Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer
Abstract
:1. Background
2. Methods
2.1. Study Design and Population
2.2. HLA-Typing
2.3. Viral Assays
2.4. Flow Cytometric Analysis
2.5. Intracellular Cytokine Staining Assays
2.6. Statistical Analysis
3. Results
3.1. Patients Characteristics
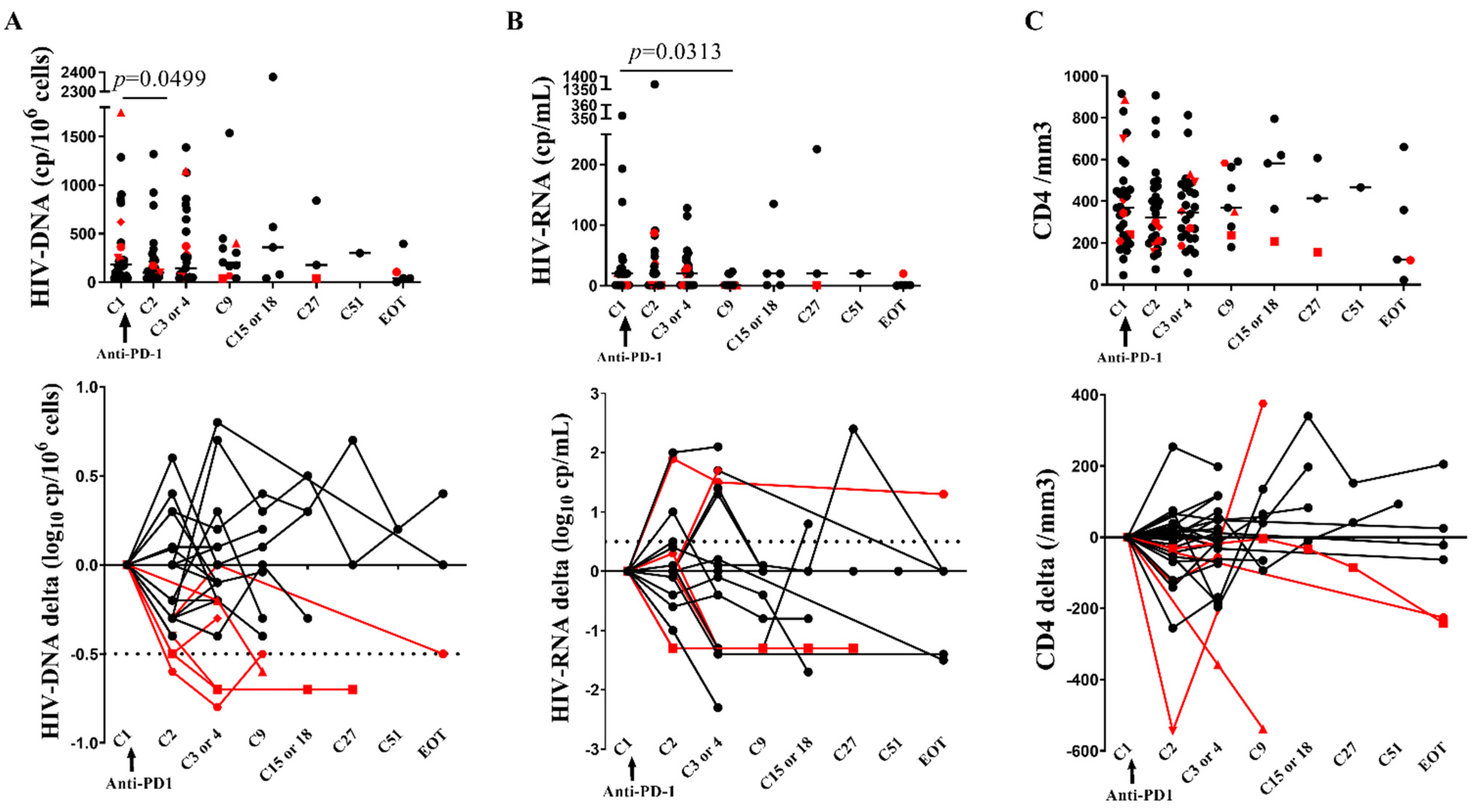
3.2. Early Two-Fold Decrease of CA-HIV-DNA following Anti-PD-1
3.3. CD4 Stability and Early T Cell Activation
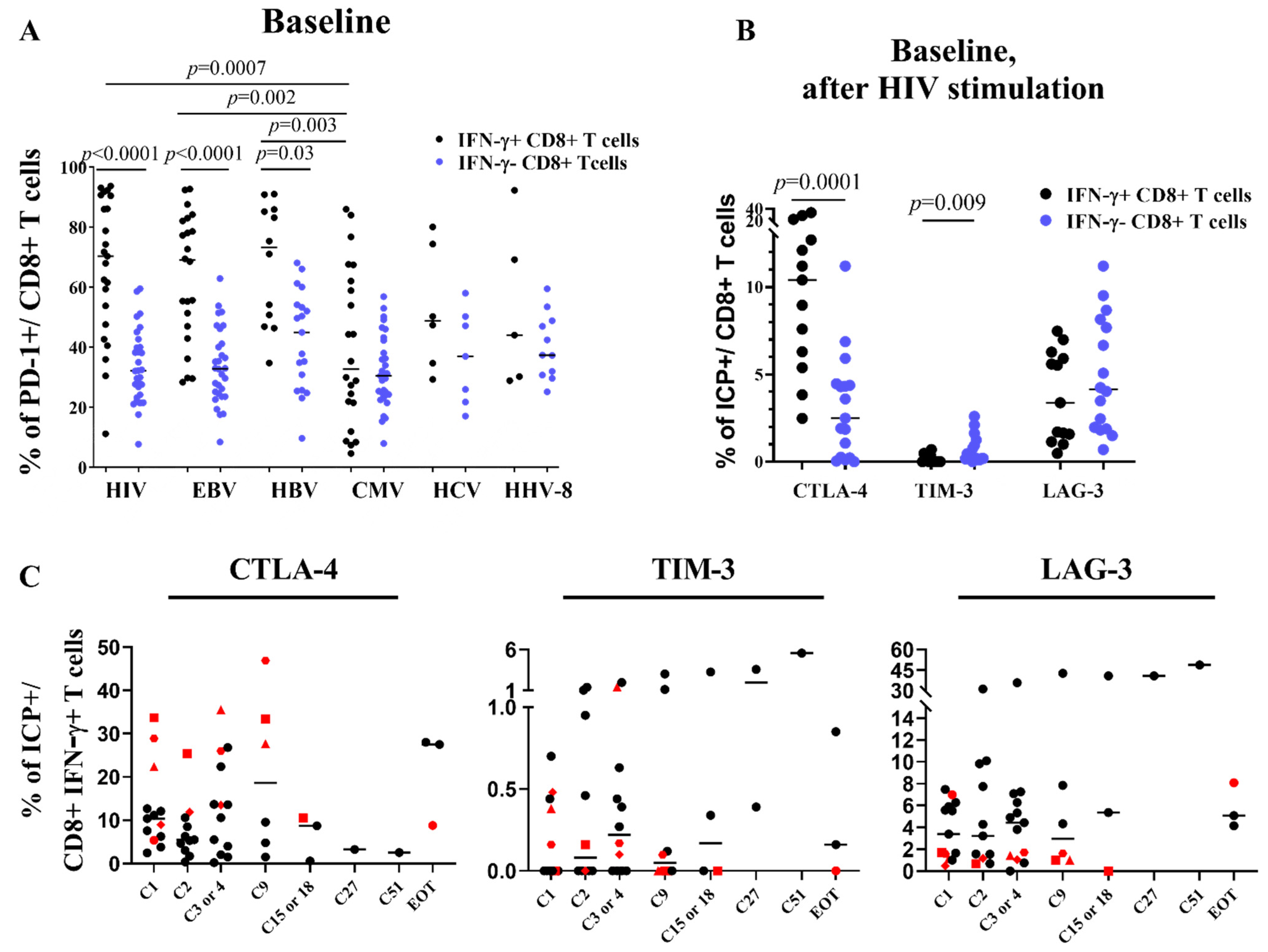
3.4. CTLA-4 Is Upregulated on CD4 Cells and ICP Compensatory Mechanisms Are Less Pronounced in Patients RR
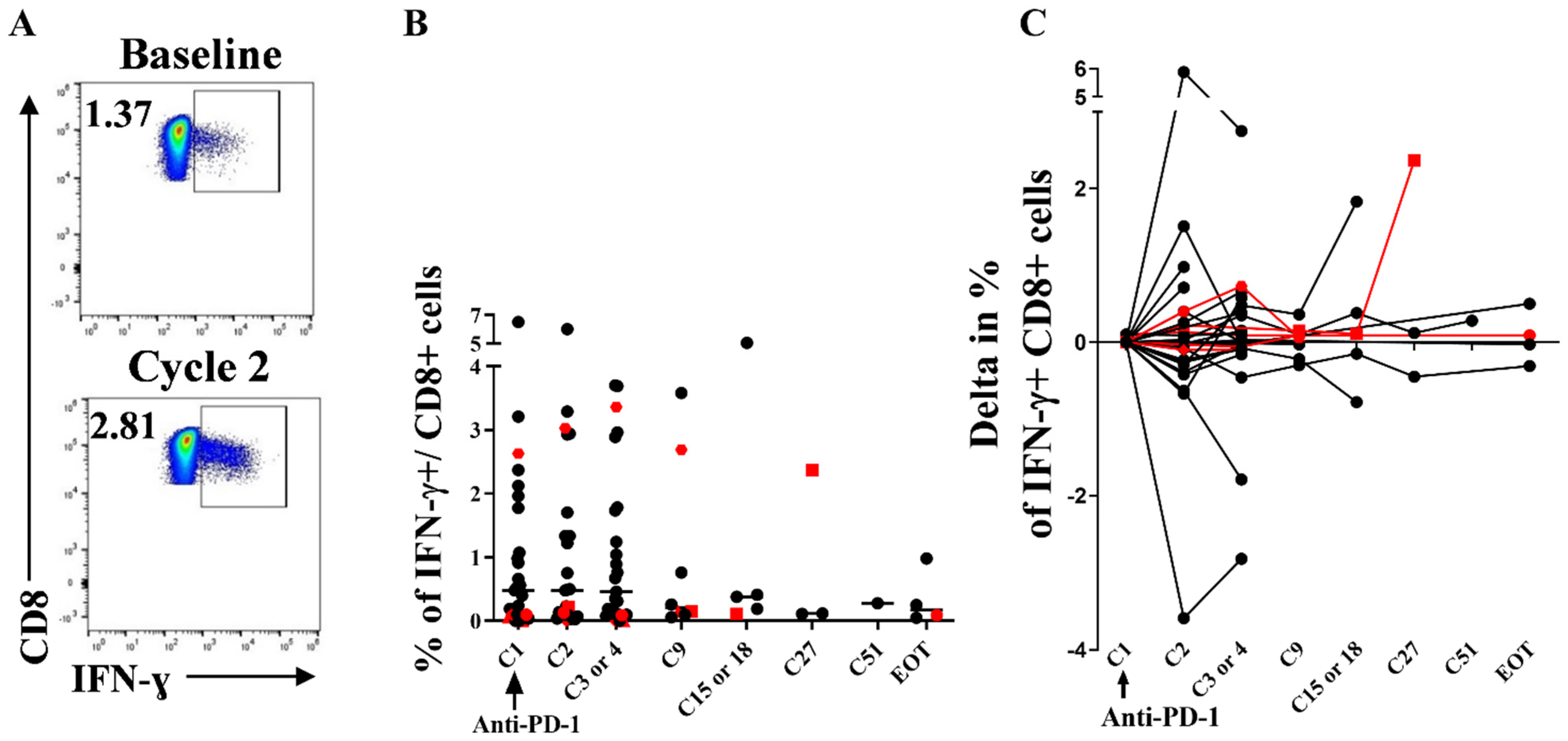
3.5. Stability of Peripheral HIV-Specific T Cells Despite PD-1 Overexpression at Baseline
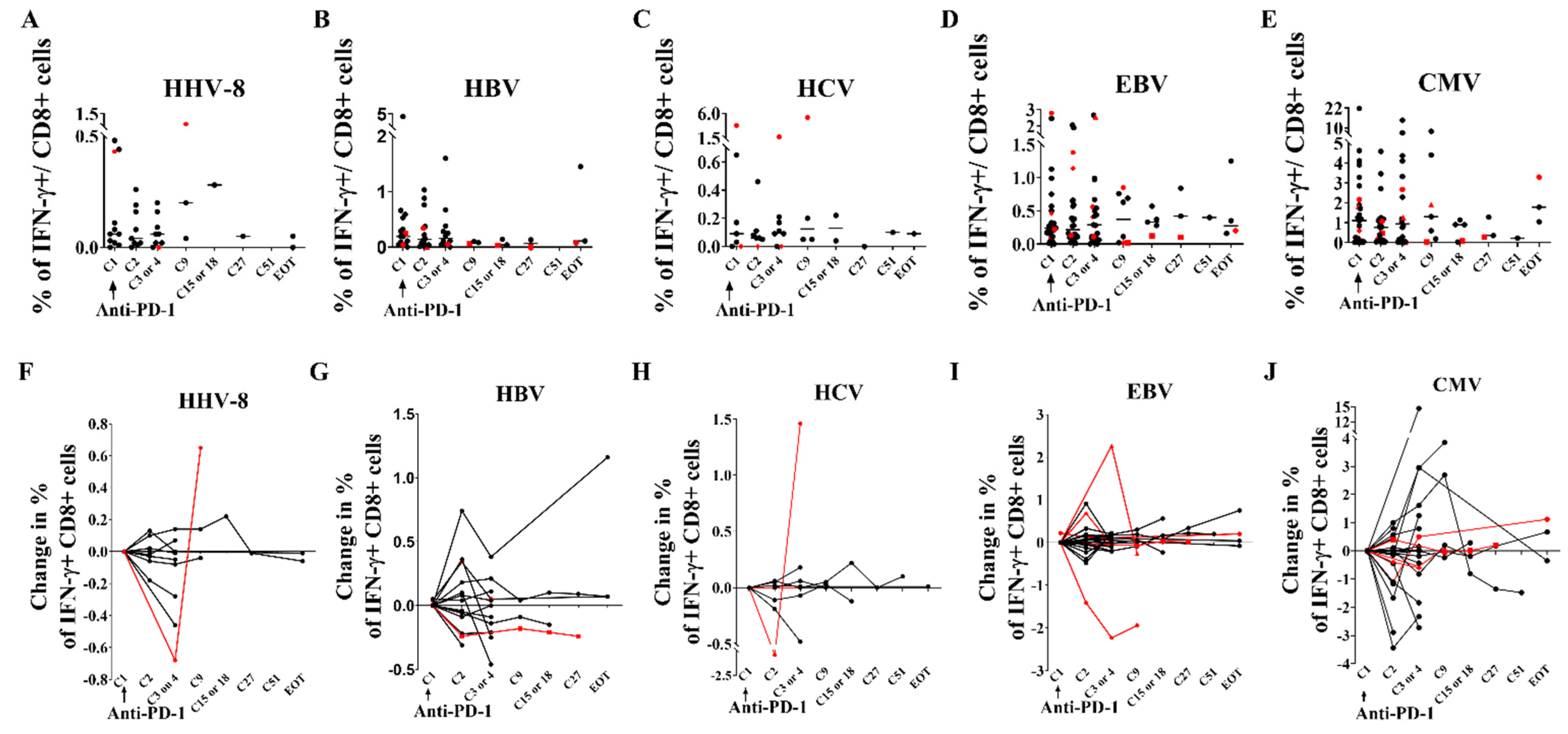
3.6. Immunological and Virological Parameters of Other Viruses Are Not Modified
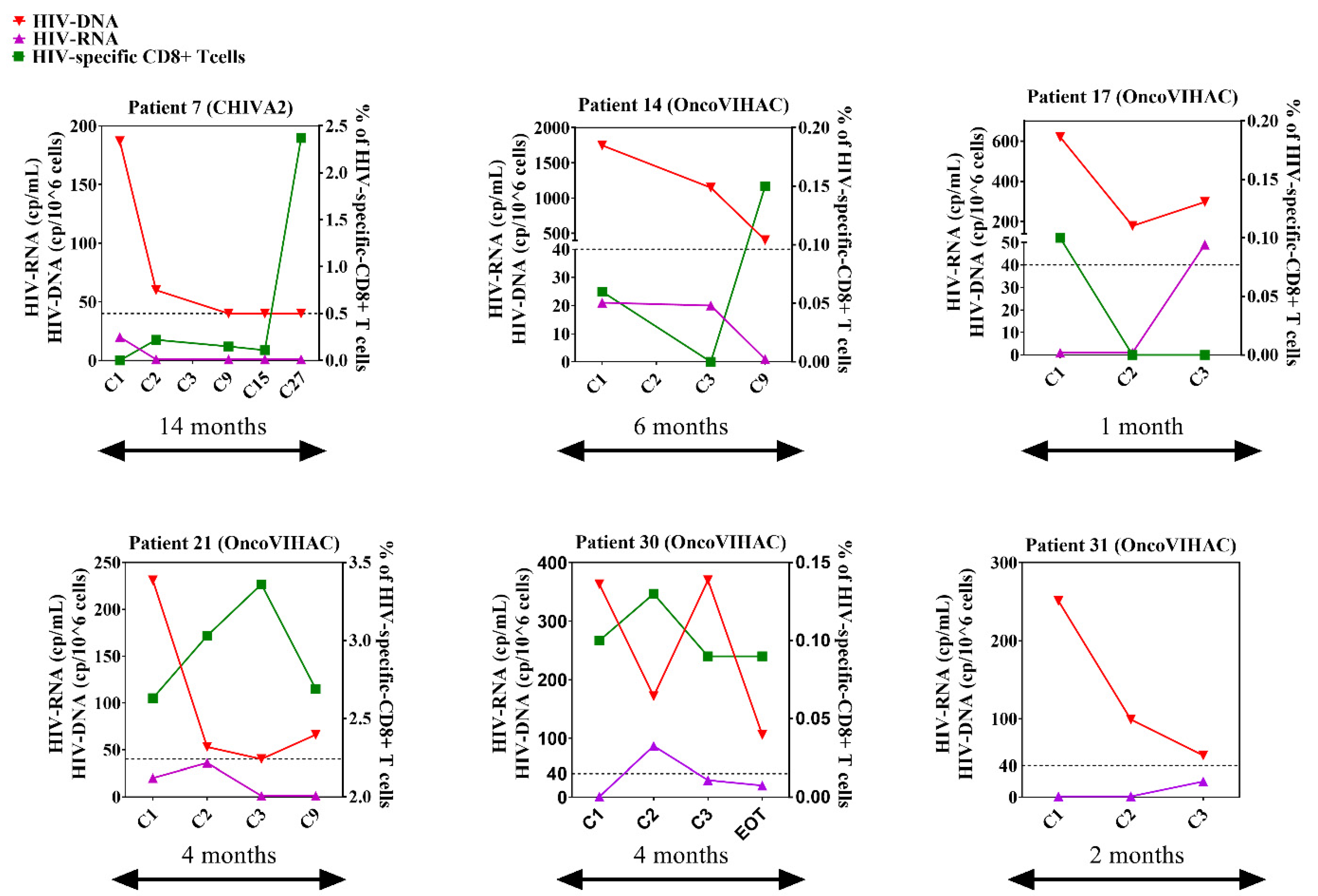
3.7. Immuno-Virological Profiling of RRs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Members of the ANRS: Oncovirim, Study Group
Members of the French Cooperative Thoracic Intergroup (IFCT) CHIVA-2 Investigators
References
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 Blockade Potentiates HIV Latency Reversal Ex Vivo in CD4+ T Cells from ART-Suppressed Individuals. Nat. Commun. 2019, 10, 814. [Google Scholar] [CrossRef]
- Fromentin, R.; Bakeman, W.; Lawani, M.B.; Khoury, G.; Hartogensis, W.; DaFonseca, S.; Killian, M.; Epling, L.; Hoh, R.; Sinclair, E.; et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLOS Pathog. 2016, 12, e1005761. [Google Scholar] [CrossRef] [PubMed]
- Evans, V.A.; van der Sluis, R.M.; Solomon, A.; Dantanarayana, A.; McNeil, C.; Garsia, R.; Palmer, S.; Fromentin, R.; Chomont, N.; Sékaly, R.-P.; et al. Programmed Cell Death-1 Contributes to the Establishment and Maintenance of HIV-1 Latency. AIDS 2018, 32, 1491–1497. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 Expression on HIV-Specific T Cells Is Associated with T-Cell Exhaustion and Disease Progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Fontenot, A.P.; Mack, D.G.; Lozupone, C.; Dillon, S.; Meditz, A.; Wilson, C.C.; Connick, E.; Palmer, B.E. Programmed Death 1 Expression on HIV-Specific CD4+ T Cells Is Driven by Viral Replication and Associated with T Cell Dysfunction. J. Immunol. 2007, 179, 1979–1987. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 Expression on HIV-Specific CD8+ T Cells Leads to Reversible Immune Dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-Specific Immunity in Vivo by PD-1 Blockade. Nature 2009, 458, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.-G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.-P. Immune Checkpoint Inhibitors in People Living with HIV: What about Anti-HIV Effects? AIDS Lond. Engl. 2019, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Guihot, A.; Marcelin, A.-G.; Massiani, M.-A.; Samri, A.; Soulié, C.; Autran, B.; Spano, J.-P. Drastic Decrease of the HIV Reservoir in a Patient Treated with Nivolumab for Lung Cancer. Ann. Oncol. 2018, 29, 517–518. [Google Scholar] [CrossRef]
- Wightman, F.; Solomon, A.; Kumar, S.S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; Mcneil, C.; Garsia, R.; Lewin, S.R. Effect of Ipilimumab on the HIV Reservoir in an HIV-Infected Individual with Metastatic Melanoma. AIDS Lond. Engl. 2015, 29, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Rajdev, L.; Rhodes, A.; Dantanarayana, A.; Tennakoon, S.; Chea, S.; Spelman, T.; Lensing, S.; Rutishauser, R.; Bakkour, S.; et al. Impact of Anti-PD-1 and Anti-CTLA-4 on the HIV Reservoir in People Living with HIV with Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium-095 Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e1973–e1981. [Google Scholar] [CrossRef] [PubMed]
- Macedo, C.; Webber, S.A.; Donnenberg, A.D.; Popescu, I.; Hua, Y.; Green, M.; Rowe, D.; Smith, L.; Brooks, M.M.; Metes, D. EBV-Specific CD8+ T Cells from Asymptomatic Pediatric Thoracic Transplant Patients Carrying Chronic High EBV Loads Display Contrasting Features: Activated Phenotype and Exhausted Function. J. Immunol. Baltim. Md 1950 2011, 186, 5854–5862. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Bord, E.; Broge, T.A.; Glotzbecker, B.; Mills, H.; Gheuens, S.; Rosenblatt, J.; Avigan, D.; Koralnik, I.J. Increased Program Cell Death-1 (PD-1) Expression on T Lymphocytes of Patients with Progressive Multifocal Leukoencephalopathy (PML). J. Acquir. Immune Defic. Syndr. 2012, 60, 244–248. [Google Scholar] [CrossRef]
- Wykes, M.N.; Lewin, S.R. Immune Checkpoint Blockade in Infectious Diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 Blockade with Nivolumab with and without Therapeutic Vaccination for Virally Suppressed Chronic Hepatitis B: A Pilot Study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Gardiner, D.; Lalezari, J.; Lawitz, E.; DiMicco, M.; Ghalib, R.; Reddy, K.R.; Chang, K.-M.; Sulkowski, M.; Marro, S.O.; Anderson, J.; et al. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection. PLoS ONE 2013, 8, e63818. [Google Scholar] [CrossRef]
- Roos-Weil, D.; Weiss, N.; Guihot, A.; Uzunov, M.; Bellanger, A.; Eymard, B.; Saadoun, D.; Houillier, C.; Idbaih, A.; Demeret, S.; et al. Immune Checkpoint Inhibitors for Progressive Multifocal Leukoencephalopathy: A New Gold Standard? J. Neurol. 2021, 268, 2458–2465. [Google Scholar] [CrossRef]
- Chen, J.; Del Valle, L.; Lin, H.-Y.; Plaisance-Bonstaff, K.; Forrest, J.C.; Post, S.R.; Qin, Z. Expression of PD-1 and PD-Ls in Kaposi’s Sarcoma and Regulation by Oncogenic Herpesvirus Lytic Reactivation. Virology 2019, 536, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Bizot, A.; Battistella, M.; Madelaine, I.; Vercellino, L.; Lebbé, C. PD-1 Blockade with Nivolumab in Endemic Kaposi Sarcoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.R.; Schulz, T.F.; Whitby, D.; Cook, P.M.; Boshoff, C.; Rainbow, L.; Howard, M.R.; Gao, S.J.; Bohenzky, R.A.; Simmonds, P.; et al. Prevalence of Kaposi’s Sarcoma Associated Herpesvirus Infection Measured by Antibodies to Recombinant Capsid Protein and Latent Immunofluorescence Antigen. Lancet Lond. Engl. 1996, 348, 1133–1138. [Google Scholar] [CrossRef]
- Lallemand, F.; Desire, N.; Rozenbaum, W.; Nicolas, J.C.; Marechal, V. Quantitative Analysis of Human Herpesvirus 8 Viral Load Using a Real-Time PCR Assay. J. Clin. Microbiol. 2000, 38, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Avettand-Fènoël, V.; Chaix, M.-L.; Blanche, S.; Burgard, M.; Floch, C.; Toure, K.; Allemon, M.-C.; Warszawski, J.; Rouzioux, C.; French Pediatric Cohort Study ANRS-CO 01 Group. LTR Real-Time PCR for HIV-1 DNA Quantitation in Blood Cells for Early Diagnosis in Infants Born to Seropositive Mothers Treated in HAART Area (ANRS CO 01). J. Med. Virol. 2009, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Nakid-Cordero, C.; Arzouk, N.; Gauthier, N.; Tarantino, N.; Larsen, M.; Choquet, S.; Burrel, S.; Autran, B.; Vieillard, V.; Guihot, A. Skewed T Cell Responses to Epstein-Barr Virus in Long-Term Asymptomatic Kidney Transplant Recipients. PLoS ONE 2019, 14, e0224211. [Google Scholar] [CrossRef]
- Guihot, A.; Oksenhendler, E.; Galicier, L.; Marcelin, A.-G.; Papagno, L.; Bedin, A.-S.; Agbalika, F.; Dupin, N.; Cadranel, J.; Autran, B.; et al. Multicentric Castleman Disease Is Associated with Polyfunctional Effector Memory HHV-8-Specific CD8+ T Cells. Blood 2008, 111, 1387–1395. [Google Scholar] [CrossRef]
- Achenbach, C.J.; Assoumou, L.; Deeks, S.G.; Wilkin, T.J.; Berzins, B.; Casazza, J.P.; Lambert-Niclot, S.; Koup, R.A.; Costagliola, D.; Calvez, V.; et al. Effect of Therapeutic Intensification Followed by HIV DNA Prime and RAd5 Boost Vaccination on HIV-Specific Immunity and HIV Reservoir (EraMune 02): A Multicentre Randomised Clinical Trial. Lancet HIV 2015, 2, e82–e91. [Google Scholar] [CrossRef]
- Calin, R.; Hamimi, C.; Lambert-Niclot, S.; Carcelain, G.; Bellet, J.; Assoumou, L.; Tubiana, R.; Calvez, V.; Dudoit, Y.; Costagliola, D.; et al. Treatment Interruption in Chronically HIV-Infected Patients with an Ultralow HIV Reservoir. AIDS Lond. Engl. 2016, 30, 761–769. [Google Scholar] [CrossRef]
- Michot, J.-M.; Mouraud, S.; Adam, J.; Lazarovici, J.; Bigenwald, C.; Rigaud, C.; Tselikas, L.; Dartigues, P.; Danu, A.; Bigorgne, A.; et al. CD8+ T Lymphocytes Immune Depletion and LAG-3 Overexpression in Hodgkin Lymphoma Tumor Microenvironment Exposed to Anti-PD-1 Immunotherapy. Cancers 2021, 13, 5487. [Google Scholar] [CrossRef] [PubMed]
- McGary, C.S.; Deleage, C.; Harper, J.; Micci, L.; Ribeiro, S.P.; Paganini, S.; Kuri-Cervantes, L.; Benne, C.; Ryan, E.S.; Balderas, R.; et al. CTLA-4+PD-1− Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 2017, 47, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, R.M.; Kumar, N.A.; Pascoe, R.D.; Zerbato, J.M.; Evans, V.A.; Dantanarayana, A.I.; Anderson, J.L.; Sékaly, R.P.; Fromentin, R.; Chomont, N.; et al. Combination Immune Checkpoint Blockade to Reverse HIV Latency. J. Immunol. 2020, 204, ji1901191. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Toor, S.M.; Khalaf, S.; Elkord, E. Breast Cancer Cells and PD-1/PD-L1 Blockade Upregulate the Expression of PD-1, CTLA-4, TIM-3 and LAG-3 Immune Checkpoints in CD4+ T Cells. Vaccines 2019, 7, 149. [Google Scholar] [CrossRef]
- Osa, A.; Uenami, T.; Koyama, S.; Fujimoto, K.; Okuzaki, D.; Takimoto, T.; Hirata, H.; Yano, Y.; Yokota, S.; Kinehara, Y.; et al. Clinical Implications of Monitoring Nivolumab Immunokinetics in Non–Small Cell Lung Cancer Patients. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Samri, A.; Durier, C.; Urrutia, A.; Sanchez, I.; Gahery-Segard, H.; Imbart, S.; Sinet, M.; Tartour, E.; Aboulker, J.P.; Autran, B.; et al. Evaluation of the interlaboratory concordance in quantification of human immunodeficiency virus-specific T cells with a gamma interferon enzyme-linked immunospot assay. Clin. Vaccine. Immunol. CVI 2006, 13, 684–697. [Google Scholar] [CrossRef]
- Sacre, K.; Carcelain, G.; Cassoux, N.; Fillet, A.-M.; Costagliola, D.; Vittecoq, D.; Salmon, D.; Amoura, Z.; Katlama, C.; Autran, B. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J. Exp. Med. 2005, 201, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; Bertoletti, A. Quantity and quality of virus-specific CD8 cell response: Relevance to the design of a therapeutic vaccine for chronic HBV infection. Mol. Immunol. 2001, 38, 467–473. [Google Scholar] [CrossRef]
- Webster, G.J.; Reignat, S.; Brown, D.; Ogg, G.S.; Jones, L.; Seneviratne, S.L.; Williams, R.; Dusheiko, G.; Bertoletti, A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: Implications for immunotherapy. J. Virol. 2004, 78, 5707–5719. [Google Scholar] [CrossRef]
- Depla, E.; Van der Aa, A.; Livingston, B.D.; Crimi, C.; Allosery, K.; De Brabandere, V.; Krakover, J.; Murthy, S.; Huang, M.; Power, S.; et al. Rational Design of a Multiepitope Vaccine Encoding T-Lymphocyte Epitopes for Treatment of Chronic Hepatitis B Virus Infections. J. Virol. 2008, 82, 435–450. [Google Scholar] [CrossRef]
- Malmassari, S.; Lone, Y.C.; Zhang, M.; Transy, C.; Michel, M.L. In vivo hierarchy of immunodominant and subdominant HLA-A*0201-restricted T-cell epitopes of HBx antigen of hepatitis B virus. Microbes Infect. 2005, 7, 626–634. [Google Scholar] [CrossRef]
- Tsai, S.L.; Lee, T.H.; Chien, R.N.; Liao, S.K.; Lin, C.L.; Kuo, G.C.; Liaw, Y.F. A method to increase tetramer staining efficiency of CD8+ T cells with MHC–peptide complexes: Therapeutic applications in monitoring cytotoxic T lymphocyte activity during hepatitis B and C treatment. J. Immunol. Methods 2004, 285, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Schnuriger, A.; Dominguez, S.; Guiguet, M.; Harfouch, S.; Samri, A.; Ouazene, Z.; Slama, L.; Simon, A.; Valantin, M.A.; Thibault, V.; et al. Acute hepatitis C in HIV-infected patients: Rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS 2009, 23, 2079–2089. [Google Scholar] [CrossRef] [PubMed]


| Pt | Cohort | Age | Sex | Type of Cancers | Pv Line | ICB Type | ART | CD4 Count (/mm3) | CD4/CD8 Ratio | HIV VL (cp/mL) | HIV-DNA(cp/10 cells) | HLA Typing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CHIVA-2 | 71 | M | NSCLC | 2 | Nivo | Rilpivirine, Dolutegravir | 183 | 0.5 | 28 | <40 | A*01:03 B*37:49 |
| 2 | CHIVA-2 | 68 | M | NSCLC | 2 | Nivo | Abacavir, Nevirapine | NA | NA | <1 | 227 | A*03:11 B*07:27 |
| 3 | CHIVA-2 | 56 | M | NSCLC | 1 | Nivo | Emtricitabine tenofovir disoproxil, Maraviroc | 831 | 0.4 | 193 | 818 | A*24:29 B*38:44 |
| 4 | CHIVA-2 | 59 | M | NSCLC | 1 | Nivo | Emtricitabine, Rilpivirine, Tenofovir alafenamide | 596 | 0.9 | <1 | <40 | A*02:29 B*49:58 |
| 5 | CHIVA-2 | 65 | M | NSCLC | 2 | Nivo | Tenofovir, Emtricitabine, Bictegravir | 451 | 0.9 * | 352 | 904 | A*02:29 B*07:15 |
| 6 | CHIVA-2 | 55 | F | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 499 | 0.6 | 138 | 1287 | A*29:30 B*37:44 |
| 7 | CHIVA-2 | 68 | M | NSCLC | 1 | Nivo | Abacavir, Efavirenz | 241 | 0.7 | <20 | 187 | A*02:11 B*39:40 |
| 8 | CHIVA-2 | 53 | F | NSCLC | 1 | Nivo | Efavirenz, Emtricitabine, Tenofovir | 291 | 0.4 | <20 | 66 | A*02:66 B*49:52 |
| 9 | CHIVA-2 | 58 | M | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 249 | 0.2 | <20 | 851 | A*01:03 B*08:51 |
| 10 | CHIVA-2 | 59 | M | NSCLC | 1 | Nivo | Lamivudine, Dolutegravir, Abacavir | 583 | 0.4 | <1 | 231 | A*02:03 B*18 27 |
| 11 | Onco VIHAC | 62 | M | Melanoma | 0 | Nivo | Elvitegravir, Emtricitabine, Tenofovir | 455 | 0.8 | <1 | 166 | A*02:26 B* 07:08 |
| 12 | Onco VIHAC | 69 | M | NSCLC | 0 | Pembro | Emtricitabine, Rilpivirine, Tenofovir | 273 | 0.4 | <1 | <40 | A*02:24 B*35:57 |
| 13 | Onco VIHAC | 75 | M | NSCLC | 1 | Nivo | Lamivudine, Dolutegravir | 217 | 1 | 42 | 218 | A*25:31 B*40:51 |
| 14 | Onco VIHAC | 60 | F | NSCLC | 0 | Pembro | Darunavir, Norvir, Raltegravir | 888 | 2.1 | 21 | 1749 | A*31:68 B*07:07 |
| 15 | Onco VIHAC | 63 | M | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 238 | 1.8 | <1 | <40 | A*03:23 B*44:53 |
| 16 | Onco VIHAC | 53 | M | HL | 3 | Nivo | Dolutegravir, Lamivudine | 373 | 0.3 | <20 | 173 | A*33:68 B*14:44 |
| 17 | Onco VIHAC | 53 | M | NSCLC | 2 | Nivo | Abacavir, Lamivudine | 405 | 0.6 | <1 | 620 | A*02:29 B*40:49 |
| 18 | Onco VIHAC | 64 | M | Bladder | 2 | Pembro | Abacavir, Lamivudine, Nivérapine | 449 | 1.1 | <1 | 213 | A*02:31 B*07:40 |
| 19 | Onco VIHAC | 62 | M | Oropharynx | 2 | Nivo | Darunavir, Ritonavir | 162 | 0.5 | <20 | 80 | A*02:11 B*15:40 |
| 20 | Onco VIHAC | 58 | M | Kaposi Sarcoma | 4 | Nivo | Dolutegravir, Abacavir, Lamivudine | 728 | 2.1 | 47 | 409 | A*33:68 B*14:44 |
| 21 | Onco VIHAC | 62 | M | Anal | 2 | Nivo | Dolutegravir, Lamivudine | 209 | 1.2 | <20 | 231 | A*02:24 B*35:44 |
| 22 | Onco VIHAC | 52 | M | Head and neck | 1 | Nivo | Darunavir, Norvir, Raltegravir | 369 | 0.8 | <20 | 191 | A*30:33 B*07:15 |
| 23 | Onco VIHAC | 71 | F | Head and neck | 1 | Nivo | Dolutegravir | 333 | 1.1 | <1 | <40 | A*02:24 B*44:50 |
| 24 | Onco VIHAC | 63 | M | Eye | 2 | Cemi | Bictegravir, Emtricitabne, Tenofovir | 45 | 0.2 | 29 | <40 | A*02:33 B*14:53 |
| 25 | Onco VIHAC | 70 | M | Melanoma | 0 | Pembro | Emtricitabine, Tenofovir, Névirapine | 434 | 0.5 | <1 | 181 | A*01:03 B*07:51 |
| 26 | Onco VIHAC | 56 | M | NSCLC | 2 | Pembro | Emtricitabine, Tenofovir, Darunavir, Ritonavir | 424 | 0.8 | <20 | 166 | A*11:11 B*15:27 |
| 27 | Onco VIHAC | 62 | M | Bladder | 1 | Pembro | Efavirenz, Emtricitabine, Tenofovir | 915 | 1,4 | <20 | 99 | A*24:24 B*44:44 |
| 28 | Onco VIHAC | 58 | M | NSCLC | 0 | Pembro | Darunavir, Doletugravir, Ritonavir, Tenofovir | 192 | 0,4 | <1 | 73 | A*02:32 B*44:51 |
| 29 | Onco VIHAC | 62 | M | NSCLC | 1 | Nivo | Raltegravir, Emtricitabine, Tenofovir | 534 | 0.3 | <1 | 99 | NA |
| 30 | Onco VIHAC | 60 | M | Bladder | 2 | Pembro | Bictegravir, Emtricitabne, Tenofovir | 969 | 0.6 | <1 | 363 | NA |
| 31 | Onco VIHAC | 59 | M | NSCLC | 1 | Nivo | Darunavir, Ritonavir | 699 | 1 | <1 | 251 | NA |
| 32 | Onco VIHAC | 60 | M | NSCLC | 0 | Pembro | Bictegravir, Emtricitabne, Tenofovir | 169 | 0.4 | <1 | 59 | NA |
| all | OncoVIHAC 69% | 61 | M 88% | NSCLC 63% Bladder 9% | 1 | Nivo 69% Pembro 28% | 369 | 1 | 20 | 184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baron, M.; Soulié, C.; Lavolé, A.; Assoumou, L.; Abbar, B.; Fouquet, B.; Rousseau, A.; Veyri, M.; Samri, A.; Makinson, A.; et al. Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer. Cells 2022, 11, 1015. https://doi.org/10.3390/cells11061015
Baron M, Soulié C, Lavolé A, Assoumou L, Abbar B, Fouquet B, Rousseau A, Veyri M, Samri A, Makinson A, et al. Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer. Cells. 2022; 11(6):1015. https://doi.org/10.3390/cells11061015
Chicago/Turabian StyleBaron, Marine, Cathia Soulié, Armelle Lavolé, Lambert Assoumou, Baptiste Abbar, Baptiste Fouquet, Alice Rousseau, Marianne Veyri, Assia Samri, Alain Makinson, and et al. 2022. "Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer" Cells 11, no. 6: 1015. https://doi.org/10.3390/cells11061015






