Vav1 Promotes B-Cell Lymphoma Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains
2.2. Mice Genotyping
2.3. Real Time
2.4. Western Blotting
2.5. Analysis of GEF Activity of Vav1
2.6. Histology, Immunohistochemical and Immunofluorescence Analysis
2.7. Histological Analysis
2.8. Statistical Analysis
3. Results
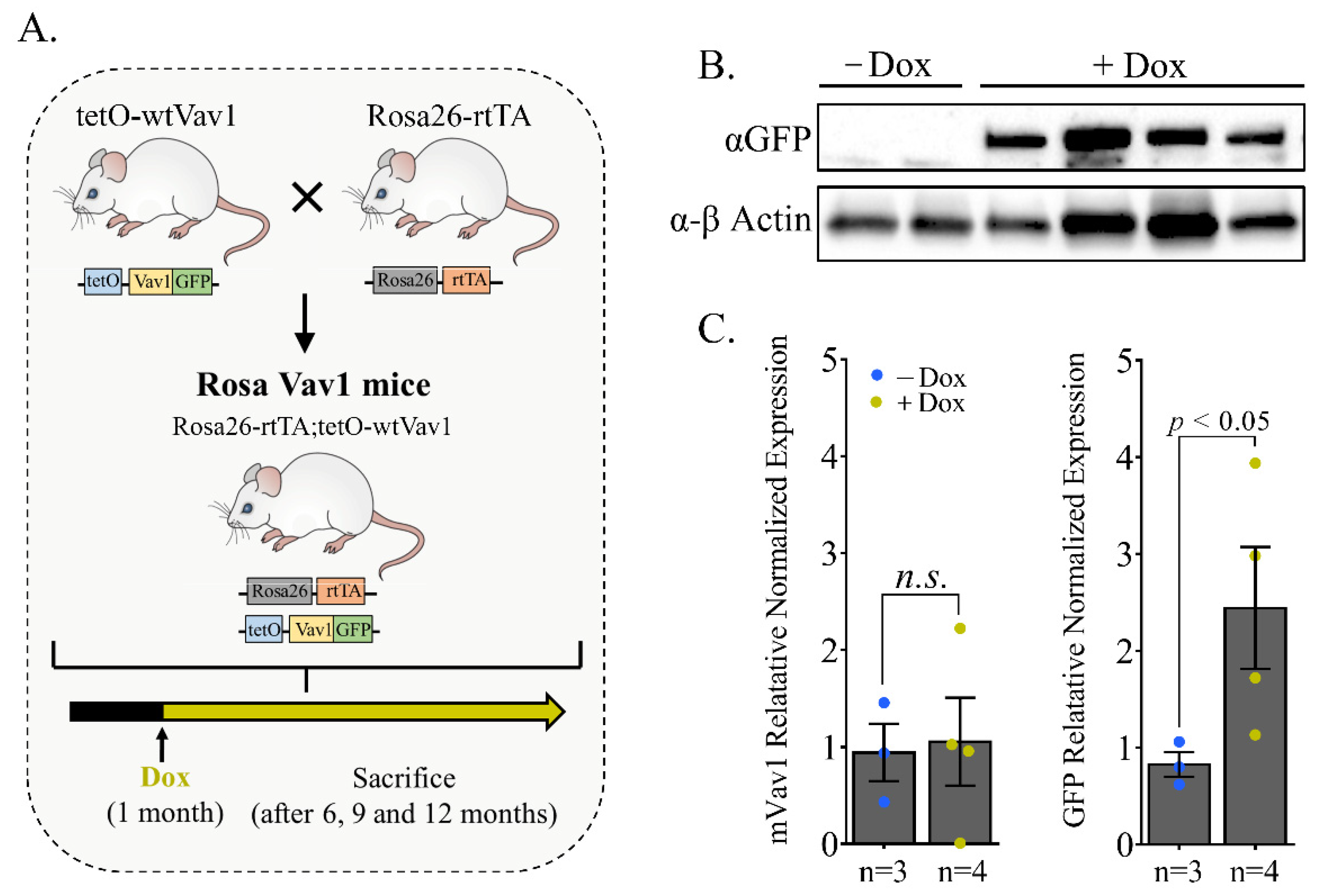
3.1. Generation of a Rosa26 Inducible Vav1 Transgenic Mouse
3.2. Malignant Lesions in Rosa/Vav1 Mice
3.3. Characterization of Lymphomas in Rosa26/Vav1 Transgenic Mice
3.4. Signaling Pathways in Rosa Vav1 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katzav, S.; Martin-Zanca, D.; Barbacid, M. Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989, 8, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Katzav, S. Flesh and blood: The story of Vav1, a gene that signals in hematopoietic cells but can be transforming in human malignancies. Cancer Lett. 2007, 255, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Katzav, S. Vav1: A hematopoietic signal transduction molecule involved in human malignancies. Int. J. Biochem. Cell Biol. 2009, 41, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Tybulewicz, V.L.J. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 2005, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Crespo, P.; Schuebel, K.E.; Ostrom, A.A.; Gutkind, J.S.; Bustelo, X.R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 1997, 385, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.-I.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Abate, F.; da Silva-Almeida, A.C.; Zairis, S.; Robles-Valero, J.; Couronne, L.; Khiabanian, H.; Quinn, S.A.; Kim, M.-Y.; Laginestra, M.A.; Kim, C.; et al. Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral T-cell lymphomas. Proc. Natl. Acad. Sci. USA 2017, 114, 764–769. [Google Scholar] [CrossRef]
- Prieto-Sánchez, R.M.; Hernández, J.A.; García, J.L.; Gutiérrez, N.C.; San Miguel, J.; Bustelo, X.R.; Hernández, J.M. Overexpression of the VAV proto-oncogene product is associated with B-cell chronic lymphocytic leukaemia displaying loss on 13q. Br. J. Haematol. 2006, 133, 642–645. [Google Scholar] [CrossRef]
- Oberley, M.J.; Wang, D.-S.; Yang, D.T. Vav1 in hematologic neoplasms, a mini review. Am. J. Blood Res. 2012, 2, 1–8. [Google Scholar]
- Hornstein, I.; Pikarsky, E.; Groysman, M.; Amir, G.; Peylan-Ramu, N.; Katzav, S. The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J. Pathol. 2003, 199, 526–533. [Google Scholar] [CrossRef]
- Lazer, G.; Idelchuk, Y.; Schapira, V.; Pikarsky, E.; Katzav, S. The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J. Pathol. 2009, 219, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zapico, M.E.; Gonzalez-Paz, N.C.; Weiss, E.; Savoy, D.N.; Molina, J.R.; Fonseca, R.; Smyrk, T.C.; Chari, S.T.; Urrutia, R.; Billadeau, D.D. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 2005, 7, 39–49. [Google Scholar] [CrossRef]
- Sebban, S.; Farago, M.; Gashai, D.; Ilan, L.; Pikarsky, E.; Ben-Porath, I.; Katzav, S. Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PLoS ONE 2013, 8, e54321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Rossi, C.; Perracchio, L.; Mottolese, M.; Marchisio, M.; Palomba, M.; Nika, E.; Natali, P.G.; et al. High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: A role in modulating genes related to the efficiency of metastatic process. Oncotarget 2014, 5, 4320–4336. [Google Scholar] [CrossRef] [PubMed]
- Wakahashi, S.; Sudo, T.; Oka, N.; Ueno, S.; Yamaguchi, S.; Fujiwara, K.; Ohbayashi, C.; Nishimura, R. VAV1 represses E-cadherin expression through the transactivation of Snail and Slug: A potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer. Transl. Res. 2013, 162, 181–190. [Google Scholar] [CrossRef]
- Kniazev, I.P.; Cheburkin, I.V.; Spikermann, K.; Peter, S.; Jenster, G.; Bangma, K.H.; Karelin, M.I.; Shkol’nik, M.I.; Urbanskiĭ, A.I.; Evtushenko, V.I.; et al. Gene expression profiles of protein kinases and phosphatases obtained by hybridization with cDNA arrays: Molecular portrait of human prostate carcinoma. Mol. Biol. 2003, 37, 97–111. [Google Scholar]
- Zhu, X.; Jin, H.; Xia, Z.; Wu, X.; Yang, M.; Zhang, H.; Shang, X.; Cheng, R.; Zhan, Z.; Yu, Z. Vav1 expression is increased in esophageal squamous cell carcinoma and indicates poor prognosis. Biochem. Biophys. Res. Commun. 2017, 486, 571–576. [Google Scholar] [CrossRef]
- Lindsey, J.C.; Kawauchi, D.; Schwalbe, E.C.; Solecki, D.J.; Selby, M.P.; McKinnon, P.J.; Olson, J.M.; Hayden, J.T.; Grundy, R.G.; Ellison, D.W.; et al. Cross-species epigenetics identifies a critical role for VAV1 in SHH subgroup medulloblastoma maintenance. Oncogene 2015, 34, 4746–4757. [Google Scholar] [CrossRef][Green Version]
- Barnes, C.J.; Vadlamudi, R.K.; Kumar, R. Novel estrogen receptor coregulators and signaling molecules in human diseases. Cell. Mol. Life Sci. 2004, 61, 281–291. [Google Scholar] [CrossRef]
- Hornstein, I.; Alcover, A.; Katzav, S. Vav proteins, masters of the world of cytoskeleton organization. Cell. Signal. 2004, 16, 1–11. [Google Scholar] [CrossRef]
- Lazer, G.; Katzav, S. Guanine nucleotide exchange factors for RhoGTPases: Good therapeutic targets for cancer therapy? Cell. Signal. 2011, 23, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Salaymeh, Y.; Farago, M.; Sebban, S.; Shalom, B.; Pikarsky, E.; Katzav, S. Vav1 and mutant K-Ras synergize in the early development of pancreatic ductal adenocarcinoma in mice. Life Sci. Alliance 2020, 3, e202000661. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Sakata-Yanagimoto, M.; Fujisawa, M.; Sakamoto, T.; Miyoshi, H.; Suehara, Y.; Nguyen, T.B.; Suma, S.; Yanagimoto, S.; Shiraishi, Y.; et al. VAV1 mutations contribute to development of T-cell neoplasms in mice. Blood 2020, 136, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Soriano, P.; Friedrich, G.; Lawinger, P. Promoter interactions in retrovirus vectors introduced into fibroblasts and embryonic stem cells. J. Virol. 1991, 65, 2314–2319. [Google Scholar] [CrossRef]
- Casola, S. Mouse models for miRNA expression: The ROSA26 locus. Methods Mol. Biol. 2010, 667, 145–163. [Google Scholar] [CrossRef]
- Hathcock, K.S.; Hirano, H.; Hodes, R.J. CD45 expression by murine B cells and T cells: Alteration of CD45 isoforms in subpopulations of activated B cells. Immunol. Res. 1993, 12, 21–36. [Google Scholar] [CrossRef]
- Villalba, M.; Coudronniere, N.; Deckert, M.; Teixeiro, E.; Mas, P.; Altman, A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity 2000, 12, 151–160. [Google Scholar] [CrossRef]
- Costello, P.S.; Walters, A.E.; Mee, P.J.; Turner, M.; Reynolds, L.F.; Prisco, A.; Sarner, N.; Zamoyska, R.; Tybulewicz, V.L. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc. Natl. Acad. Sci. USA 1999, 96, 3035–3040. [Google Scholar] [CrossRef]
- Lee, I.S.; Liu, Y.; Narazaki, M.; Hibi, M.; Kishimoto, T.; Taga, T. Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6. FEBS Lett. 1997, 401, 133–137. [Google Scholar] [CrossRef]
- Sebban, S.; Farago, M.; Rabinovich, S.; Lazer, G.; Idelchuck, Y.; Ilan, L.; Pikarsky, E.; Katzav, S. Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget 2014, 5, 9214–9226. [Google Scholar] [CrossRef]
- Hollmann, A.; Aloyz, R.; Baker, K.; Dirnhofer, S.; Owens, T.; Sladek, R.; Tzankov, A. Vav-1 expression correlates with NFκB activation and CD40-mediated cell death in diffuse large B-cell lymphoma cell lines. Hematol. Oncol. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wan, Y.; Li, S.; Du, M.; Zhang, C.; Zhou, X.; Cao, Y. The distinct role of guanine nucleotide exchange factor Vav1 in Bcl-2 transcription and apoptosis inhibition in Jurkat leukemia T cells. Acta Pharmacol. Sin. 2011, 32, 99–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertagnolo, V.; Grassilli, S.; Bavelloni, A.; Brugnoli, F.; Piazzi, M.; Candiano, G.; Petretto, A.; Benedusi, M.; Capitani, S. Vav1 modulates protein expression during ATRA-induced maturation of APL-derived promyelocytes: A proteomic-based analysis. J. Proteome Res. 2008, 7, 3729–3736. [Google Scholar] [CrossRef]
- Bertagnolo, V.; Brugnoli, F.; Grassilli, S.; Nika, E.; Capitani, S. Vav1 in differentiation of tumoral promyelocytes. Cell. Signal. 2012, 24, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Lu, P.J.; Ding, L.Y.; Chu, P.C.; Hsu, W.Y.; Chen, C.S.; Tsao, C.C.; Chen, B.H.; Lee, C.T.; Shan, Y.S.; et al. TGFβ promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1. Oncogene 2017, 36, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Ilan, L.; Katzav, S. Human Vav1 expression in hematopoietic and cancer cell lines is regulated by c-Myb and by CpG methylation. PLoS ONE 2012, 7, e29939. [Google Scholar] [CrossRef]
- Zugaza, J.L.; López-Lago, M.A.; Caloca, M.J.; Dosil, M.; Movilla, N.; Bustelo, X.R. Structural determinants for the biological activity of Vav proteins. J. Biol. Chem. 2002, 277, 45377–45392. [Google Scholar] [CrossRef]
- Shalom, B.; Farago, M.; Pikarsky, E.; Katzav, S. Vav1 mutations identified in human cancers give rise to different oncogenic phenotypes. Oncogenesis 2018, 7, 80. [Google Scholar] [CrossRef]
- Strathdee, D.; Ibbotson, H.; Grant, S.G.N. Expression of transgenes targeted to the Gt(ROSA)26Sor locus is orientation dependent. PLoS ONE 2006, 1, e4. [Google Scholar] [CrossRef]
- Feng, Y.-Q.; Warin, R.; Li, T.; Olivier, E.; Besse, A.; Lobell, A.; Fu, H.; Lin, C.M.; Aladjem, M.I.; Bouhassira, E.E. The human beta-globin locus control region can silence as well as activate gene expression. Mol. Cell. Biol. 2005, 25, 3864–3874. [Google Scholar] [CrossRef]
- Mullangi, S.; Lekkala, M.R. Mucosa-associated lymphoma tissue. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Alvarez-Lesmes, J.; Chapman, J.R.; Poveda, J.C. Pitfalls in gastrointestinal tract haematopoietic lesions. Pathology 2021, 54, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanian, A.; Heil, J.R.; Sharma, S. Anatomy, head and neck, adenoids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Farooq, U.; Chakinala, R.C. MALToma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Genco, I.S.; Gur, H.; Hajiyeva, S. Lymphoma of the breast: A clinicopathologic analysis of 51 cases with a specific emphasis on patients with a history of breast carcinoma. Breast J. 2021, 27, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Borie, R.; Wislez, M.; Antoine, M.; Copie-Bergman, C.; Thieblemont, C.; Cadranel, J. Pulmonary mucosa-associated lymphoid tissue lymphoma revisited. Eur. Respir. J. 2016, 48, 1252. [Google Scholar] [CrossRef] [PubMed]
- Rivière, E.; Pascaud, J.; Tchitchek, N.; Boudaoud, S.; Paoletti, A.; Ly, B.; Dupré, A.; Chen, H.; Thai, A.; Allaire, N.; et al. Salivary gland epithelial cells from patients with Sjögren’s syndrome induce B-lymphocyte survival and activation. Ann. Rheum. Dis. 2020, 79, 1468–1477. [Google Scholar] [CrossRef]
- Ahmed, S.; Shahid, R.K.; Sison, C.P.; Fuchs, A.; Mehrotra, B. Orbital lymphomas: A clinicopathologic study of a rare disease. Am. J. Med. Sci. 2006, 331, 79–83. [Google Scholar] [CrossRef]
- Kogame, T.; Kabashima, K.; Egawa, G. Putative Immunological Functions of Inducible Skin-Associated Lymphoid Tissue in the Context of Mucosa-Associated Lymphoid Tissue. Front. Immunol. 2021, 12, 733484. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Jones, B.E.; Maerz, M.D.; Buckner, J.H. IL-6: A cytokine at the crossroads of autoimmunity. Curr. Opin. Immunol. 2018, 55, 9–14. [Google Scholar] [CrossRef]
- Batten, M.; Groom, J.; Cachero, T.G.; Qian, F.; Schneider, P.; Tschopp, J.; Browning, J.L.; Mackay, F. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 2000, 192, 1453–1466. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Gourzi, V.C.; Konsta, O.D.; Baltatzis, G.E.; Tzioufas, A.G. Toll-like receptor 3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway. Clin. Exp. Immunol. 2014, 178, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Ittah, M.; Miceli-Richard, C.; Gottenberg, E.; Lavie, F.; Lazure, T.; Ba, N.; Sellam, J.; Lepajolec, C.; Mariette, X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren’s syndrome. Arthritis Res. Ther. 2006, 8, R51. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.-E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef] [PubMed]
- Kapsogeorgou, E.K.; Manoussakis, M.N. Salivary gland epithelial cells (SGEC): Carriers of exquisite B7-2 (CD86) costimulatory molecules. J. Autoimmun. 2010, 35, 188–191. [Google Scholar] [CrossRef]
- Kim, N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J. Gastroenterol. Hepatol. 2019, 34, 1287–1295. [Google Scholar] [CrossRef]
- Sinkovics, J.G. Molecular biology of oncogenic inflammatory processes. I. Non-oncogenic and oncogenic pathogens, intrinsic inflammatory reactions without pathogens, and microRNA/DNA interactions (Review). Int. J. Oncol. 2012, 40, 305–349. [Google Scholar] [CrossRef]
- Wilsbacher, J.L.; Moores, S.L.; Brugge, J.S. An active form of Vav1 induces migration of mammary epithelial cells by stimulating secretion of an epidermal growth factor receptor ligand. Cell Commun. Signal. 2006, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Schapira, V.; Lazer, G.; Katzav, S. Osteopontin is an oncogenic Vav1- but not wild-type Vav1-responsive gene: Implications for fibroblast transformation. Cancer Res. 2006, 66, 6183–6191. [Google Scholar] [CrossRef][Green Version]
- Lin, E.Y.; Nguyen, A.V.; Russell, R.G.; Pollard, J.W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001, 193, 727–740. [Google Scholar] [CrossRef]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef]
- Stanley, E.R.; Berg, K.L.; Einstein, D.B.; Lee, P.S.; Pixley, F.J.; Wang, Y.; Yeung, Y.G. Biology and action of colony--stimulating factor-1. Mol. Reprod. Dev. 1997, 46, 4–10. [Google Scholar] [CrossRef]
- Achkova, D.; Maher, J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem. Soc. Trans. 2016, 44, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ingman, W.V. Cytokine networks that mediate epithelial cell-macrophage crosstalk in the mammary gland: Implications for development and cancer. J. Mammary Gland Biol. Neoplasia 2014, 19, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, L.J.; Stanley, E.R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J. Cell Biol. 1980, 85, 153–159. [Google Scholar] [CrossRef]
- Mo, H.; Hao, Y.; Lv, Y.; Chen, Z.; Shen, J.; Zhou, S.; Yin, M. Overexpression of macrophage-colony stimulating factor-1 receptor as a prognostic factor for survival in cancer: A systematic review and meta-analysis. Medicine 2021, 100, e25218. [Google Scholar] [CrossRef]
- Dos Anjos Cassado, A. F4/80 as a major macrophage marker: The case of the peritoneum and spleen. Results Probl. Cell Differ. 2017, 62, 161–179. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Roncador, G.; Maestre, L.; Mata, E.; Jiménez, S.; Martínez-Torrecuadrada, J.L.; Reyes-García, A.I.; Rubio, C.; Tomás, J.F.; Estévez, M.; et al. CSF1R protein expression in reactive lymphoid tissues and lymphoma: Its relevance in classical hodgkin lymphoma. PLoS ONE 2015, 10, e0125203. [Google Scholar] [CrossRef]
- Ullrich, K.; Wurster, K.D.; Lamprecht, B.; Köchert, K.; Engert, A.; Dörken, B.; Janz, M.; Mathas, S. BAY 43-9006/Sorafenib blocks CSF1R activity and induces apoptosis in various classical Hodgkin lymphoma cell lines. Br. J. Haematol. 2011, 155, 398–402. [Google Scholar] [CrossRef]
- Lamprecht, B.; Walter, K.; Kreher, S.; Kumar, R.; Hummel, M.; Lenze, D.; Köchert, K.; Bouhlel, M.A.; Richter, J.; Soler, E.; et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat. Med. 2010, 16, 571–579. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.; Rolland, D.C.M.; Polk, A.; Wolfe, A.; Dewar, H.; Chowdhury, P.; Onder, O.; Dewar, R.; Brown, N.A.; Bailey, N.G.; et al. Colony-Stimulating Factor 1 Receptor (CSF1R) Activates AKT/mTOR Signaling and Promotes T-Cell Lymphoma Viability. Clin. Cancer Res. 2020, 26, 690–703. [Google Scholar] [CrossRef]
- Krause, G.; Hassenrück, F.; Hallek, M. Relevant Cytokines in the B Cell Lymphoma Micro-Environment. Cancers 2020, 12, 2525. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalom, B.; Farago, M.; Salaymeh, Y.; Sebban, S.; Pikarsky, E.; Katzav, S. Vav1 Promotes B-Cell Lymphoma Development. Cells 2022, 11, 949. https://doi.org/10.3390/cells11060949
Shalom B, Farago M, Salaymeh Y, Sebban S, Pikarsky E, Katzav S. Vav1 Promotes B-Cell Lymphoma Development. Cells. 2022; 11(6):949. https://doi.org/10.3390/cells11060949
Chicago/Turabian StyleShalom, Batel, Marganit Farago, Yaser Salaymeh, Shulamit Sebban, Eli Pikarsky, and Shulamit Katzav. 2022. "Vav1 Promotes B-Cell Lymphoma Development" Cells 11, no. 6: 949. https://doi.org/10.3390/cells11060949
APA StyleShalom, B., Farago, M., Salaymeh, Y., Sebban, S., Pikarsky, E., & Katzav, S. (2022). Vav1 Promotes B-Cell Lymphoma Development. Cells, 11(6), 949. https://doi.org/10.3390/cells11060949





