Transposable Elements: Major Players in Shaping Genomic and Evolutionary Patterns
Abstract
:1. Introduction
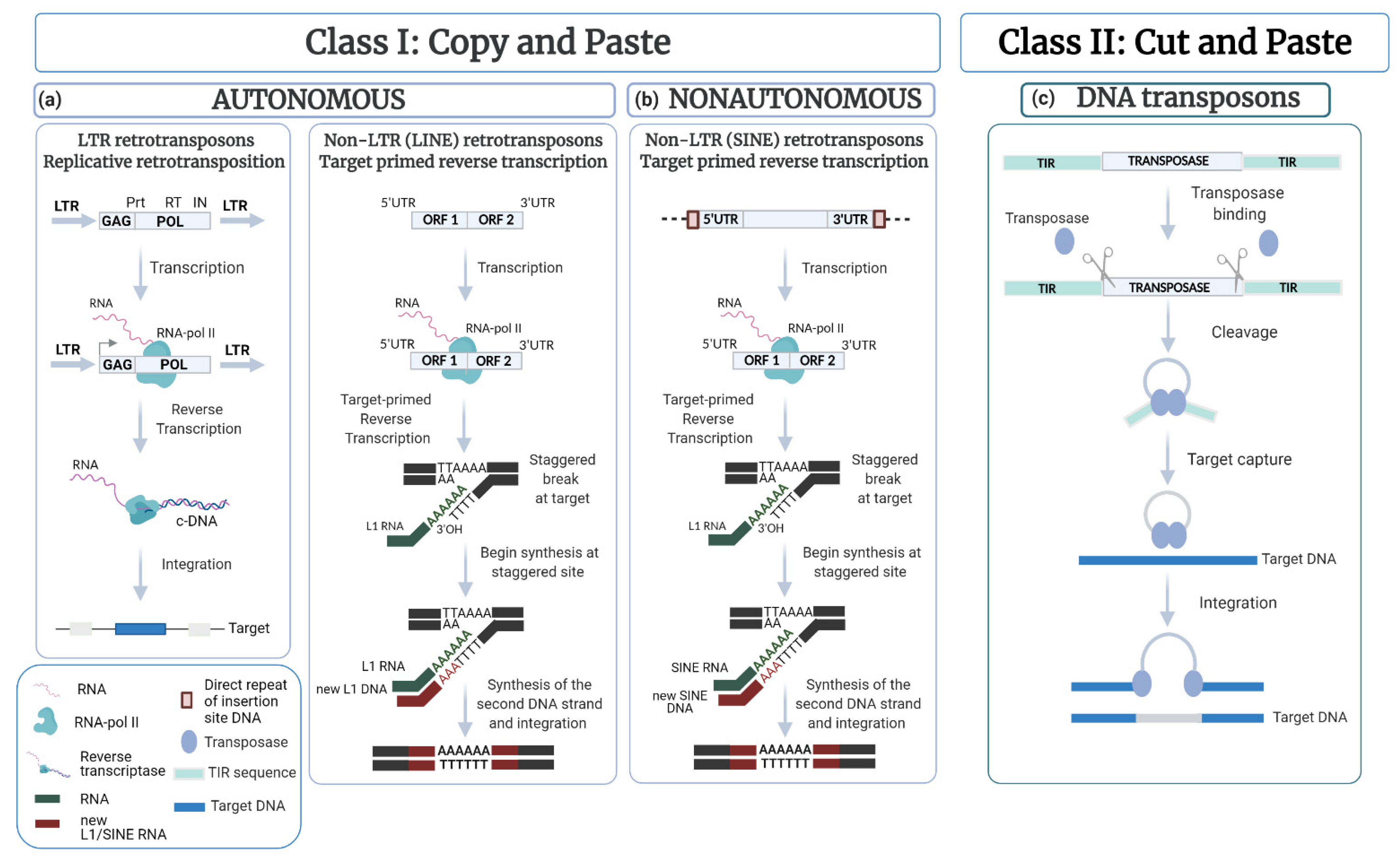
2. TEs as Parasites of the Genome
3. TEs as Symbionts within the Host Genome
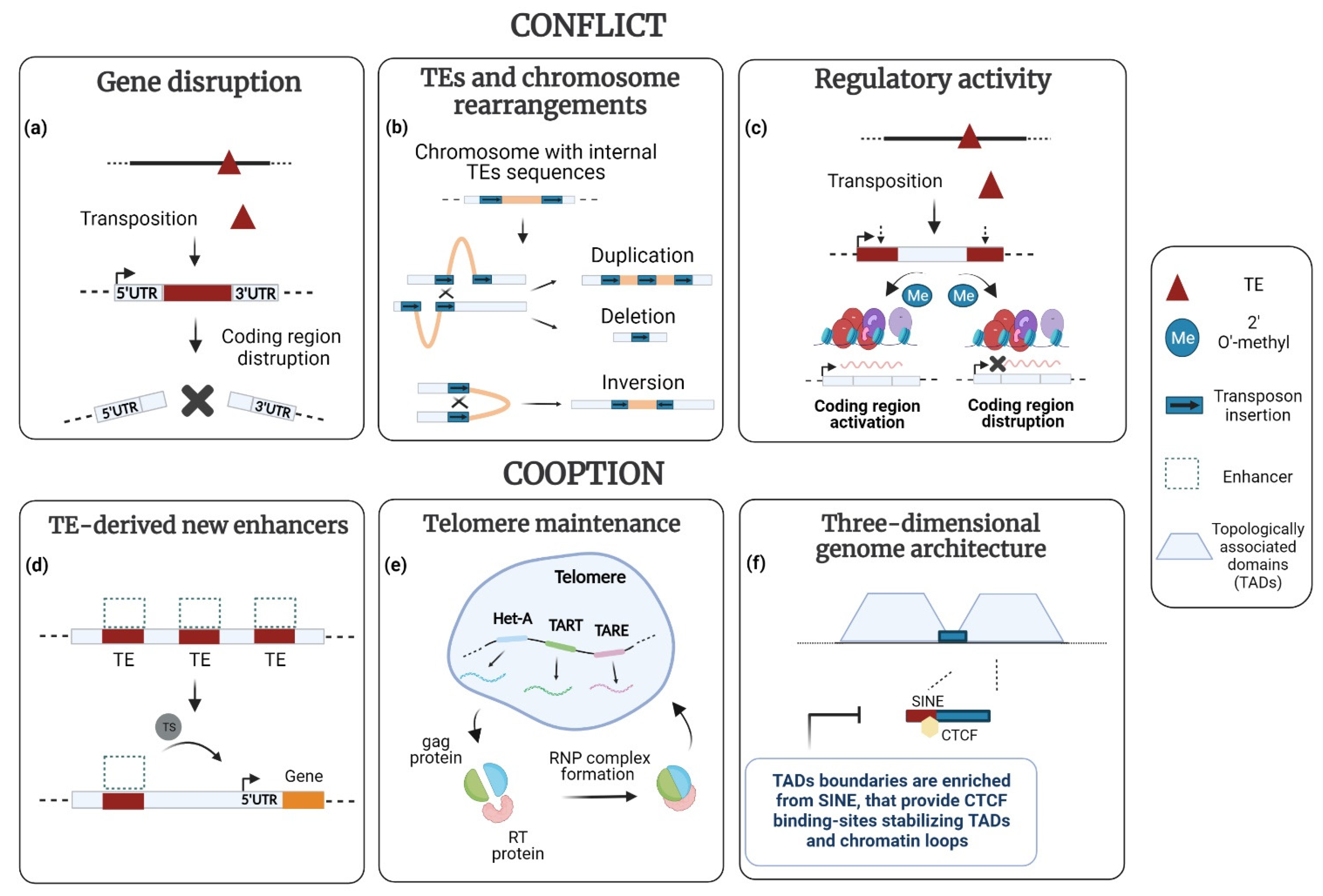
3.1. TEs and New Regulatory Programs
3.2. TEs and New Epigenetic Landscapes
3.3. TEs and Chromosome Structure
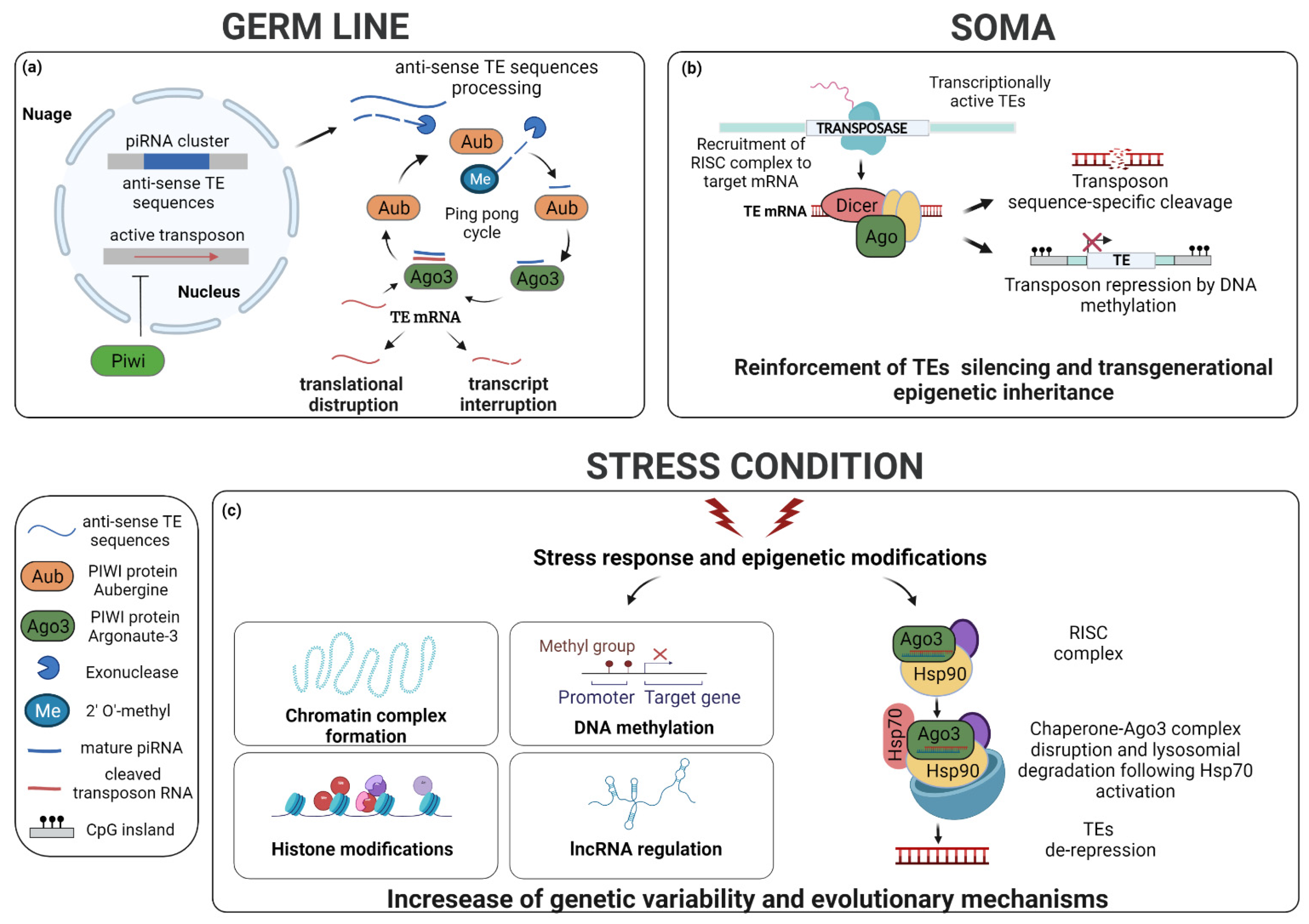
4. Regulation of the Transposition of TEs
5. Environmental Stress and Evolution
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanley, S.M. Speciation, and the fossil record. Prog. Clin. Biol. Res. 1982, 96, 41–49. [Google Scholar]
- Maynard Smith, J. Games, Sex, and Evolution; Harvester-Wheatsheaf: Hoboken, NJ, USA, 1988; p. 264. [Google Scholar]
- Gould, S.J. Wonderful Life: The Burgess Shale and the Nature of History; W. W. Norton and Company: New York, NY, USA, 1989; p. 347. [Google Scholar]
- Schindewolf, O.H. Basic Questions in Paleontology: Geologic Time, Organic Evolution, and Biological Systematics; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Rudwick, M.J.S.; Cuvier, G. Fossil Bones, and Geological Catastrophes; University of Chicago Press: Chicago, IL, USA, 1997; p. 301. [Google Scholar]
- Eldrege, N. The Pattern of Evolution; W.H. Freeman in New York: Gordonsville, VA, USA, 1999. [Google Scholar]
- Erwin, D.; Valentine, J.W. The Cambrian Explosion: The Construction of Animal Biodiversity; Roberts and Company Publishers: Greenwood Village, CO, USA, 2013. [Google Scholar]
- Cuvier, G.; Valenciennes, A. Histoire Naturelle des Poissons; Des Sciénoïdes, F.G., Levrault, P., Eds.; Livre cinquième; Bertrand: Paris, France, 1830; pp. 1–499. [Google Scholar]
- Eldredge, N.; Stanley, S.M. Living Fossils; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Valentine, J.W. How good was the fossil record? Clues from the California Pleistocene. Paleobiology 1989, 15, 83–94. [Google Scholar] [CrossRef]
- Stanley, S.M. Macroevolution: Pattern and Process; Baltimore University Press: Baltimore, MD, USA, 1998; p. 332. [Google Scholar]
- Gould, S.J. Punctuated equilibrium in fact and theory. J. Soc. Biol. Struct. 1989, 12, 117–136. [Google Scholar] [CrossRef]
- Gould, S.J.; Eldredge, N. Punctuated Equilibrium Comes of Age. Nature 1993, 366, 223–227. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 16, 792–801. [Google Scholar] [CrossRef] [Green Version]
- SanMiguel, P.; Tikhonov, A.; Jin, Y.K.; Motchoulskaia, N.; Zakharov, D.; Melake-Berhan, A.; Springer, P.S.; Edwards, K.J.; Lee, M.; Avramova, Z.; et al. Nested Retrotransposons in the Intergenic Regions of the Maize Genome. Science 1996, 274, 765–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science 2009, 326, 1178534. [Google Scholar] [CrossRef] [Green Version]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Merel, V.; Boulesteix, M.; Fablet, M.; Vieira, C. Transposable elements in Drosophila. Mob. DNA 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Pritham, E. Helitrons, the Eukaryotic Rolling-circle Transposable Elements. Microbiol. Spectr. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Campbell, S.; Aswad, A.; Katzourakis, A. Disentangling the origins of virophages and polintons. Curr. Opin. Virol. 2017, 25, 59–65. [Google Scholar] [CrossRef]
- Craig, N.L.; Chandler, M.; Gellert, M.; Lambowitz, A.M.; Rice, P.A.; Sandmeyer, S.B. Mobile DNA III; ASM Press: Washington, DC, USA, 2015; pp. 1100–1350. [Google Scholar]
- Hickey, D.A. Selfish DNA: A sexually-transmitted nuclear parasite. Genetics 1982, 101, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B.; Charlesworth, D. The population dynamics of transposable elements. Genet. Res. 1983, 42, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Feschotte, C.; Pritham, E.J. DNA Transposons and the Evolution of Eukaryotic Genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvàk, Z.; Levin, H.L.; Macfarlan, T.S.; et al. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 199. [Google Scholar] [CrossRef]
- Zhang, H.H.; Peccoud, J.; Xu, M.R.X.; Zhang, X.G.; Gilbert, G. Horizontal transfer and evolution of transposable elements in vertebrates. Nat. Commun. 2020, 11, 1362. [Google Scholar] [CrossRef] [Green Version]
- Gray, Y. It takes two transposons to tango: Transposable element mediated chromosomal rearrangements. Trends Genet. 2000, 16, 461–468. [Google Scholar] [CrossRef]
- Gilbert, N.; Lutz-Priggem, S.; Moran, J.V. Genomic deletions created upon LINE-1 retrotransposition. Cell 2002, 110, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Xing, J.; Wang, H.; Hedges, D.J.; Garber, R.K.; Cordaux, R.; Batzer, M.A. Under the genomic radar: The stealth model of Alu amplification. Genome Res. 2005, 15, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geurts, A.M.; Collier, L.S.; Geurts, J.L.; Oseth, L.L.; Bell, M.L.; Mu, D.; Lucito, R.; Godbout, S.A.; Green, L.E.; Lowe, S.W.; et al. Gene Mutations and Genomic Rearrangements in the Mouse as a Result of Transposon Mobilization from Chromosomal Concatemers. PLoS Genet. 2006, 2, 0020156. [Google Scholar] [CrossRef]
- Zhang, Y.; Romanish, M.T.; Mager, D.L. Distributions of Transposable Elements reveal Hazardous Zones in Mammalian Intron. PLoS Comput. Biol. 2011, 7, e1002046. [Google Scholar] [CrossRef] [Green Version]
- Kidwell, M.G. Hybrid dysgenesis in Drosophila melanogaster: The relationship between the P-M and I-R interac-tion systems. Genet. Res. 1979, 33, 105–117. [Google Scholar] [CrossRef]
- Shaefer, Z.; Ruth, E.; Kidwell, M.G.; Sterling, A.F. Hybrid dysgenesis in Drosophila melanogaster: Morphological and cytological studies of ovarian dysgenesis. Genetics 1979, 92, 1141–1152. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. Roles for retrotransposon insertions in human disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payer, L.M.; Burns, K.H. Transposable elements in human genetic disease. Nat. Rev. Genet. 2019, 20, 760–772. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. Active human retrotransposons: Variation and disease. Curr. Opin. Genet. Dev. 2012, 3, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Sorek, R.; Ast, G.; Graur, D. Alu-containing exons are alternatively spliced. Genome Res. 2002, 12, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi-Ikeda, M.; Kobayashi, K.; Kanagawa, M.; Yu, C.C.; Mori, K.; Oda, T.; Kuga, A.; Kurahashi, H.; Akman, H.O.; DiMauro, S.; et al. Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature 2011, 478, 127–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zheng, X.; Xiao, D.; Zheng, Y. Age-associated de-repression of retrotransposons in the Drosophila fat- body, its potential cause and consequences. Aging Cell 2016, 15, 542–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, J.G.; Jones, B.C.; Jiang, N.; Chang, C.; Hosier, S.; Wickremesinghe, P.; Garcia, M.; Hartnett, D.A.; Burhenn, L.; Neretti, N.; et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 11277–11282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordani, G.; Cavaliere, V.; Gargiulo, G.; Lattanzi, G.; Andrenacci, D. Retrotransposons Down- and Up- Regula-tion in Aging Somatic Tissues. Cells 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Andrenacci, D.; Cavaliere, V.; Lattanzi, G. The role of transposable elements activity in aging and their possible in-volvement in laminopathic diseases. Ageing Res. Rev. 2020, 57, 100995. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A.; Mita, P.; McKerrow, W.; Fenyo, D.; Boeke, J.D.; Linker, S.B.; Gage, F.H.; Kreiling, J.A.; Petrashen, A.P.; et al. The role of retrotransposable ele-ments in aging and age-associated diseases. Nature 2021, 596, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, H.A.; McBryan, T.; Nelson, D.M.; Vanderkraats, N.D.; Shah, P.P.; van Tuyn, J.; Singh Rai, T.; Brock, C.; Donahue, G.; Dunican, D.S.; et al. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 2013, 15, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Van Meter, M.; Ablaeva, J.; Ke, Z.; Gonzalez, R.S.; Taguchi, T.; De Cecco, M.; Leonova, K.I.; Kogan, V.; Helfand, S.L.; et al. LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab. 2019, 29, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.A.; Liu, G.; Eichler, E.E. An Alu transposition model for the origin and expansion of human segmental duplications. Am. J. Hum. Genet. 2003, 73, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Han, K.; Meyer, T.J.; Kim, H.S.; Batzer, M.A. Chromosomal inversions between human and chimpanzee lineages caused by retrotransposons. PLoS ONE. 2008, 3, 4047. [Google Scholar] [CrossRef] [Green Version]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Presneau, N.; Laplace-Marieze, V.; Sylvain, V.; Lortholary, A.; Hardouin, A.; Bernard-Gallon, D.; Bignon, J.Y. New mechanism of BRCA-1 mutation by deletion/insertion at the same nucleotide position in three unrelated French breast/ovarian cancer families. Hum. Genet. 1998, 103, 334–339. [Google Scholar] [CrossRef]
- Belancio, V.P.; Roy-Engel, A.M.; Deininger, P.L. All y’all need to know about retroelements in cancer. Semin. Cancer Biol. 2010, 20, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Babaian, A.; Romanish, M.T.; Gagnier, L.; Kuo, L.Y.; Karimi, M.M.; Steidl, C.; Mager, D.L. Onco-exaptation of an endogenous retroviral LTR drives IRF5 expression in Hodgkin lymphoma. Oncogene 2016, 35, 2542–2546. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Sutherland, C.F.; Chatterjee, A.; Stockwell, P.A.; Eccles, M.R.; Macaulay, E.C. Reawakening the Develop-mental origins of Cancer Through Transposable Elements. Front. Oncol. 2020, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Gilbert, M.; Ivan, M.E.; Komotar, R.J.; Heiss, J.; Nath, A. The role of human endogenous retrovirus in gliomas: From etiological perspectives and therapeutic implications. Neuro Oncol. 2021, 23, 1647–1655. [Google Scholar] [CrossRef]
- Pradhan, R.K.; Ramakrishna, W. Transposons: Unexpected players in cancer. Gene 2022, 808, 145975. [Google Scholar] [CrossRef]
- Levine, A.J.; Ting, D.T.; Greenbaum, B.D. P53 and the defenses against genome instability caused by transposons and repetitive elements. Bioessays 2016, 38, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Pimpinelli, S.; Piacentini, L. Environmental change and the evolution of genomes: Transposable elements as trans-lators of phenotypic plasticity into genotypic variability. Funct. Ecol. 2020, 34, 428–441. [Google Scholar] [CrossRef]
- Minervini, C.F.; Marsano, R.M.; Casieri, P.; Fanti, L.; Caizzi, R.; Pimpinelli, S.; Rocchi, M.; Viggiano, L. Hetero-chromatin protein 1 interacts with 5’UTR of transposable element ZAM in a sequence-specific fashion. Gene 2007, 393, 1–10. [Google Scholar] [CrossRef]
- Kunarso, G.; Chia, N.; Jeyakani, J.; Hwang, C.; Lu, X.; Chan, Y.; Ng, H.; Bourque, G. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat. Genet. 2010, 42, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-mediated rewiring of gene regulatory networks con-tributed to the evolution of pregnancy in mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Chuong, E.B.; Rumi, M.A.K.; Soares, M.J.; Bake, J.C. Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat. Genet. 2013, 45, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, V.; Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Phil. Trans. R. Soc. B 2020, 375, 20190347. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Han, K.; Liang, P. Role of Transposable Elements in Gene Regulation in the Human Genome. Life 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Chasing, L.A. Comparison of multiple vertebrate genomes reveals the birth and evolution of hu-man exons. Proc. Natl. Acad. Sci. USA 2006, 103, 13427–13432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev-Maor, G.; Sorek, R.; Levanon, E.Y.; Paz, N.; Eisenberg, E.; Ast, G. RNA-editing-mediated exon evolution. Genome Biol. 2007, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Warren, I.A.; Naville, M.; Chalopin, D.; Levin, P.; Berger, C.S.; Galiana, D.; Volff, J.N. Evolutionary impact of trans-posable elements on genomic diversity and lineage-specific innovation in vertebrates. Chromosome Res. 2015, 23, 505–531. [Google Scholar] [CrossRef]
- Pantzartzi, C.N.; Pergner, J.; Kozmik, Z. The role of transposable elements in functional evolution of amphyoxus genome: The case of opsin gene family. Sci. Rep. 2018, 8, 2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosby, R.L.; Judd, J.; Zhang, R.; Zhong, A.; Garry, N.; Pritham, E.J.; Feschotte, C. Recurrent evolution of verte-brate transcription factors by transposase capture. Science 2021, 371, abc6405. [Google Scholar] [CrossRef]
- Feschotte, C. Transposable elements and the evolution of regulatory networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Cohen, C.J.; Lock, W.M.; Mager, D.L. Endogenous retroviral LTRs as promoters for human genes: A critical assessment. Gene 2009, 448, 105–114. [Google Scholar] [CrossRef]
- Rebollo, R.; Farivar, S.; Mager, D.L. C-GATE-catalogue of genes affected by transposable elements. Mob. DNA 2012, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.J.; Macfarlan, T.S.; Lorincz, M.C. Long terminal repeats: From parasitic elements to building blocks of the transcriptional regulatory repertoire. Mol. Cell 2016, 62, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, C.D.; Springer, N.M. Transposable element influences on gene expression in plants. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cheng, T.C.; Huang, G.; Lu, Q.; Surleac, M.D.; Mandell, J.D.; Pontarotti, P.; Petrescu, A.J.; Xu, A.; Xiong, Y.; et al. Transposon molecular domestication and the evolution of the RAG recombinase. Nature 2019, 569, 79–84. [Google Scholar] [CrossRef]
- Capy, P. Taming, domestication and exaptation: Trajectories of transposable elements in genomes. Cells 2021, 10, 13390. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zeng, J.; Lowe, C.B.; Sellers, R.G.; Salama, S.R.; Yang, M.; Burgess, S.M.; Brachmann, R.K.; Haussler, D. Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc. Natl. Acad. Sci. USA 2007, 104, 18613–18618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, P.; Meile, L.; Plissonneau, C.; Ma, X.; Hartmann, F.E.; Crol, D.; McDonald, B.A.; Sánchez-Vallet, A. Transposable element insertions shape gene regulation and melanin production in a fungal pathogen of wheat. BMC Biol. 2018, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Zhang, F.; Tsukiyama, T.; Saito, H.; Hancock, C.N.; Richardson, A.O.; Okumoto, Y.; Tanisaka, T.; Wessler, S.R. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 2009, 461, 1130–1134. [Google Scholar] [CrossRef]
- Kispert, A.; Koschorz, B.; Herrmann, B.G. The T protein encoded by Brachyury is a tissue-specific transcription fac-tor. EMBO J. 1995, 14, 4763–4772. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, W.; Wudzinska, A.; Huang, E.; Brosh, R.; Pour, M.; Miller, A.; Dasen, J.S.; Maurano, M.T.; Kim, S.Y.; et al. The genetic basis of tail-loss evolution in humans and apes. bioRxiv 2021. preprint. [Google Scholar]
- Nicolau, M.; Picault, N.; Moissiard, G. The Evolutionary Volte-Face of Transposable Elements: From Harmful Jumping Genes to Major Drivers of Genetic Innovation. Cells 2021, 10, 2952. [Google Scholar] [CrossRef] [PubMed]
- Piriyapongsa, J.; Jordan, I.K. Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 2008, 14, 814–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.; Guigo, R. The RIDL hypothesis: Transposable elements as functional domains of long noncoding RNAs. RNA 2014, 20, 959–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J. Transposon-Derived Non-coding RNAs and Their Function in Plants. Front. Plant Sci. 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Dupressoir, A.; Lavialle, C.; Heidmann, T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Feschotte, C. Co-option of endogenous viral sequences for host cell function. Curr. Opin. Virol. 2017, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hermant, C.; Torres-Padilla, M.E. TFs for TEs: The transcription factor repertoire of mammalian transposable elements. Genes Dev. 2021, 35, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Cheng, Y.; Ma, Z.; Li, D.; Xing, X.; Edge, P.; Snyder, M.P.; Wang, T. Widespread contribution of transposable elements to the innovation of gene regulatory networks. Genome Res. 2014, 24, 1963–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, J.H.; O’Sullivan, R.J.; Braunschweig, U.; Opravil, S.; Radolf, M.; Steinlein, P.; Jenuwein, T. The profile of re-peat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 2005, 24, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadrana, L.; Silveira, A.B.; George, F.M.; LeBlanc, C.; Martienssen, R.A.; Jeddeloh, J.A.; Colot, V. The Arabidopsis thaliana Mobilome and Its Impact at the Species Level. eLife 2016, 5, e15716. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Zhao, X.; Tock, A.J.; Lambing, C.; Underwood, C.J.; Hardcastle, T.J.; Serra, H.; Kim, J.; Cho, H.S.; Kim, J.; et al. Nucleosomes and DNA methyl-ation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res. 2018, 28, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guio, L.; Vieira, C.; Gonzales, J. Stress affects the epigenetic marks added by natural transposable element inser-tions in Drosophila melanogaster. Sci. Rep. 2018, 8, 12197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noshay, J.M.; Anderson, S.N.; Zhou, P.; Ji, L.; Ricci, W.; Lu, Z.; Stitzer, M.C.; Crisp, P.A.; Hirsch, C.N.; Zhang, X.; et al. Monitoring the interplay between transposable element families and DNA methylation in maize. PLoS Genet. 2019, 15, e1008291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.M.J.; Greenberg, D.; Nguyen, N.; Haeussler, M.; Ewing, A.D.; Katzman, S.; Paten, B.; Salama, S.R.; Haussler, D. An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 2014, 516, 242–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molaro, A.; Malik, H.S. Hide and seek: How chromatin-based pathways silence retroelements in the mammalian germline. Curr. Opin. Genet. Dev. 2016, 37, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Senft, A.D.; Macfarlan, T.S. Transposable elements shape the evolution of mammalian development. Nat. Rev. Genet. 2021, 22, 691–711. [Google Scholar] [CrossRef]
- Lippman, Z.; Gendrel, A.V.; Black, M.; Vaughn, M.W.; Dedhia, N.; McCombie, W.R.; Lavine, K.; Mittal, V.; May, B.; Kasschau, K.D.; et al. Role of transposable elements in hetero-chromatin and epigenetic control. Nature 2004, 430, 471–476. [Google Scholar] [CrossRef]
- Matzke, N.J.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Phan Tran, L.S.; Qin, F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef] [Green Version]
- Ichiyanagi, T.; Katoh, H.; Mori, Y.; Hirafuku, K.; Boyboy, B.A.; Kavase, M.; Ichiyanagi, K. B2 SINE Copies Serve as a Transposable Boundary of DNA Methylation and Histone Modifications in the Mouse. Mol. Biol. Evol. 2021, 38, 2380–2395. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V. Transposable elements, epigenetics, and genome evolution. Science 2012, 338, 758–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlan, T.S.; Gifford, W.D.; Driscoll, S.; Lettieri, K.; Rowe, H.M.; Bonanomi, D.; Firth, A.; Singer, O.; Tr, D.; Pfaff, S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 2012, 487, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, M.T.; Faulkner, G.J.; Dubnau, J.; Ponomarev, I.; Gage, F.H. The role of transposable elements in health and diseases of the central nervous system. J. Neurosci. 2013, 33, 17577–17586. [Google Scholar] [CrossRef] [Green Version]
- Todd, C.D.; Deniz, Ö.; Taylor, D.; Branco, M.R. Functional evaluation of transposable elements as enhancers in mouse embryonic and trophoblast stem cells. eLife 2019, 8, e44344. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Cong, Y.S. Human endogenous retroviruses in development and disease. Comput. Struct. Biotechnol. J. 2021, 19, 5978–5986. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, R.N.; Khusnutdinova, E.K. Involvement of transposable elements in neurogenesis. Vavilov J. Genet. Breed. 2020, 24, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Valdebenito-Maturana, B.; Torres, F.; Carrasco, M.; Tapla, J.C. Differential regulation of transposable elements (TEs) during the murine submandibolar gland development. Mob. DNA 2021, 12, 23. [Google Scholar] [CrossRef]
- Ferrari, R.; Grandi, N.; Tramontano, E.; Dieci, G. Retrotransposons as Drivers of mammalian Brain Evolution. Life 2021, 11, 376. [Google Scholar] [CrossRef]
- Huang, S.; Tao, X.; Yuan, S.; Zhang, Y.; Li, P.; Beilinson, H.A.; Zhang, Y.; Yu, W.; Pontarotti, P.; Escriva, H.; et al. Discovery of an Active RAG Transposon Illuminates the Ori-gins of V(D)J Recombination. Cell 2016, 166, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Ono, R.; Nakamura, K.; Inoue, K.; Naruse, M.; Usami, T.; Wakisaka-Saito, N.; Hino, T.; Suzuki-Migishima, R.; Ogo-nuki, N.; Miki, H.; et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2006, 38, 101–106. [Google Scholar] [CrossRef]
- Breitling, R.; Gerber, J.K. Origin of the paired domain. Dev. Genes Evol. 2000, 210, 644–650. [Google Scholar] [CrossRef]
- Erwin, J.A.; Marchetto, M.C.; Gage, F.H. Mobile DNA elements in the generation of diversity and complexity in the brain. Nat. Rev. Neurosci. 2014, 15, 497–506. [Google Scholar] [CrossRef]
- Paquola, A.C.M.; Erwin, J.A.; Gage, F.H. Insight into the role of somatic mosaicism in the brain. Curr. Opin. Syst. Biol. 2017, 1, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Bodea, G.O.; McKelvey, E.G.Z.; Faulkner, G.J. Retrotransposon-induced mosaicism in the neural Genome. Open Biol. 2018, 8, 180074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muotri, A.R.; Chu, V.T.; Marchetto, M.C.N.; Deng, W.; Moran, J.V.; Gage, F.H. Somatic mosaicism in neuronal pre-cursor cells mediated by L1 retrotransposition. Nature 2005, 435, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Coufal, N.G.; Garcia-Perez, H.L.; Peng, G.R.; Yeo, G.W.; Mu, Y.; Lovci, M.T.; Morell, M.; O’Shea, K.S.; Moran, J.V.; Gage, F.G. L1 retrotransposition in human neural progenitor cells. Nature 2009, 460, 1127–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muotri, A.R.; Marchetto, M.C.N.; Coufal, N.G.; Oefner, R.; Yeo, G.; Nakashima, K.; Gage, F.H. L1 retrotransposition in neurons is modulated by MeCP2. Nature 2010, 18, 09544. [Google Scholar] [CrossRef] [PubMed]
- Perrat, P.N.; Gupta, S.; Wang, J.; Theurkauf, W.; Weng, Z.; Rosbash, M.; Waddell, S. Transposition driven genomic heterogeneity in the Drosophila brain. Science 2013, 340, 1231965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapitonov, V.V.; Jurka, J. Molecular paleontology of transposable elements in the Drosophila melanogaster genome. Proc. Natl. Acad. Sci. USA 2003, 100, 6569–6574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; Fitz-Hugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed] [Green Version]
- Pimpinelli, S.; Berloco, M.; Fanti, L.; Dimitri, P.; Bonaccorsi, S.; Marchetti, E.; Caizzi, R.; Caggese, C.; Gatti, M. Transposable elements are stable structural components of Drosophila melanogaster heterochromatin. Proc. Natl. Acad. Sci. USA 1995, 92, 3804–3808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copenhaver, G.P.; Preuss, D. Centromeres in the genomic era: Unraveling paradoxes. Curr. Opin. Plant Biol. 1999, 2, 104–108. [Google Scholar] [CrossRef]
- Dorer, D.R.; Henikoff, S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 1994, 77, 993–1002. [Google Scholar] [CrossRef]
- Fanti, L.; Dorer, D.R.; Berloco, M.; Henikoff, S.; Pimpinelli, S. The heterochromatin protein 1 binds transgene arrays. Chromosoma 1998, 107, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, M.; Steinemann, S. Enigma of Y chromosome degeneration: Neo-Y and Neo-X chromosomes of Drosophila miranda a model for sex chromosome evolution. Genetica 1998, 102, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, M.; Lisch, D. Transposable elements as sources of genomic variation. Nature 2002, 416, 601–602. [Google Scholar]
- Meštrović, N.; Mravinac, B.; Pavlek, M.; Vojvoda-Zeljko, T.; Satovic, E.; Plohl, M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res. 2015, 23, 583–596. [Google Scholar] [CrossRef]
- Traverse, K.L.; Pardue, M.L. A spontaneously opened ring chromosome of Drosophila melanogaster has acq-uired He-T DNA sequences at both new telomeres. Proc. Natl. Acad. Sci. USA 1988, 85, 8116–8120. [Google Scholar] [CrossRef] [Green Version]
- Biessmann, H.; Carter, S.B.; Mason, J.M. Chromosome ends in Drosophila without telomeric DNA sequences. Proc. Natl. Acad. Sci. USA 1990, 87, 1758–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, F.M.; Levis, R.W. Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. Proc. Natl. Acad. Sci. USA 1994, 91, 12510–12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, J.M.; Biessmann, H. The unusual telomeres of Drosophila. Trends Genet. 1995, 11, 58–62. [Google Scholar] [CrossRef]
- Abad, J.P.; De Pablos, B.; Osoegawa, K.; De Jong, P.J.; Martin-Gallardo, A.; Villasante, A. TAHRE, a novel telo-meric retrotransposon from Drosophila melanogaster, reveals the origin of Drosophila telomeres. Mol. Biol. Evol. 2004, 21, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.L.; DeBaryshe, P.G. Drosophila telomeres: A variation on the telomerase theme. Fly 2008, 2, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markova, D.N.; Christensen, S.M.; Betrán, E. Telomere-Specialized Retroelements in Drosophila: Adaptive Symbionts of the Genome, Neutral, or in Conflict? BioEssays 2020, 42, e1900154. [Google Scholar] [CrossRef] [PubMed]
- Biessmann, H.; Champion, L.E.; O’Hair, M.; Ikenaga, K.; Kasravi, B.; Mason, J.M. Frequent transpositions of Drosophila melanogaster HeT-A elements to receding chromosome ends. EMBO J. 1992, 11, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Levis, R.W.; Ganesan, R.; Houtchens, K.; Tolar, L.A.; Sheen, F.M. Transposons in place of telomeric repeats at a Drosophila telomere. Cell 1993, 75, 1083–1093. [Google Scholar] [CrossRef]
- Cacchione, S.; Cenci, G.; Raffa, G.D. Silence at the end: How Drosophila regulates expression and transposition of telomeric retroelements. J. Mol. Biol. 2020, 432, 4305–4321. [Google Scholar] [CrossRef]
- Saint-Leandre, B.; Christopher, C.; Levine, M.T. Adaptive evolution of an essential telomere protein restricts telomeric retrotransposons. eLife 2020, 9, e60987. [Google Scholar] [CrossRef]
- Fanti, L.; Giovinazzo, G.; Berloco, M.; Pimpinelli, S. The Heterochromatin protein 1 Prevents Telomere Fusions in Drosophila. Mol. Cell 1998, 2, 527–538. [Google Scholar] [CrossRef]
- Perrini, B.; Piacentini, L.; Fanti, L.; Altieri, F.; Chichiarelli, S.; Berloco, M.; Turano, C.; Ferraro, A.; Pimpinelli, S. HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell 2004, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Casacuberta, E.; Pardue, M.L. HeT-A elements in Drosophila virilis: Retrotransposon telomeres are conserved across the Drosophila genus. Proc. Natl. Acad. Sci. USA 2003, 100, 14091–14096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenaillon, M.I.; Hollister, J.D.; Gaut, B.S. A triptych of the evolution of plant transposable elements. Trends Plant Sci. 2010, 15, 471–478. [Google Scholar] [CrossRef] [PubMed]
- The International Rice Genome Sequencing Project; Sasaki, T. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Bowers, J.E.; Rokhsar, D.S. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajkus, J.; Sýkorová, E.; Leitch, A.R. Telomeres in evolution and evolution of telomeres. Chromosome Res. 2005, 13, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chavan, A.; Palladino, J.; Wei, X.; Martins, N.M.C.; Santinello, B.; Chen, C.; Erceg, J.; Beliveau, B.J.; Wu, C.; et al. Islands of retroelements are major components of Drosophila centromeres. PLoS Biol. 2019, 17, e3000241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueh, A.C.; Northrop, E.L.; Brettingham-Moore, K.H.; Choo, K.H.A.; Wong, L.H. LINE Retrotransposon RNA is an essential structural and functional epigenetic component of a core neocentromeric chromatin. PLoS Genet. 2009, 5, e1000354. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Zhang, J.; Liu, Y.; Feng, C.; Han, F. Back-spliced RNA from retrotransposon binds to centromere and regulates centromeric chromatin loops in maize. PLoS Biol. 2020, 18, e3000582. [Google Scholar] [CrossRef]
- Brown, J.D.; O’Neill, R.J. Chromosomes, conflict, and epigenetics: Chromosomal speciation revisited. Annu. Rev. Genom. Hum. Genet. 2010, 11, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.; O’Neill, R.J. Centromere repeats: Hidden gems of the genome. Genes 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, S.; Koo, H.L.; Jenkins, J.; Rizzo, M.; Rooney, T.; Tallon, L.J.; Feldblyum, T.; Nierman, W.; Benito, M.I.; Lin, X.; et al. Analysis of the genome sequence of the flowering plants Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Agudo, M.; Losada, A.; Abad, J.P.; Pimpinelli, S.; Ripoll, P.; Villasante, A. Centromeres from telomeres? The centromeric region of the Y chromosome of Drosophila melanogaster contains a tandem array of telomeric HeT-A- and TART-related sequences. Nucleic Acids Res. 1999, 27, 3318–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villasante, A.; Abad, J.P.; Planelló, R.; Méndez-Lago, M.; Celniker, S.E.; de Pablos, B. Drosophila telomeric re-trotransposons derived from an ancestral element that was recruited to replace telomerase. Genome Res. 2007, 17, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilder, J.; Hollocher, H. Mobile elements and the genesis of microsatellites in Dipterans. Mol. Biol. Evol. 2001, 18, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.K.; Simmons, M.J. Gross chromosome rearrangements mediated by transposable elements in Drosophila melanogaster. BioEssays 1994, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Ranz, J.M.; Barbadilla, A.; Long, M.; Ruiz, A. Generation of a widespread Drosophila inversion by a transposable element. Science 1999, 285, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Roeder, G.S.; Fink, G.R. Movement of yeast transposable elements by gene conversion. Proc. Natl. Acad. Sci. USA 1988, 79, 5621–5625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakich, D.; Kazazian, H.H.; Antonarakis, S.E.; Gitschier, S. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat. Genet. 1997, 5, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Puig, M.; Ruiz, A. Molecular Characterization of Two Natural Hotspots in the Drosophila buzzatii Genome Induced by Transposon Insertions. Genome Res. 2001, 11, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousios, A.; Nützmann, H.W.; Buck, D.; Michieletto, D. Integrating transposable elements in the 3D genome. Mob. DNA 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.S.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef] [Green Version]
- Ciabrelli, F.; Cavalli, G. Chromatin-driven behavior of topologically associating domains. J. Mol. Biol. 2015, 427, 608–625. [Google Scholar] [CrossRef] [Green Version]
- Penagos-Puig, A.; Furlan-Magaril, M. Heterochromatin as an Important Driver of Genome Organization. Front. Cell. Dev. Biol. 2020, 8, 579137. [Google Scholar] [CrossRef]
- Belton, J.M.; McCord, R.P.; Gibcus, J.H.; Naumova, N.; Zhan, Y.; Dekker, Y. Hi-C: A comprehensive technique to capture the conformation of genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Kruse, K.; Díaz, N.; Enriquez-Gasca, R.E.; Gaume, X.; Torres-Padilla, M.E.; Vaquerizas, J.M. Transposable elements drive reorganisation of 3D chromatin during early embryogenesis. bioRxiv 2019, 1, 523712. [Google Scholar]
- SanMiguel, P.; Gaut, B.S.; Tikhonov, A.; Nakajima, Y.; Bennetzen, J.L. The paleontology of intergene retrotrans-posons of maize. Nat. Genet. 1998, 20, 43–45. [Google Scholar] [CrossRef]
- Buchon, N.; Vaury, C. RNAi: A defensive RNA-silencing against viruses and transposable elements. Heredity 2006, 96, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Castel, S.E.; Martienssen, R.A. RNA interference in the nucleus: Roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Bozzetti, M.P.; Fanti, L.; Di Tommaso, S.; Piacentini, L.; Berloco, M.; Tritto, P.; Specchia, V. The “Special” crystal-Stellate System in Drosophila melanogastert Reveals Mechanisms Underlying piRNA Pathway-Mediated Canalization. Genet. Res. Int. 2012, 2012, 324293. [Google Scholar]
- Specchia, V.; Piacentini, L.; Tritto, P.; Fanti, L.; D’Alessandro, R.; Palumbo, G.; Pimpinelli, S.; Bozzetti, M.P. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 2010, 463, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.; Lu, W.J.; D’Brot, A.; Buszczak, M.; Abrams, J.M. p53 activity is selectively licensed in the Drosophila stem cell compartment. elife 2014, 3, e01530. [Google Scholar] [CrossRef]
- Wylie, A.; Jones, A.E.; D’Brot, A.; Lu, W.J.; Kurtz, P.; Moran, J.V.; Rakheja, D.; Chen, K.S.; Hammer, R.E.; Comerford, S.A.; et al. p53 genes function to restrain mobile elements. Genes Dev. 2016, 30, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Ghildiyal, M.; Seitz, H.; Horwich, M.D.; Li, C.; Du, T.; Lee, S.; Xu, J.; Kittler, E.L.; Zapp, M.L.; Weng, Z. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 2008, 320, 1077–1081. [Google Scholar] [CrossRef] [Green Version]
- Hirochika, H.; Okamoto, H.; Kakutani, T. Silencing of retrotransposons in Arabidopsis and reactivation by the ddm1 mutation. Plant Cell 2000, 12, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Miura, A.; Yonebayashi, S.; Watanabe, K.; Toyama, T.; Shimada, H.; Kakutani, T. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 2001, 411, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.P.; Chaillet, J.R.; Bestor, T.H. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 1998, 20, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, M.C.; Schubeler, D.; Hutchinson, S.R.; Dickerson, D.R.; Groudine, M. DNA methylation density influences the stability of an epigenetic imprint and Dnmt3a/-independent de novo methylation. Mol. Cell. Biol. 2002, 22, 7572–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, M.; Chuma, S.; Tanaka, T.; Franz, T.; Stark, A.; Pillai, R.S. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat. Struct. Mol. Biol. 2009, 16, 639–646. [Google Scholar] [CrossRef]
- Yang, F.; Wang, P.J. Multiple LINEs of retrotransposon silencing mechanisms in the mammalian germline. Semin. Cell Dev. Biol. 2016, 59, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moissiard, G.; Cokus, S.J.; Cary, J.; Feng, S.; Billi, A.C.; Stroud, H.; Husmann, D.; Zhan, Y.; Lajoie, B.R.; McCord, R.P.; et al. MORC Family ATPases Required for Heterochromatin Condensation and Gene Silencing. Science 2012, 336, 1448–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, W.A.; Stroud, H.; Nee, K.; Liu, W.; Pezic, D.; Manakov, S.; Lee, S.A.; Moissiard, G.; Zamudio, N.; Bourc’his, D.; et al. MORC1 represses transposable elements in the mouse male germline. Nat. Commun. 2014, 5, 5795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindič, N.; Budič, M.; Petan, T.; Knisbacher, B.A.; Levanon, E.Y.; Lovšin, N. Differential inhibition of LINE1 and LINE2 retrotransposition by vertebrate AID/APOBEC proteins. Retrovirology 2013, 10, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, T.; Arias, J.F.; Iwabu, Y.; Yokoyama, M.; Fujita, H.; Sato, H.; Tokunaga, K. APOBEC3G Oligomerization is associated with the inhibition of both Alu and LINE-1 retrotransposition. PLoS ONE 2013, 8, e84228. [Google Scholar] [CrossRef]
- Bogerd, H.P.; Wiegand, H.L.; Hulme, A.E.; Garcia-perez, J.L.; O’Shea, K.S.; Moran, J.V.; Cullen, B.R. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. USA 2006, 103, 8780–8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heras, S.R.; Macias, S.; Plass, M.; Fernandez, N.; Cano, D.; Eyras, E.; Garcia-Perez, J.L.; Caceres, J.F. The Micro-processor controls the activity of mammalian retrotransposons. Nat. Struct. Mol. Biol. 2013, 20, 2658. [Google Scholar] [CrossRef] [PubMed]
- Hamdorf, M.; Idica, A.; Zisoulis, D.G.; Gamelin, L.; Martin, C.; sanders, K.J.; Pedersen, I.M. miR-128 represses L1 retrotransposition by binding directly to L1 RNA. Nat. Struct. Mol. Biol. 2015, 22, 3090. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, M.; Kashyap, M.; Rezazadeh, S.; Geneva, A.J.; Morello, T.D.; Seluanov, A.; Gorbunova, V. SIRT6 re-presses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat. Commun. 2014, 5, 5011. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.L.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.K.; Koche, R.P.; et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Maupetit-Mehouas, S.; Vaury, C. Transposon Reactivation in the Germline May Be Useful for Both Transposons and Their Host Genomes. Cells 2020, 9, 1172. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.P.; Ronsseray, S.; Boivin, A. From embryo to adult: piRNA-mediated silencing throughout germline development in Drosophila. G3 Genes Genomes Genet. 2017, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Voronova, A.; Belevich, V.; Jansons, A.; Rungis, D. Stress induced transcriptional activation of retrotransposon-like sequences in the Scots pine (Pinus sylvestris L.) genome. Tree Genet. Genomes 2014, 10, 937–951. [Google Scholar] [CrossRef]
- Ryan, C.P.; Brownlie, J.C.; Whyard, S. Hsp90 and physiological stress are linked to autonomous transposon mobility and heritable genetic change in nematodes. Genome Biol. Evol. 2016, 8, 3794–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera-Soriano, V.; Guerreiro, M. Expression of the retrotransposon Helena reveals a complex pattern of TE de-regulation in Drosophila hybrids. PLoS ONE 2016, 11, e0147903. [Google Scholar]
- Fanti, L.; Piacentini, L.; Cappucci, C.; Casale, A.M.; Pimpinelli, S. Canalization by Selection of de Novo Induced Mutations. Genetics 2017, 206, 1995–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, B.; Hansen, E.C.; Yoveva, A.; Aprile-Garcia, F.; Hussong, R.; Sawarkar, R. The evolutionary capacitor HSP90 buffers the regulatory effects of mammalian endogenous retroviruses. Nat. Struct. Mol. Biol. 2017, 24, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Roquis, D.; Robertson, M.; Yu, L.; Thieme, M.; Julkowska, M.; Bucher, E. Genomic impact of stress-induced trans-posable element mobility in Arabidopsis. Nucleic Acids Res. 2021, 49, 10431–10447. [Google Scholar] [CrossRef]
- Pappalardo, A.M.; Ferrito, V.; Biscotti, M.A.; Canapa, A.; Capriglione, T. Transposable Elements and Stress in Vertebrates: An Overview. Int. J. Mol. Sci. 2021, 22, 1970. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, A.E.; Campagne, P.; Rigden, D.J.; Yung, C.J.; Lingley, J.; Quail, M.A.; Hall, N.; Darby, A.C.; Saccheri, I.J. The industrial melanism mutation in British peppered moths is a transposable element. Nature 2016, 534, 17951. [Google Scholar]
- Hou, J.; Lu, D.; Mason, A.S.; Li, B.; Xiao, M.; Fu, D. Non-coding RNAs and transposable elements in plant genomes: Emergence, regulatory mechanisms and roles in plant development and stress responses. Planta 2019, 250, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, L.; Barreiro, L.; Bourque, G. Transposable elements have contributed human regulatory regions that are activated upon bacterial infection. Phil. Trans. R. Soc. B 2020, 375, 20190332. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, V.A.; McEntee, K. DNA damage activates transcription and transposition of yeast Ty retrotransposons. Mol. Gen. Genet. 1989, 218, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Capy, P.; Gasperi, G.; Biemont, C.; Bazin, C. Stress and transposable elements: Co-evolution or useful parasites? Heredity 2000, 85, 101–106. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakayashiki, H.; Takagi, M.; Tosa, Y.; Mayama, S. Heat shock, copper sulfate and oxidative stress activate the retrotransposon MAGGY resident in the plant pathogenic fungus Magnaporthe grisea. Mol. Genet. Genom. 2001, 266, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sawy, M.; Kale, S.P.; Dugan, C.; Nguyen, T.Q.; Belancio, V.; Bruch, H.; Roy-Engel, A.M.; Deininger, P.L. Nickel stimulates L1 retrotransposition by a post-transcriptional mechanism. J. Mol. Biol. 2005, 354, 246–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, S.P.; Moore, L.; Deininger, P.L.; Roy-Engel, A.M. Heavy metals stimulate human LINE-1 retrotransposition. Int. J. Environ. Res. Public Health 2005, 2, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stribinskis, V.; Ramos, K.S. Activation of human long interspersed nuclear element 1 retrotransposition by ben-zo(a)pyrene, an ubiquitous environmental carcinogen. Cancer Res. 2006, 66, 2616–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoycheva, T.; Pesheva, M.; Venkov, P. The role of reactive oxygen species in the induction of Ty1 retrotranspo-sition in Saccharomyces cerevisiae. Yeast 2010, 27, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakatani, Y.; Hamada, N.; Jinno-Oue, A.; Shimizu, N.; Wada, S.; Funayama, T.; Mori, T.; Islam, S.; Hoque, S.A.; et al. Ionising irradiation alters the dynamics of human long interspersed nuclear elements 1 (LINE1) retrotransposon. Mutagenesis 2012, 27, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jardim, S.S.; Passaglia Schuch, A.; Moura Pereira, C.; Silva Loreto, E.L. Effects of heat and UV radiation on the mo-bilization of transposon mariner-Mos1. Cell Stress Chaperones 2015, 20, 843–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueria de Oliveira, D.; Trindade Rosa, M.; Vieira, C.; Loreto, E.L.S. Oxidative and radiation stress induces trans-posable element transcription in Drosophila melanogaster. J. Evol. Biol. 2021, 34, 628–638. [Google Scholar] [CrossRef]
- Horváth, V.; Merenciano, M.; González, J. Revisiting the relationship between transposable elements and the eukaryotic stress response. Trends in Genetics 2017, 33, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Cappucci, U.; Noro, F.; Casale, A.M.; Fanti, L.; Berloco, M.; Alagia, A.A.; Grassi, L.; Le Pera, L.; Piacentini, L.; Pimpinelli, S. The Hsp70 chaperone is a major player in stress-induced transposable element activation. Proc. Natl. Acad. Sci. USA 2019, 116, 17943–17950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, O.; Danchin, E.; Mirouze, M.; Loot, C.; Blanchet, S. Adaptation to global Change: A Transposable Element-Epigenetics Perspective. Trends Ecol. Evol. 2016, 31, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Forsman, A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, R.J.; Donelson, J.M.; Schunter, C.; Ravasi, T.; Gaitan-Espitia, J.D. Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change. Phil. Trans. R. Soc. B 2019, 374, 20180174. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Arnold, S.J. The Measurement of Selection on Correlated Characters. Evolution 1983, 37, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Karasov, T.L.; Messer, P.W.; Petrov, D.A. Genome-wide Patterns of Adaptation to Temperate Environments Associated with Transposable Elements in Drosophila. PLoS Genet. 2010, 6, e1000905. [Google Scholar]
- Schrader, L.; Schmitz, J. The impact of transposable elements in adaptive evolution. Mol. Ecol. 2018, 28, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.G. Stress, Adaptation, and the Deep Genome: Why Transposons Matter. Integr. Comp. Biol. 2020, 60, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Catlin, N.S.; Josephs, E.B. The important contribution of transposable elements to phenotypic variation and evolution. Curr. Opin. Plant Biol. 2022, 65, 102140. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, L.; Fanti, L.; Specchia, V.; Bozzetti, M.P.; Berloco, M.; Palumbo, G.; Pimpinelli, S. Transposons, environmental changes, and heritable induced phenotypic variability. Chromosoma 2014, 123, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddington, C.H. Canalization of development and the inheritance of acquired characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Waddington, C.H. Genetic assimilation of an acquired character. Evolution 1953, 7, 118–126. [Google Scholar] [CrossRef]
- Waddington, C.H. Canalization of development and genetic assimilation of acquired characters. Nature 1959, 183, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loison, L. Canalization and genetic assimilation: Reassessing the radicality of the Waddingtonian concept of inheritance of acquired characters. Semin. Cell Dev. Biol. 2019, 88, 4–13. [Google Scholar] [CrossRef]
- Spirov, A.V.; Levchenko, V.F.; Sabirov, M.A. Concepts of Canalization and Genetic Assimilation in Deve-lopmental Biology: Current Approaches and Studies. J. Evol. Biochem. Physiol. 2021, 57, 1–15. [Google Scholar] [CrossRef]
- Marzec, S.R.; Pelletier, K.; Chang, A.H.; Dworkin, I. Reexamining Waddington: Canalization and new muta-tions are not required for the evolution of genetic assimilation. bioRxiv 2022, 88, 475581. [Google Scholar]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, Y.C.G. Double-edged sword: The evolutionary consequences of the epigenetic silencing of trans-posable elements. PLoS Genet. 2020, 16, e1008872. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Schock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Lis, J.; Wu, C. Protein traffic on the heat shock promoter: Parking, stalling, and trucking along. Cell 1993, 74, 1–4. [Google Scholar] [CrossRef]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, R. Transcriptional regulation of the metazoan stress protein response. Prog. Nucleic Acid Res. 2004, 78, 143–185. [Google Scholar]
- Feder, J.H.; Rossi, J.M.; Solomon, J.; Solomon, N.; Lindquist, S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992, 6, 1402–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock fac-tors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fablet, M.; Vieirà, C. Evolvability, epigenetics and transposable elements. BioMol. Concepts 2011, 2, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Mourier, T.; Nielsen, L.P.; Hansen, A.J.; Willerslev, E. Transposable elements in cancer as a by-product of stress-induced evolvability. Front. Genet. 2014, 5, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colonna Romano, N.; Fanti, L. Transposable Elements: Major Players in Shaping Genomic and Evolutionary Patterns. Cells 2022, 11, 1048. https://doi.org/10.3390/cells11061048
Colonna Romano N, Fanti L. Transposable Elements: Major Players in Shaping Genomic and Evolutionary Patterns. Cells. 2022; 11(6):1048. https://doi.org/10.3390/cells11061048
Chicago/Turabian StyleColonna Romano, Nunzia, and Laura Fanti. 2022. "Transposable Elements: Major Players in Shaping Genomic and Evolutionary Patterns" Cells 11, no. 6: 1048. https://doi.org/10.3390/cells11061048
APA StyleColonna Romano, N., & Fanti, L. (2022). Transposable Elements: Major Players in Shaping Genomic and Evolutionary Patterns. Cells, 11(6), 1048. https://doi.org/10.3390/cells11061048






