Docosapentaenoic n6 | −0.008 | −0.132 |
| Docosapentaneoic n3 | 0.132 | −0.134 |
| Lignoceric | 0.048 | 0.481 |
| Docosahexaneoic | −0.090 | −0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumpper-Fedus, K.; Hart, P.A.; Belury, M.A.; Crowe, O.; Cole, R.M.; Pita Grisanti, V.; Badi, N.; Liva, S.; Hinton, A.; Coss, C.; et al. Altered Plasma Fatty Acid Abundance Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer. Cells 2022, 11, 910. https://doi.org/10.3390/cells11050910
Gumpper-Fedus K, Hart PA, Belury MA, Crowe O, Cole RM, Pita Grisanti V, Badi N, Liva S, Hinton A, Coss C, et al. Altered Plasma Fatty Acid Abundance Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer. Cells. 2022; 11(5):910. https://doi.org/10.3390/cells11050910
Chicago/Turabian StyleGumpper-Fedus, Kristyn, Phil A. Hart, Martha A. Belury, Olivia Crowe, Rachel M. Cole, Valentina Pita Grisanti, Niharika Badi, Sophia Liva, Alice Hinton, Christopher Coss, and et al. 2022. "Altered Plasma Fatty Acid Abundance Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer" Cells 11, no. 5: 910. https://doi.org/10.3390/cells11050910
APA StyleGumpper-Fedus, K., Hart, P. A., Belury, M. A., Crowe, O., Cole, R. M., Pita Grisanti, V., Badi, N., Liva, S., Hinton, A., Coss, C., Ramsey, M. L., Noonan, A., Conwell, D. L., & Cruz-Monserrate, Z. (2022). Altered Plasma Fatty Acid Abundance Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer. Cells, 11(5), 910. https://doi.org/10.3390/cells11050910






