A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Processing and Cell Isolation
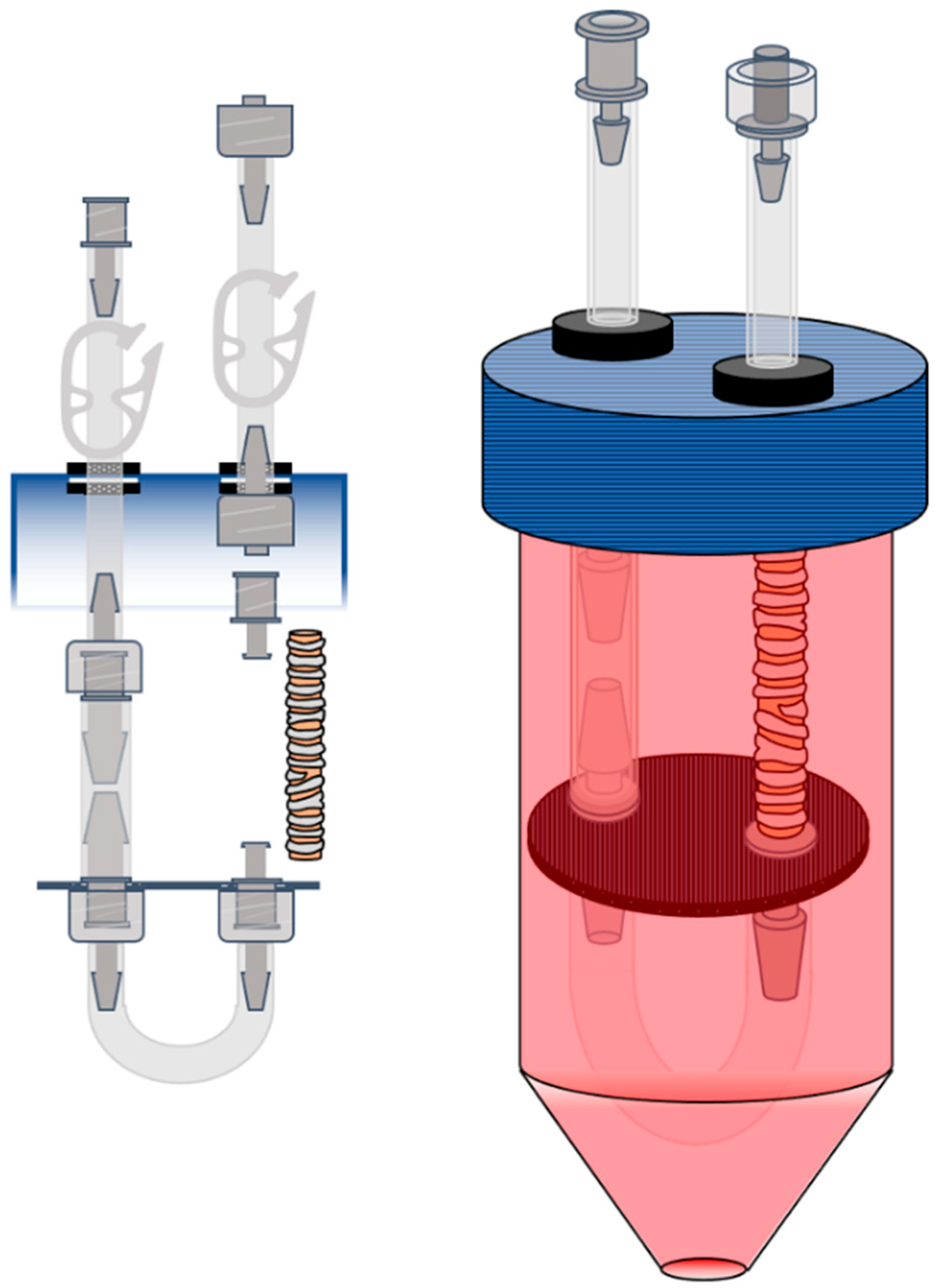
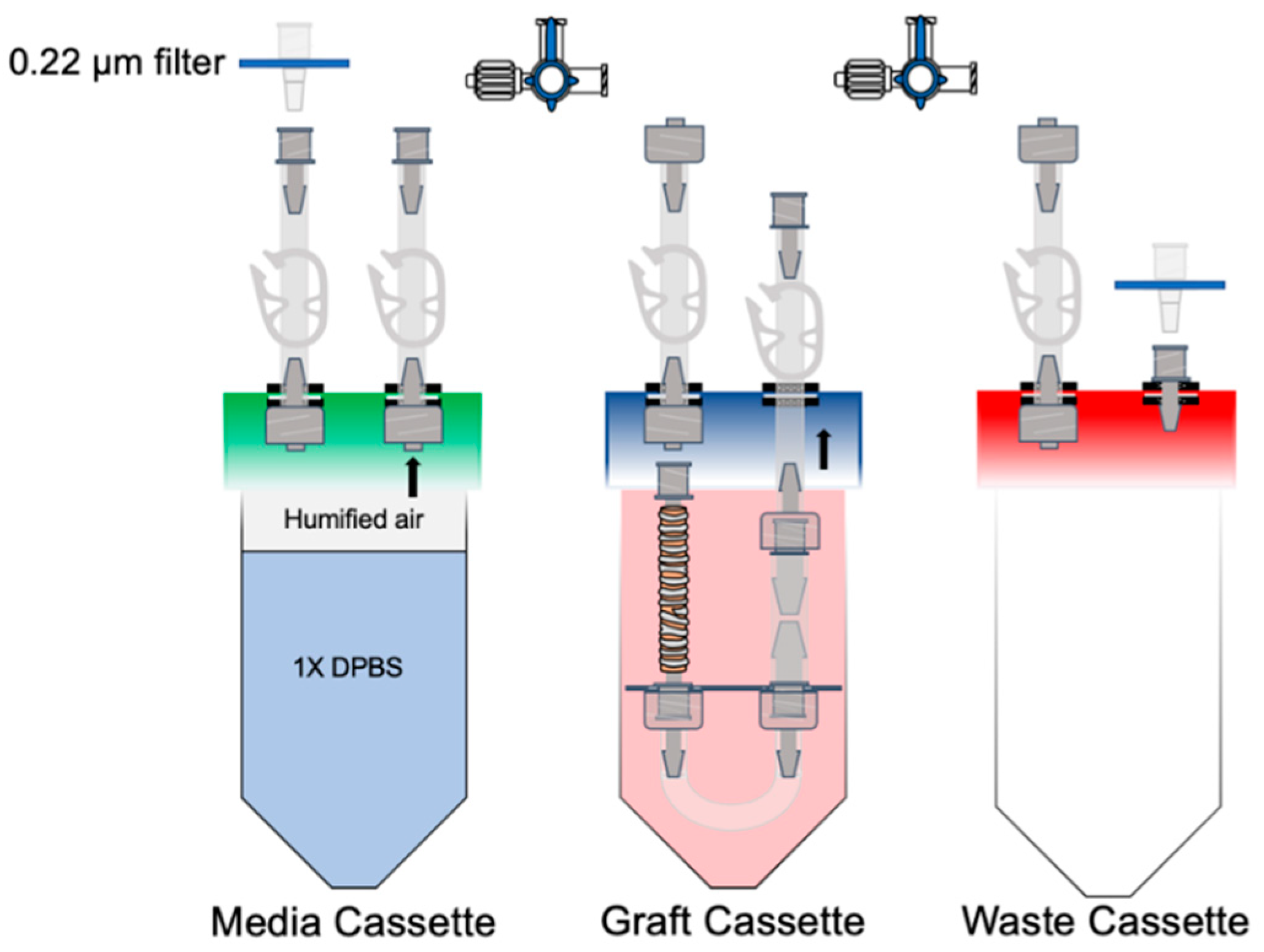
2.3. Bioreactor Design
2.4. Decellularization Protocol
2.5. Recellularization Protocol
2.6. Establishing an Air–Liquid Interface within the Bioreactor
2.7. Live Viability Assay
2.8. Compliance Testing
2.9. DNA Quantification
2.10. Immunofluorescence and Histology
3. Results
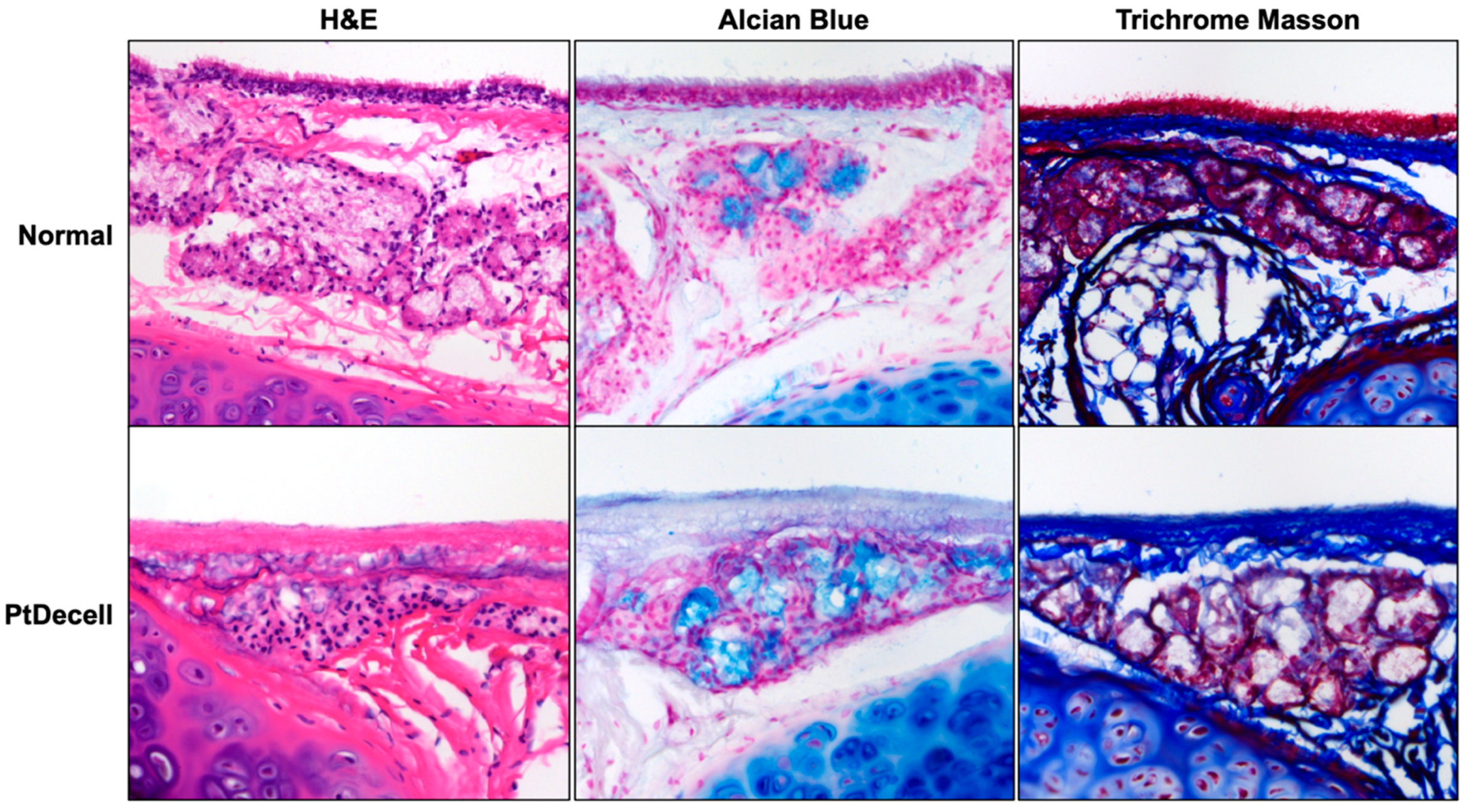
3.1. Tracheal De-Epithelialization and Assessment of Cellular Presence
3.2. Mechanical Analysis
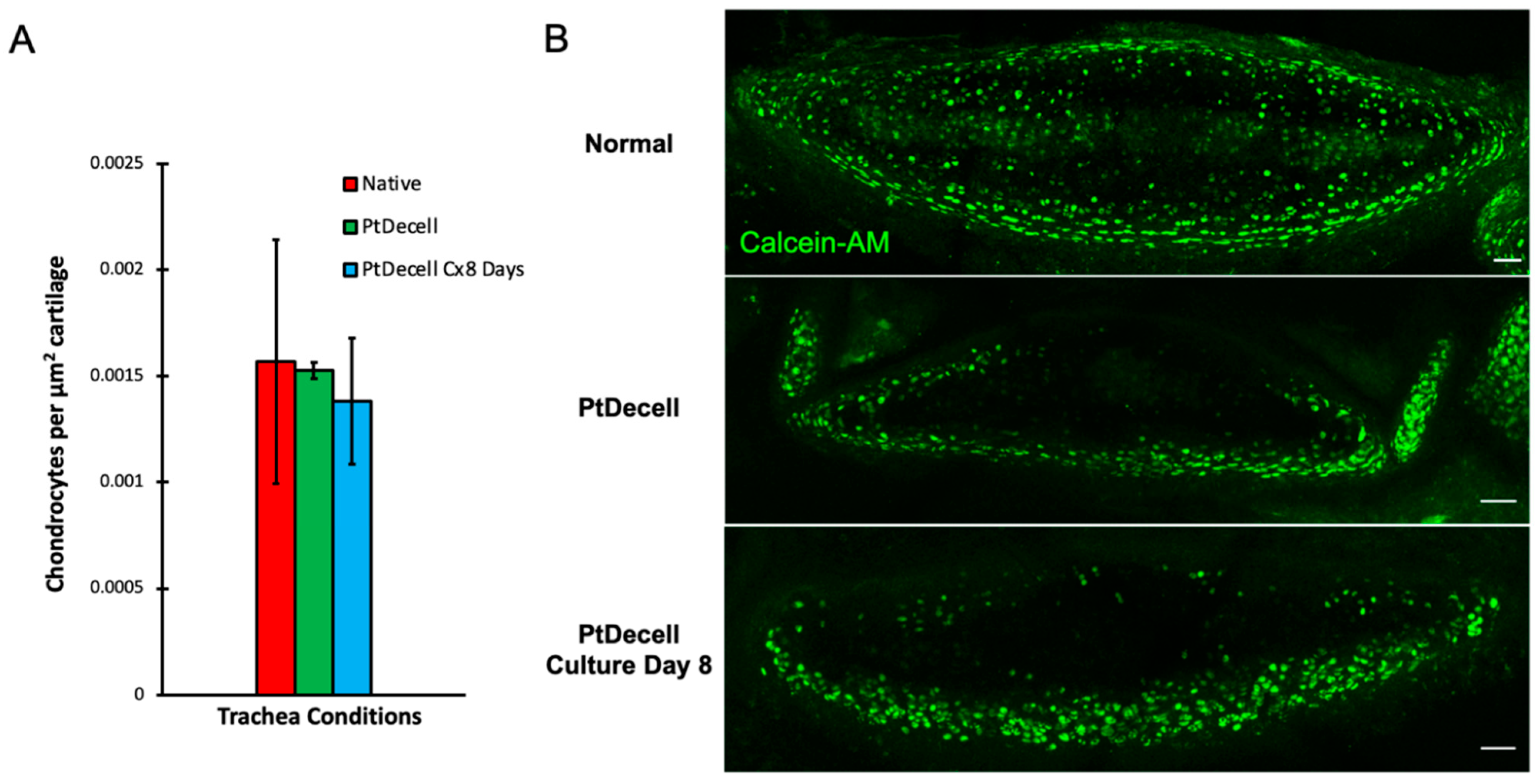
3.3. Chondrocyte Viability
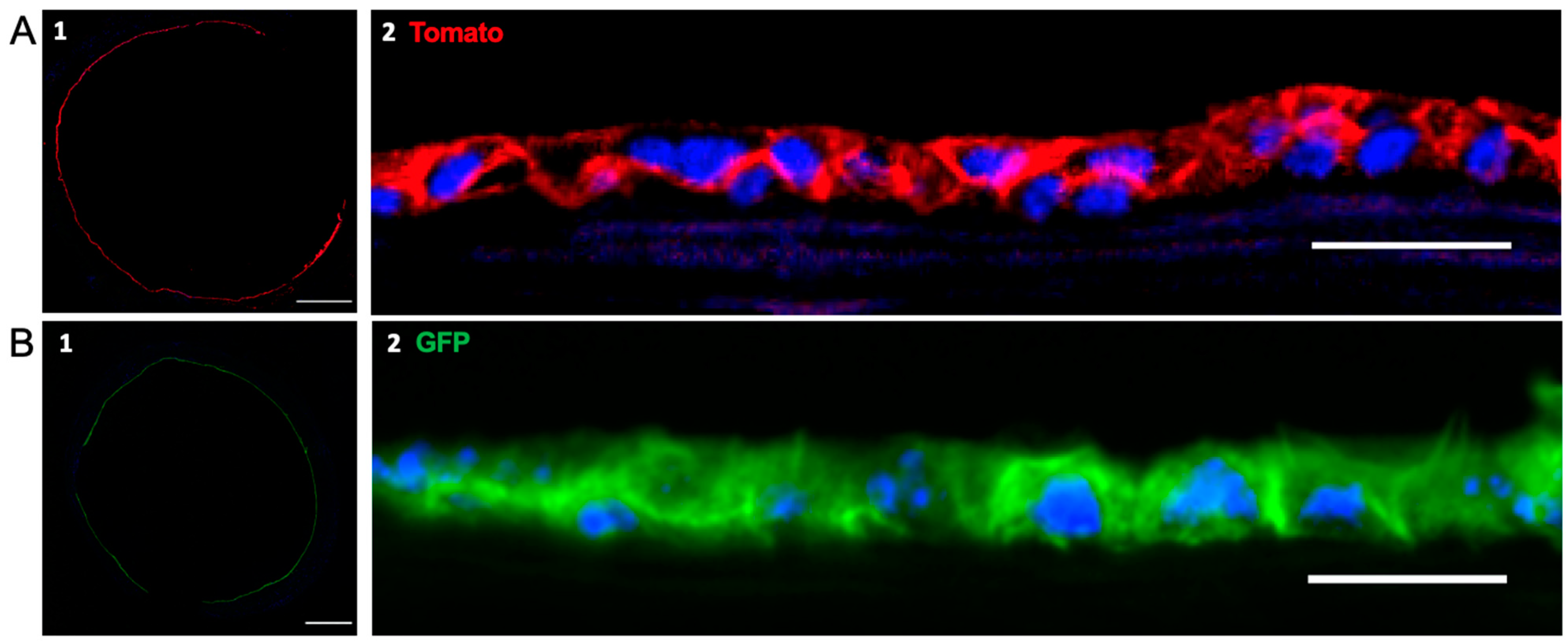
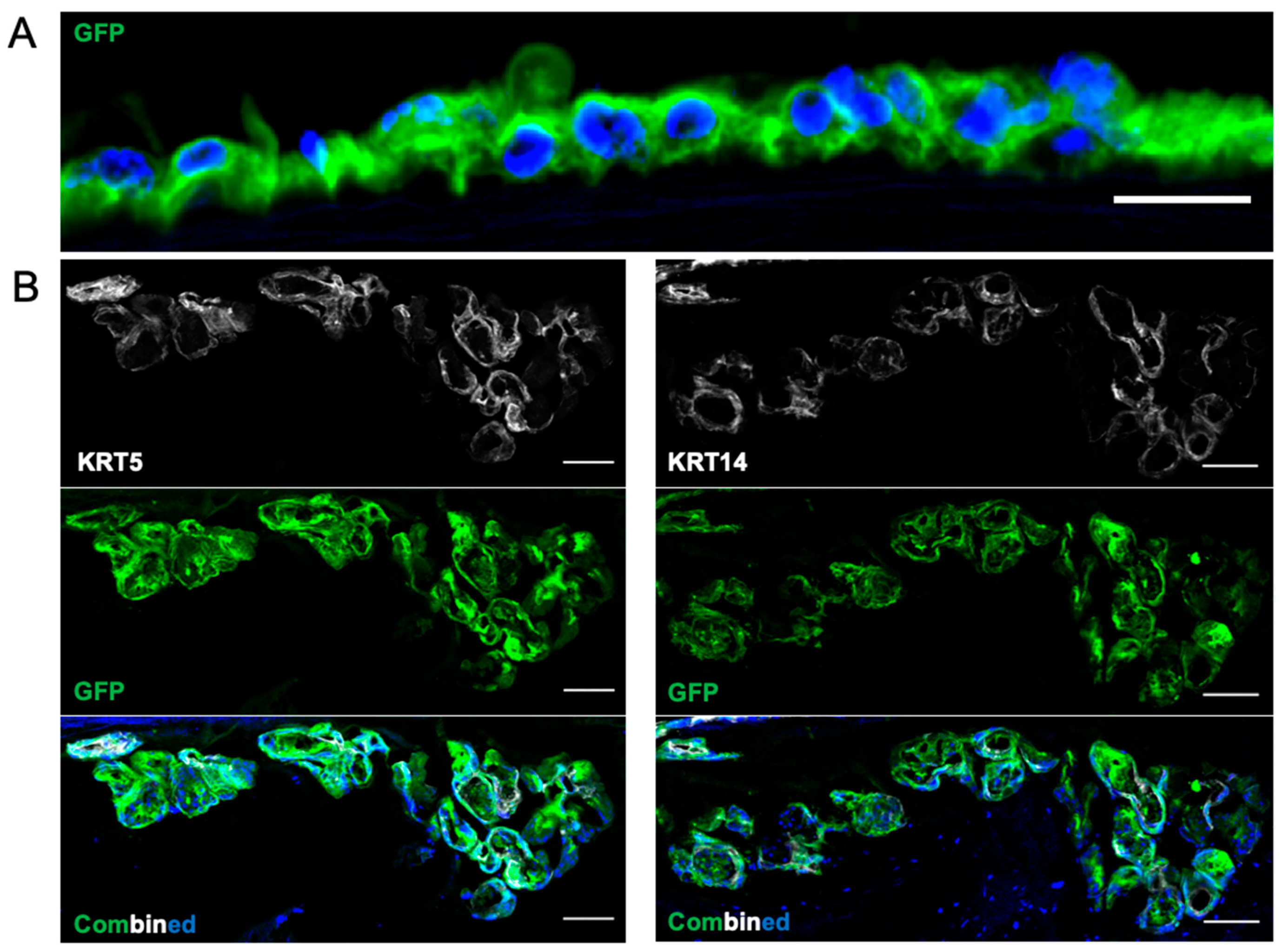
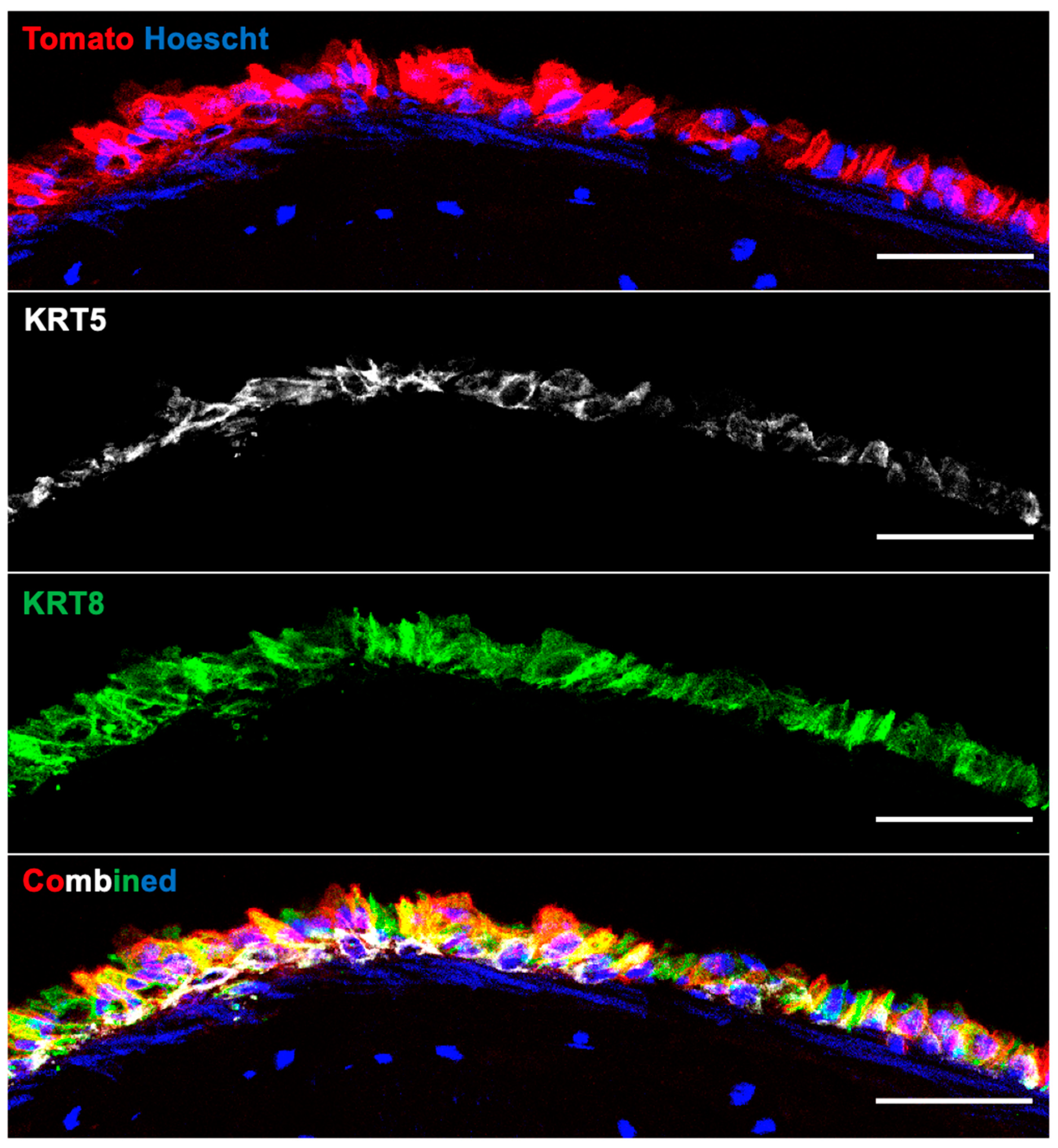
3.4. Recellularization of Decellularized Grafts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etienne, H.; Fabre, D.; Gomez Caro, A.; Kolb, F.; Mussot, S.; Mercier, O.; Mitilian, D.; Stephan, F.; Fadel, E.; Dartevelle, P. Tracheal replacement. Eur. Respir. J. 2018, 51, 1702211. [Google Scholar] [CrossRef] [PubMed]
- Delaere, P.; Van Raemdonck, D. Tracheal replacement. J. Thorac. Dis. 2016, 8 (Suppl. 2), S186–S196. [Google Scholar] [CrossRef] [PubMed]
- Moser, P.T.; Ott, H.C. Recellularization of organs: What is the future for solid organ transplantation? Curr. Opin. Organ. Transplant. 2014, 19, 603–609. [Google Scholar] [CrossRef]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Kutten, J.C.; McGovern, D.; Hobson, C.M.; Luffy, S.A.; Nieponice, A.; Tobita, K.; Francis, R.J.; Reynolds, S.D.; Isenberg, J.S.; Gilbert, T.W. Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function. Tissue Eng. Part A 2015, 21, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roomans, G.M. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur. Cell Mater. 2010, 19, 284–299. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Hintze, J.M.; Hayden, R.E.; Lott, D.G. Tracheal decellularization using a combination of chemical, physical and bioreactor methods. Int. J. Artif. Organs 2017, 41, 100–107. [Google Scholar] [CrossRef]
- Hong, P.; Bezuhly, M.; Graham, M.E.; Gratzer, P.F. Efficient decellularization of rabbit trachea to generate a tissue engineering scaffold biomatrix. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 67–74. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Gong, D.; Xia, C.; Xu, Z. Comparison of detergent-based decellularization protocols for the removal of antigenic cellular components in porcine aortic valve. Xenotransplantation 2018, 25, e12380. [Google Scholar] [CrossRef]
- Vavken, P.; Joshi, S.; Murray, M.M. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J. Orthop. Res. 2009, 27, 1612–1618. [Google Scholar] [CrossRef] [Green Version]
- Wallis, J.M.; Borg, Z.D.; Daly, A.B.; Deng, B.; Ballif, B.A.; Allen, G.B.; Jaworski, D.M.; Weiss, D.J. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. Part C Methods 2012, 18, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrini, J.L.; Gard, A.L.; Liu, A.; Leiby, K.L.; Schwan, J.; Kunkemoeller, B.; Calle, E.A.; Sivarapatna, A.; Lin, T.; Dimitrievska, S.; et al. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr. Biol. 2015, 7, 1598–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Batioglu-Karaaltin, A.; Karaaltin, M.V.; Ovali, E.; Yigit, O.; Kongur, M.; Inan, O.; Bozkurt, E.; Cansiz, H. In vivo tissue-engineered allogenic trachea transplantation in rabbits: A preliminary report. Stem Cell Rev. Rep. 2015, 11, 347–356. [Google Scholar] [CrossRef]
- Liu, Y.; Nakamura, T.; Sekine, T.; Matsumoto, K.; Ueda, H.; Yoshitani, M.; Toba, T.; Shimizu, Y. New type of tracheal bioartificial organ treated with detergent: Maintaining cartilage viability is necessary for successful immunosuppressant free allotransplantation. ASAIO J. 2002, 48, 21–25. [Google Scholar] [CrossRef]
- Aoki, F.G.; Varma, R.; Marin-Araujo, A.E.; Lee, H.; Soleas, J.P.; Li, A.H.; Soon, K.; Romero, D.; Moriya, H.T.; Haykal, S.; et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci Rep. 2019, 9, 12034. [Google Scholar] [CrossRef]
- Delaere, P.; Vranckx, J.; Verleden, G.; De Leyn, P.; Van Raemdonck, D.; Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N. Engl. J. Med. 2010, 362, 138–145. [Google Scholar] [CrossRef]
- Kuo, E.; Bharat, A.; Shih, J.; Street, T.; Norris, J.; Liu, W.; Parks, W.; Walter, M.; Patterson, G.A.; Mohanakumar, T. Role of airway epithelial injury in murine orthotopic tracheal allograft rejection. Ann. Thorac. Surg. 2006, 82, 1226–1233. [Google Scholar] [CrossRef]
- Fernández, F.G.; Jaramillo, A.; Chen, C.; Liu, D.Z.; Tung, T.; Patterson, G.A.; Mohanakumar, T. Airway epithelium is the primary target of allograft rejection in murine obliterative airway disease. Am. J. Transplant. 2004, 4, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Ikonen, T.S.; Brazelton, T.R.; Berry, G.J.; Shorthouse, R.S.; Morris, R.E. Epithelial re-growth is associated with inhibition of obliterative airway disease in orthotopic tracheal allografts in non-immunosuppressed rats. Transplantation 2000, 70, 857–863. [Google Scholar] [CrossRef]
- Qu, N.; de Vos, P.; Schelfhorst, M.; de Haan, A.; Timens, W.; Prop, J. Integrity of airway epithelium is essential against obliterative airway disease in transplanted rat tracheas. J. Heart Lung Transplant. 2005, 24, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Haykal, S.; Zhou, Y.; Marcus, P.; Salna, M.; Machuca, T.; Hofer, S.O.P.; Waddell, T.K. The effect of decellularization of tracheal allografts on leukocyte infiltrationand of recellularization on regulatory T cell recruitement. Biomaterials 2013, 34, 5821–5832. [Google Scholar] [CrossRef] [PubMed]
- Dajani, R.; Zhang, Y.; Taft, P.J.; Travis, S.M.; Starner, T.D.; Olsen, A.; Zabner, J.; Welsh, M.J.; Engelhardt, J.F. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am. J. Respir. Cell. Mol. Biol. 2005, 32, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Mimms, R.; Banigan, M.; Lee, M.; Elkis, V.; Peters-Hall, J.R.; Mubeen, H.; Joselow, A.; Peña, M.T.; Rose, M.C. Development of glandular models from human nasal progenitor cells. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, T.J.; Anderson, P.J.; Rotti, P.G.; Tyler, S.R.; Crooke, A.K.; Choi, S.H.; Montoro, D.T.; Silverman, C.L.; Shahin, W.; Zhao, R.; et al. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell 2018, 22, 653–667.e5, Erratum in Cell Stem Cell 2018, 22, 779. [Google Scholar] [CrossRef] [Green Version]
- Tata, A.; Kobayashi, Y.; Chow, R.D.; Tran, J.; Desai, A.; Massri, A.J.; McCord, T.J.; Gunn, M.D.; Tata, P.R. Myoepithelial Cells of Submucosal Glands Can Function as Reserve Stem Cells to Regenerate Airways after Injury. Cell Stem Cell 2018, 22, 668–683.e6. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sui, H.; Fisher, J.T.; Yan, Z.; Liu, X.; Cho, H.J.; Joo, N.S.; Zhang, Y.; Zhou, W.; Yi, Y.; et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Investig. 2010, 120, 3149–3160. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Olivier, A.K.; Liang, B.; Yi, Y.; Sui, H.; Evans, T.I.; Zhang, Y.; Zhou, W.; Tyler, S.R.; Fisher, J.T.; et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 502–512. [Google Scholar] [CrossRef]
- Sun, X.; Yi, Y.; Yan, Z.; Rosen, B.H.; Liang, B.; Winter, M.C.; Evans, T.I.A.; Rotti, P.G.; Yang, Y.; Gray, J.S.; et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019, 11, eaau7531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swatek, A.M.; Lynch, T.J.; Crooke, A.K.; Anderson, P.J.; Tyler, S.R.; Brooks, L.; Ivanovic, M.; Klesney-Tait, J.A.; Eberlein, M.; Pena, T.; et al. Depletion of Airway Submucosal Glands and TP63+KRT5+Basal Cells in Obliterative Bronchiolitis. Am. J. Respir. Crit. Care Med. 2018, 197, 1045–1057. [Google Scholar] [CrossRef]
- Sui, H.; Olivier, A.K.; Klesney-Tait, J.A.; Brooks, L.; Tyler, S.R.; Sun, X.; Skopec, A.; Kline, J.; Sanchez, P.G.; Meyerholz, D.K.; et al. Ferret lung transplant: An orthotopic model of obliterative bronchiolitis. Am. J. Transplant. 2013, 13, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.C.; Parekh, K.R.; Engelhardt, J.F. Ferret respiratory disease models for the study of lung stem cells. In Lung Stem Cells in Development, Health and Disease (ERS Monograph); Nikolić, M.Z., Hogan, B.L.M., Eds.; European Respiratory Society: Sheffield, UK, 2021; pp. 273–289. [Google Scholar] [CrossRef]
- Yu, M.; Sun, X.; Tyler, S.R.; Liang, B.; Swatek, A.M.; Lynch, T.J.; He, N.; Yuan, F.; Feng, Z.; Rotti, P.G.; et al. Highly Efficient Transgenesis in Ferrets Using CRISPR/Cas9-Mediated Homology-Independent Insertion at the ROSA26 Locus. Sci. Rep. 2019, 9, 1971. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.J.; Lynch, T.J.; Engelhardt, J.F. Multipotent Myoepithelial Progenitor Cells Are Born Early during Airway Submucosal Gland Development. Am. J. Respir. Cell. Mol. Biol. 2017, 56, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Loy, C.; Pezzoli, D.; Candiani, G.; Mantovani, D. A Cost-Effective Culture System for the In Vitro Assembly, Maturation, and Stimulation of Advanced Multilayered Multiculture Tubular Tissue Models. Biotechnol. J. 2018, 13, 1700359. [Google Scholar] [CrossRef]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weymann, A.; Patil, N.P.; Sabashnikov, A.; Korkmaz, S.; Li, S.; Soos, P.; Ishtok, R.; Chaimow, N.; Pätzold, I.; Czerny, N.; et al. Perfusion-Decellularization of Porcine Lung and Trachea for Respiratory Bioengineering. Artif. Organs 2015, 39, 1024–1032. [Google Scholar] [CrossRef]
- Hung, S.H.; Su, C.H.; Lin, S.E.; Tseng, H. Preliminary experiences in trachea scaffold tissue engineering with segmental organ decellularization. Laryngoscope 2016, 126, 2520–2527. [Google Scholar] [CrossRef]
- Remlinger, N.T.; Czajka, C.A.; Juhas, M.E.; Vorp, D.A.; Stolz, D.B.; Badylak, S.F.; Gilbert, S.; Gilbert, T.W. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials 2010, 31, 3520–3526. [Google Scholar] [CrossRef]
- Huang, Z.; Godkin, O.; Schulze-Tanzil, G. The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage. Stem Cell Rev. Rep. 2017, 13, 50–67. [Google Scholar] [CrossRef]
- Lu, T.; Huang, Y.; Qiao, Y.; Zhang, Y.; Liu, Y. Evaluation of changes in cartilage viability in detergent-treated tracheal grafts for immunosuppressant-free allotransplantation in dogs. Eur. J. Cardiothorac. Surg. 2018, 53, 672–679. [Google Scholar] [CrossRef]
- Kuo, E.; Maruyama, T.; Fernandez, F.; Mohanakumar, T. Molecular mechanisms of chronic rejection following transplantation. Immunol. Res. 2005, 32, 179–185. [Google Scholar] [CrossRef]
- Lane, B.P.; Habicht, G.S.; Jasper, G.S. Lymphocyte-epithelium interaction during rejection of nonisogeneic rat tracheal grafts. Am. J. Pathol. 1977, 86, 71–80. [Google Scholar] [PubMed]
- Qu, N.; de Haan, A.; Harmsen, M.C.; Kroese, F.G.; de Leij, L.F.; Prop, J. Specific immune responses against airway epithelial cells in a transgenic mouse-trachea transplantation model for obliterative airway disease. Transplantation 2003, 76, 1022–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauck, K.A.; Hosenpud, J.D. The bronchial epithelium: A potential allogeneic target for chronic rejection after lung transplantation. J. Heart Lung Transplant. 1996, 15, 709–714. [Google Scholar]
- Genden, E.M.; Iskander, A.J.; Bromberg, J.S.; Mayer, L. Orthotopic tracheal allografts undergo reepithelialization with recipient-derived epithelium. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Genden, E.M.; Govindaraj, S.; Chaboki, H.; Cleven, H.; Fedorova, E.; Bromberg, J.S.; Mayer, L. Reepithelialization of orthotopic tracheal allografts prevents rejection after withdrawal of immunosuppression. Ann. Otol. Rhinol. Laryngol. 2005, 114, 279–288. [Google Scholar] [CrossRef]
- Genden, E.M.; Iskander, A.; Bromberg, J.S.; Mayer, L. The kinetics and pattern of tracheal allograft re-epithelialization. Am. J. Respir. Cell. Mol. Biol. 2003, 28, 673–681. [Google Scholar] [CrossRef]
- Kobayashi, K.; Suzuki, T.; Nomoto, Y.; Tada, Y.; Miyake, M.; Hazama, A.; Nakamura, T.; Omori, K. Potential of heterotopic fibroblasts as autologous transplanted cells for tracheal epithelial regeneration. Tissue Eng. 2007, 13, 2175–2184. [Google Scholar] [CrossRef]
- Levardon, H.; Yonker, L.M.; Hurley, B.P.; Mou, H. Expansion of Airway Basal Cells and Generation of Polarized Epithelium. Bio-Protocol 2018, 8, e2877. [Google Scholar] [CrossRef]
- Pardo-Saganta, A.; Law, B.M.; Tata, P.R.; Villoria, J.; Saez, B.; Mou, H.; Zhao, R.; Rajagopal, J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015, 16, 184–197. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, A.C.; Lynch, T.J.; Ahlers, B.A.; Ievlev, V.; Engelhardt, J.F.; Parekh, K.R. A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas. Cells 2022, 11, 1027. https://doi.org/10.3390/cells11061027
Pai AC, Lynch TJ, Ahlers BA, Ievlev V, Engelhardt JF, Parekh KR. A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas. Cells. 2022; 11(6):1027. https://doi.org/10.3390/cells11061027
Chicago/Turabian StylePai, Albert C., Thomas J. Lynch, Bethany A. Ahlers, Vitaly Ievlev, John F. Engelhardt, and Kalpaj R. Parekh. 2022. "A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas" Cells 11, no. 6: 1027. https://doi.org/10.3390/cells11061027
APA StylePai, A. C., Lynch, T. J., Ahlers, B. A., Ievlev, V., Engelhardt, J. F., & Parekh, K. R. (2022). A Novel Bioreactor for Reconstitution of the Epithelium and Submucosal Glands in Decellularized Ferret Tracheas. Cells, 11(6), 1027. https://doi.org/10.3390/cells11061027





