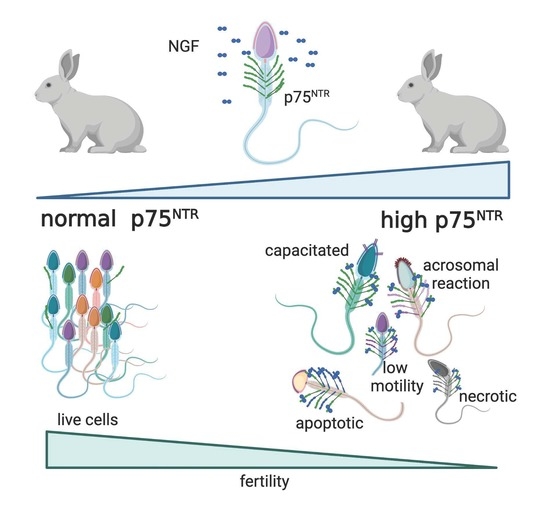
The Effect of Interaction NGF/p75NTR in Sperm Cells: A Rabbit Model
Abstract
:1. Introduction
2. Materials and Methods
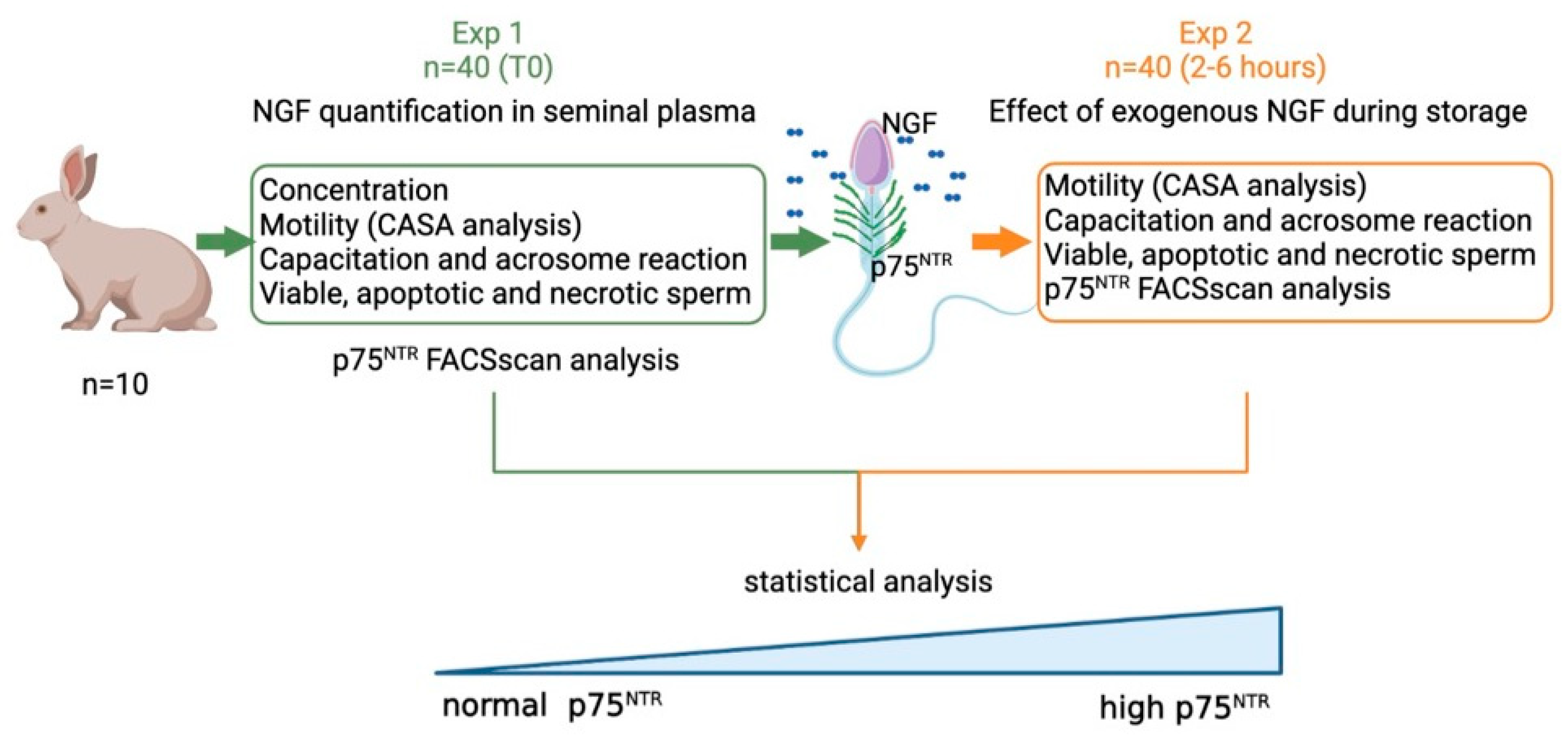
2.1. Experimental Design
- Exp 1. Quantification of NGF concentration and p75NTR expression in epididymal and in ejaculated sperm, and the effect of p75NTR expression at time 0 on the main sperm outcomes.
- Exp 2. Effects of exogenous NGF (100 ng/mL) during storage (up to 6 h) on ejaculated sperm.
2.2. Animals and Semen Sampling
2.3. Semen Handling
2.4. NGF Quantification in Seminal Plasma
2.5. Motility (CASA Analysis)
2.6. Sperm Capacitation and Acrosomal Reaction
2.7. Viable, Apoptotic, and Necrotic Sperm Analysis
2.8. p75NTR FACScan Analysis
2.9. Statistical Design
3. Results
3.1. Exp 1. Quantification of NGF Concentration and p75NTR Expression in Epididymal and Ejaculated Sperm and the Effect of p75NTR Expression on Main Sperm Outcomes
3.2. Exp 2. p75NTR Expression in Ejaculated Sperm and Impact of Exogenous NGF (100 ng/mL) during Storage (up to 6 h) on H and N Sperm Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tessarollo, L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998, 9, 125–137. [Google Scholar] [CrossRef]
- Lima, F.S.; Stewart, J.L.; Canisso, I.F. Insights into nerve growth factor-β role in bovine reproduction-Review. Theriogenology 2020, 150, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.A.; Singh, J.; Ratto, M.H.; Adams, G.P. Neuroanatomical basis of the nerve growth factor ovulation–induction pathway in llamas†. Biol. Reprod. 2020, 104, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Palermo, F.A.; Bufalari, A.; Mercati, F.; Paoloni, D.; Cocci, P.; Moretti, G.; Crotti, S.; Zerani, M.; Dall’Aglio, C. Seasonal Expression of NGF and Its Cognate Receptors in the Ovaries of Grey Squirrels (Sciurus carolinensis). Animals 2020, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Ratto, M.H.; Berland, M.; Silva, M.E.; Adams, G.P. New insights of the role of β-NGF in the ovulation mechanism of induced ovulating species. Reproduction 2019, 157, R199–R207. [Google Scholar] [CrossRef] [Green Version]
- Adams, G.P.; Ratto, M.H.; Silva, M.E.; Carrasco, R.A. Ovulation-inducing factor (OIF/NGF) in seminal plasma: A review and update. Reprod. Domest. Anim. 2016, 51 (Suppl. S2), 4–17. [Google Scholar] [CrossRef]
- Maranesi, M.; Zerani, M.; Leonardi, L.; Pistilli, A.; Arruda-Alencar, J.; Stabile, A.; Rende, M.; Castellini, C.; Petrucci, L.; Parillo, F. Gene expression and localization of NGF and its cognate receptors NTRK 1 and NGFR in the sex organs of male rabbits. Reprod. Domest. Anim. 2015, 50, 918–925. [Google Scholar] [CrossRef]
- Bothwell, M. Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. F1000Research 2019, 8, 1658. [Google Scholar] [CrossRef]
- Freund-Michel, V.; Frossard, N. The nerve growth factor and its receptors in airway inflammatory diseases. Pharmacol. Ther. 2008, 117, 52–76. [Google Scholar] [CrossRef]
- Pula, G.; Pistilli, A.; Montagnoli, C.; Stabile, A.M.; Rambotti, M.G.; Rende, M. The tricyclic antidepressant amitriptyline is cytotoxic to HTB114 human leiomyosarcoma and induces p75NTR-dependent apoptosis. Anti-Cancer Drugs 2013, 24, 899–910. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Arias-Álvarez, M.; Millán, P.; Lorenzo, P.L.; García-García, R.M.; Rebollar, P.G. Physiological effects on rabbit sperm and reproductive response to recombinant rabbit beta nerve growth factor administered by intravaginal route in rabbit does. Theriogenology 2020, 157, 327–334. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Arias-Alvarez, M.; Timón, P.; Bautista, J.M.; Rebollar, P.G.; Lorenzo, P.L.; Garcia-Garcia, R.M. Characterization of β-Nerve Growth Factor-TrkA system in male reproductive tract of rabbit and the relationship between β-NGF and testosterone levels with seminal quality during sexual maturation. Theriogenology 2019, 126, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Dal Bosco, A.; Collodel, G.; Pistilli, A.; Stabile, A.M.; Macchioni, L.; Mancuso, F.; Luca, G.; Rende, M. In vitro effect of nerve growth factor on the main traits of rabbit sperm. Reprod. Biol. Endocrinol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Bosco, A.D.; Cotozzolo, E.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Nerve growth factor receptor role on rabbit sperm storage. Theriogenology 2020, 153, 54–61. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [Green Version]
- Schecterson, L.C.; Bothwell, M. Neurotrophin receptors: Old friends with new partners. Dev. Neurobiol. 2010, 70, 332–338. [Google Scholar] [CrossRef]
- Ayer-LeLievre, C.; Olson, L.; Ebendal, T.; Hallböök, F.; Persson, H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc. Natl. Acad. Sci. USA 1988, 85, 2628–2632. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zheng, L.; Wang, C.; Zhou, X. Absence of nerve growth factor and comparison of tyrosine kinase receptor A levels in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clin. Chim. Acta 2010, 411, 1482–1486. [Google Scholar] [CrossRef]
- Lin, K.; Ding, X.F.; Shi, C.G.; Zeng, D.; QuZong, S.; Liu, S.H.; Wu, Y.; LuoBu, G.; Fan, M.; Zhao, Y.Q. Nerve growth factor promotes human sperm motility in vitro by increasing the movement distance and the number of A grade spermatozoa. Andrologia 2015, 47, 1041–1046. [Google Scholar] [CrossRef]
- Jin, W.; Tanaka, A.; Watanabe, G.; Matsuda, H.; Taya, K. Effect of NGF on the motility and acrosome reaction of golden hamster spermatozoa in vitro. J. Reprod. Dev. 2010, 56, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maranesi, M.; Petrucci, L.; Leonardi, L.; Piro, F.; Rebollar, P.G.; Millán, P.; Cocci, P.; Vullo, C.; Parillo, F.; Moura, A. New insights on a NGF-mediated pathway to induce ovulation in rabbits (Oryctolagus cuniculus). Biol. Reprod. 2018, 98, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellini, C.; Mattioli, S.; Dal Bosco, A.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Role of NGF on sperm traits: A review. Theriogenology 2020, 150, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Boiti, C.; Castellini, C.; Besenfelder, U.; Theau-Clément, M.; Liguori, L.; Renieri, T.; Pizzi, F. Guidelines for the handling of rabbit bucks and semen. World Rabbit. Sci. 2005, 13, 71–91. [Google Scholar]
- Albus, U. Guide for the Care and Use of Laboratory Animals, 8th ed.; SAGE Publications Sage UK: London, UK, 2012. [Google Scholar]
- Castellini, C.; Dal Bosco, A.; Ruggeri, S.; Collodel, G. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil. Steril. 2011, 96, 24–27. [Google Scholar] [CrossRef]
- Cocchia, N.; Pasolini, M.; Mancini, R.; Petrazzuolo, O.; Cristofaro, I.; Rosapane, I.; Sica, A.; Tortora, G.; Lorizio, R.; Paraggio, G. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology 2011, 75, 1201–1210. [Google Scholar] [CrossRef]
- Altman, D.G. Categorising continuous variables. Br. J. Cancer 1991, 64, 975. [Google Scholar] [CrossRef] [Green Version]
- Barbato, O.; De Felice, E.; Todini, L.; Menchetti, L.; Malfatti, A.; Scocco, P. Effects of Feed Supplementation on Nesfatin-1, Insulin, Glucagon, Leptin, T3, Cortisol, and BCS in Milking Ewes Grazing on Semi-Natural Pastures. Animals 2021, 11, 682. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using Spss, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- StataCorp, L. StataCorp. Stata Statistical Software: Release 10; StataCorp LP: College Station, TX, USA, 2007. [Google Scholar]
- Pistilli, A.; Rende, M.; Crispoltoni, L.; Montagnoli, C.; Stabile, A.M. LY294002 induces in vitro apoptosis and overexpression of p75NTR in human uterine leiomyosarcoma HTB 114 cells. Growth Factors 2015, 33, 376–383. [Google Scholar] [CrossRef]
- Hempstead, B.; Birge, R.; Fajardo, J.; Glassman, R.; Mahadeo, D.; Kraemer, R.; Hanafusa, H. Expression of the v-crk oncogene product in PC12 cells results in rapid differentiation by both nerve growth factor-and epidermal growth factor-dependent pathways. Mol. Cell. Biol. 1994, 14, 1964–1971. [Google Scholar]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR--live or let die. Curr. Opin. Neurobiol. 2005, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pizzari, T.; Dean, R.; Pacey, A.; Moore, H.; Bonsall, M.B. The evolutionary ecology of pre-and post-meiotic sperm senescence. Trends Ecol. Evol. 2008, 23, 131–140. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Group | p Value | |
|---|---|---|---|
| Normal p75NTR (n = 21, 52.5%) | High p75NTR (n = 19, 47.5%) | ||
| Concentration (n × 106) | 234 ± 92 | 197 ± 87 | 0.198 |
| Deadcells (%) | 21.45 ± 10.76 | 33.88 ± 11.04 | 0.06 |
| Motility rate # (%) | 49.15 ± 23.91 | 42.79 ± 18.35 | 0.403 |
| VCL (μm/s) | 169.44 ± 55.71 | 169.12 ± 39.01 | 0.983 |
| Apoptotic cells † (%) | 15.94 ± 8.50 | 21.60 ± 5.38 | 0.06 |
| NCP † (%) | 74 ± 10 | 62 ± 19 | 0.092 |
| CP #,† (%) | 2 ± 3 | 2 ± 2 | 0.388 |
| Ar † (%) | 0.00 ± 0.00 | 0.05 ± 0.22 | 0.331 |
| NGF (pg/mL) | 1591.71 ± 413.07 | 1621.13 ± 428.86 | 0.336 |
| Dead | Motility # | VCL | Apoptotic Cells | NCP | CP# | Ar | NGF | p75NTR | |
|---|---|---|---|---|---|---|---|---|---|
| Concentration | 0.172 | 0.097 | −0.140 | 0.261 | 0.011 | −0.141 | −0.242 | −0.175 | −0.403 ** |
| Dead | −0.324 * | −0.233 | 0.951 ** | −0.638 ** | −0.164 | 0.065 | −0.130 | 0.098 | |
| Motility # | 0.716 ** | −0.380 * | 0.163 | −0.258 | −0.289 | 0.116 | −0.183 | ||
| VCL | −0.294 | −0.032 | −0.140 | 0.045 | 0.135 | −0.071 | |||
| Apoptotic cells | −0.583 ** | −0.187 | 0.065 | −0.170 | 0.062 | ||||
| NCP | −0.310 | −0.203 | 0.008 | −0.134 | |||||
| CP# | 0.297 | 0.022 | 0.136 | ||||||
| Ar | 0.112 | 0.124 | |||||||
| NGF | −0.119 |
| Parameters | NGF Treatment | Groups | p Value | ||||
|---|---|---|---|---|---|---|---|
| Normal p75NTR (n = 21, 52.5%) | High p75NTR (n = 19, 47.5%) | N vs. H | C vs. NGF | N vs. H (C vs. NGF) | Time (0-2-4-6 h) | ||
| Concentration (n. ×106) | C | 222.46 ± 3.28 | 214.70 ± 2.87 | 0.156 | 0.870 | 0.236 | 0.125 |
| NGF | 219.45 ± 2.95 | 218.69 ± 2.95 | |||||
| Deadcells (%) | C | 40.80 ± 2.59 | 42.94 ± 2.28 | 0.001 | 0.389 | 0.012 | <0.001 |
| NGF | 32.88 ± 2.04 | 46.93 ± 2.25 | |||||
| Motility # (%) | C | 46.24 ± 2.36 | 35.48 ± 1.64 | <0.001 | 0.706 | 0.162 | 0.120 |
| NGF | 56.23 ± 2.42 | 31.70 ± 1.50 | |||||
| VCL (mm/s) | C | 172.05 ± 10.07 | 180.25 ± 8.63 | 0.789 | 0.054 | 0.252 | 0.477 |
| NGF | 200.02 ± 8.12 | 187.09 ± 8.82 | |||||
| Apoptotic cells † (%) | C | 25.58 ± 1.65 | 27.27 ± 1.45 | 0.001 | 0.279 | 0.028 | <0.001 |
| NGF | 20.70 ± 1.30 | 28.99 ± 1.44 | |||||
| NCP (%) † | C | 57.91 ± 2.42 | 53.10 ± 2.17 | <0.001 | 0.992 | 0.142 | <0.001 |
| NGF | 61.15 ± 1.94 | 49.90 ± 2.13 | |||||
| CP (%) #,† | C | 3.69 ± 0.29 | 4.28 ± 0.28 | 0.190 | 0.528 | 0.733 | <0.001 |
| NGF | 3.88 ± 0.25 | 5.02 ± 0.34 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellini, C.; Mattioli, S.; Cotozzolo, E.; Pistilli, A.; Rende, M.; Bartolini, D.; Di Sante, G.; Menchetti, L.; Dal Bosco, A.; Stabile, A.M. The Effect of Interaction NGF/p75NTR in Sperm Cells: A Rabbit Model. Cells 2022, 11, 1035. https://doi.org/10.3390/cells11061035
Castellini C, Mattioli S, Cotozzolo E, Pistilli A, Rende M, Bartolini D, Di Sante G, Menchetti L, Dal Bosco A, Stabile AM. The Effect of Interaction NGF/p75NTR in Sperm Cells: A Rabbit Model. Cells. 2022; 11(6):1035. https://doi.org/10.3390/cells11061035
Chicago/Turabian StyleCastellini, Cesare, Simona Mattioli, Elisa Cotozzolo, Alessandra Pistilli, Mario Rende, Desirée Bartolini, Gabriele Di Sante, Laura Menchetti, Alessandro Dal Bosco, and Anna Maria Stabile. 2022. "The Effect of Interaction NGF/p75NTR in Sperm Cells: A Rabbit Model" Cells 11, no. 6: 1035. https://doi.org/10.3390/cells11061035
APA StyleCastellini, C., Mattioli, S., Cotozzolo, E., Pistilli, A., Rende, M., Bartolini, D., Di Sante, G., Menchetti, L., Dal Bosco, A., & Stabile, A. M. (2022). The Effect of Interaction NGF/p75NTR in Sperm Cells: A Rabbit Model. Cells, 11(6), 1035. https://doi.org/10.3390/cells11061035