Thyroidal Transcriptomic Profiles of Pathoadaptive Responses to Congenital Hypothyroidism in XB130 Knockout Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
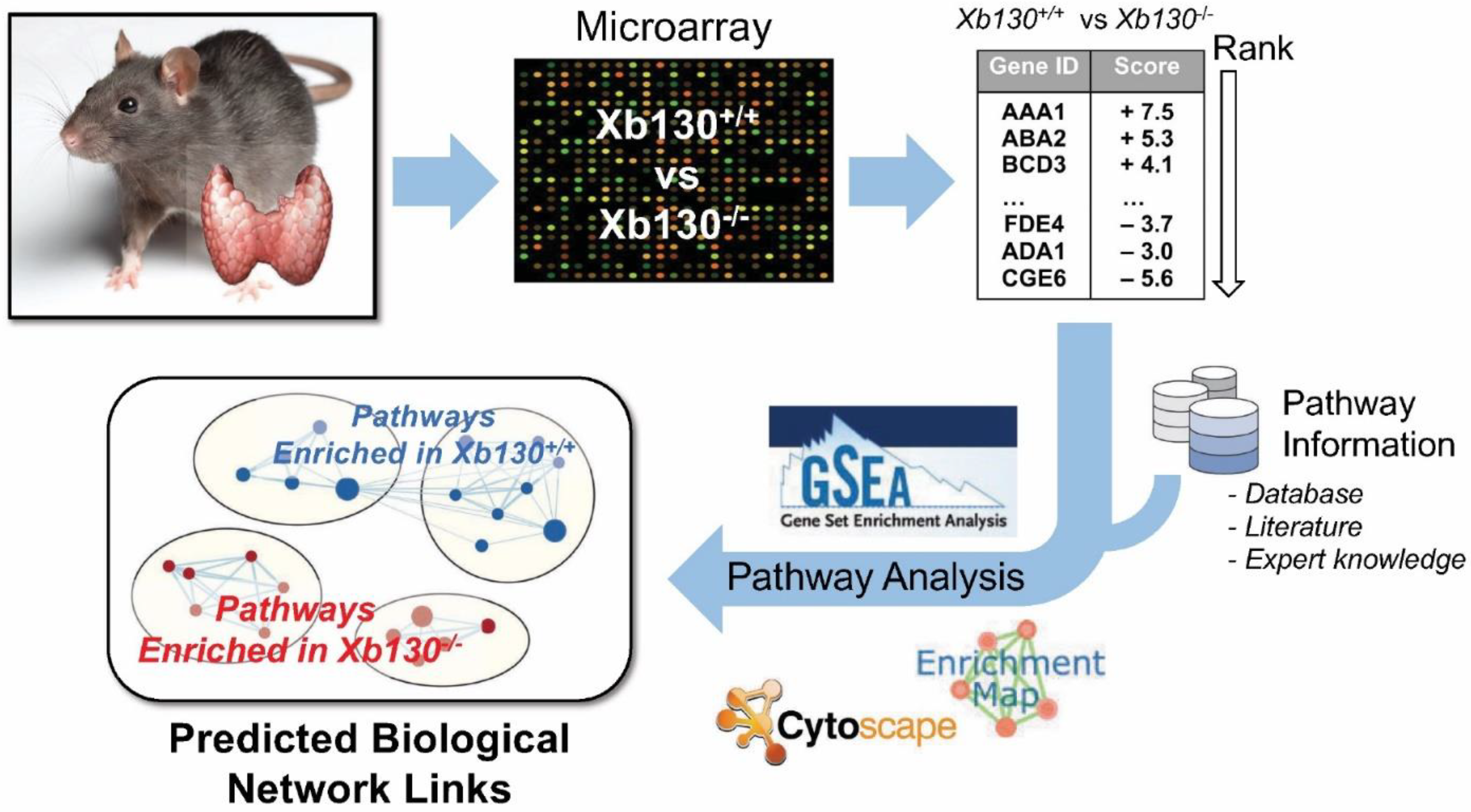
2.2. RNA Extraction and Microarray Analyses
2.3. Gene Set Enrichment Analysis
3. Results
3.1. Transcriptomic Profiling of Thyroid Gland during Postnatal Development
3.2. Downregulated Gene Clusters in Xb130−/− Thyroid Glands at Early Postnatal Stages Are Related to Mitochondrial Energetics
3.3. Upregulation of Gene Clusters Related to Tissue Development in Xb130−/− Thyroid Glands at W4 and W12
3.4. Elevated Inflammatory Response-Related Pathways in Xb130−/− Thyroid Glands at W12
4. Discussion
4.1. Effects of TH Deficiency on Cellular Metabolism in the Thyroid Glands at Early Postnatal Stages
4.2. Compensation for Defects in Thyroid Epithelial Polarity
4.3. Thyroid Gland Growth and Remodeling
4.4. Early Hypothyroid-Triggered Inflammatory Responses in Thyroid Glands
4.5. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassner, A.J. Congenital Hypothyroidism. Clin. Perinatol. 2018, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H.; Refetoff, S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr. Opin. Pediatr. 2011, 23, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, A.C.; Guedes, D.R.; Santos, C.S.; Knobel, M.; Rubio, I.G.S.; Medeiros-Neto, G. Thyroperoxidase gene mutations in congenital goitrous hypothyroidism with total and partial iodide organification defect. Thyroid 2003, 13, 1145–1151. [Google Scholar] [CrossRef]
- Rigutto, S.; Hoste, C.; Grasberger, H.; Milenkovic, M.; Communi, D.; Dumont, J.E.; Corvilain, B.; Miot, F.; De Deken, X. Activation of dual oxidases Duox1 and Duox2: Differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J. Biol. Chem. 2009, 284, 6725–6734. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, E.; Gayral, S.; Massart, C.; Van Sande, J.; Reiter, J.F.; Dumont, J.E.; Robaye, B.; Schurmans, S. Thyroid-specific inactivation of KIF3A alters the TSH signaling pathway and leads to hypothyroidism. J. Mol. Endocrinol. 2013, 50, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Villacorte, M.; Delmarcelle, A.; Lernoux, M.; Bouquet, M.; Lemoine, P.; Bolsée, J.; Umans, L.; de Sousa Lopes, S.C.; Van Der Smissen, P.; Sasaki, T.; et al. Thyroid follicle development requires Smad1/5- and endothelial cell-dependent basement membrane assembly. Development 2016, 143, 1958–1970. [Google Scholar] [PubMed] [Green Version]
- Xu, J.; Bai, X.; Lodyga, M.; Han, B.; Xiao, H.; Keshavjee, S.; Hu, J.; Zhang, H.; Yang, B.B.; Liu, M. XB130, a novel adaptor protein for signal transduction. J. Biol. Chem. 2007, 282, 16401–16412. [Google Scholar] [CrossRef] [Green Version]
- Lodyga, M.; De Falco, V.; Bai, X.H.; Kapus, A.; Melillo, R.M.; Santoro, M.; Liu, M. XB130, a tissue-specific adaptor protein that couples the RET/PTC oncogenic kinase to PI 3-kinase pathway. Oncogene 2009, 28, 937–949. [Google Scholar] [CrossRef]
- Shiozaki, A.; Lodyga, M.; Bai, X.H.; Nadesalingam, J.; Oyaizu, T.; Winer, D.; Asa, S.L.; Keshavjee, S.; Liu, M. XB130, a novel adaptor protein, promotes thyroid tumor growth. Am. J. Pathol. 2011, 178, 391–401. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, Y.; Sujihara, J.; Lu, W.; Liao, X.; Arvan, P.; Refetoff, S.; Liu, M. XB130 plays an essential role in folliculogenesis through mediating interactions between microfilament and microtubule systems in thyrocytes. Thyroid 2022, 32, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shimizu, H.; Xiang, Y.; Sugihara, J.; Lu, W.; Liao, X.; Cho, H.; Toba, H.; Bai, H.X.; Asa, S.L.; et al. XB130 deficiency causes congenital hypothyroidism in mice due to disorganized apical membrane structure and function of thyrocytes. Thyroid 2021, 31, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Sugihara, J.; Wang, Y.; Shimizu, H.; Xiang, Y.; Newbigging, S.; Liao, X.; Asa, S.; Refetoff, S.; Liu, M. Pathogenesis of multinodular goiter in elderly XB130 deficient mice: Alteration of thyroperoxidase affinity with iodide and hydrogen peroxide. Thyroid. 2021. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Zamel, R.; Yeung, J.; Bader, G.D.; Dos Santos, C.C.; Bai, X.; Wang, Y.; Keshavjee, S.; Liu, M. Potential therapeutic targets for lung repair during human ex vivo lung perfusion. Eur. Respir. J. 2020, 55, 1902222. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Wakeham, A.; Hao, Z.; Toba, H.; Bai, X.; Keshavjee, S.; Mak, T.; Liu, M. XB130 deficiency affects tracheal epithelial differentiation during airway repair. PLoS ONE 2014, 9, e108952. [Google Scholar] [CrossRef] [PubMed]
- Toba, H.; Wang, Y.; Bai, X.; Zamel, R.; Cho, H.R.; Liu, H.; Lira, A.; Keshavjee, S.; Liu, M. XB130 promotes bronchioalveolar stem cell and Club cell proliferation in airway epithelial repair and regeneration. Oncotarget 2015, 6, 30803–30817. [Google Scholar] [CrossRef] [PubMed]
- Merico, D.; Isserlin, R.; Stueker, O.; Emili, A.; Bader, G.D. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 2010, 5, e13984. [Google Scholar] [CrossRef]
- Kucera, M.; Isserlin, R.; Arkhangorodsky, A.; Bader, G.D. AutoAnnotate: A Cytoscape app for summarizing networks with semantic annotations. F1000Research 2016, 5, 1717. [Google Scholar] [CrossRef]
- Harper, M.E.; Seifert, E.L. Thyroid hormone effects on mitochondrial energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef]
- van de Weerdt, B.C.; Medema, R.H. Polo-like kinases: A team in control ivision. Cell Cycle. 2006, 5, 853–864. [Google Scholar] [CrossRef]
- Zhang, X.; Kellogg, A.P.; Citterio, C.E.; Zhang, H.; Larkin, D.; Morishita, Y.; Targovnik, H.M.; Balbi, V.A.; Arvan, P. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. JCI Insight 2021, 6, e148496. [Google Scholar] [CrossRef] [PubMed]
- Tata, J.R. Looking for the mechanism of action of thyroid hormone. J. Thyroid Res. 2011, 2011, 730630. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, F.; Senese, R.; Lanni, A.; Goglia, F. Thyroid hormones and mitochondria: With a brief look at derivatives and analogues. Mol. Cell. Endocrinol. 2013, 379, 51–61. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [Green Version]
- Martino, G.; Covello, C.; De Giovanni, R.; Filippelli, R.; Pitrelli, G. Direct in vitro action of thyroid hormones on mitochondrial RNA-polymerase. Mol. Biol. Rep. 1986, 11, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, J.A.; Fernández-Silva, P.; Garrido-Pérez, N.; López-Pérez, M.J.; Pérez-Martos, A.; Montoya, J. Direct regulation of mitochondrial RNA synthesis by thyroid hormone. Mol. Cell. Biol. 1999, 19, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsden, J.D.; Buchanan, M.A.; Egginton, S.; Watkinson, J.C.; Mautner, V.; Eggo, M.C. Complete inhibition of goiter in mice requires combined gene therapy modification of angiopoietin, vascular endothelial growth factor, and fibroblast growth factor signaling. Endocrinology 2005, 146, 2895–2902. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, D.; Akama, T.; Fukushima, T.; Nedachi, T.; Kawasaki, C.; Chida, K.; Minami, S.; Suzuki, K.; Hakuno, F.; Takahashi, S. Phosphatidylinositol 3-kinase-binding protein, PI3KAP/XB130, is required for cAMP-induced amplification of IGF mitogenic activity in FRTL-5 thyroid cells. Mol. Endocrinol. 2012, 26, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsden, J.D. Angiogenesis in the thyroid gland. J. Endocrinol. 2000, 166, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arntzenius, A.B.; Smit, L.J.; Schipper, J.; van der Heide, D.; Meinders, A.E. Inverse relation between iodine intake and thyroid blood flow: Color Doppler flow imaging in euthyroid human. J. Clin. Endocrinol. Metab. 1991, 73, 1051–1055. [Google Scholar] [CrossRef]
- Glaser, C.; Marti, U.; Bürgi-Saville, M.E.; Ruchti, C.; Gebauer, M.; Büchler, M.W.; Gerber, H.; Bürgi, U.; Peter, H.J. Inhibition of iodine organification and regulation of follicular size in rat thyroid tissue in vitro. Endocrine 1999, 11, 165–170. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, J.H.; Haegele, H.; Muller, S.; Anders, H.J. Danger control programs cause tissue injury and remodeling. Int. J. Mol. Sci. 2013, 14, 11319–11346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, O.P.; Lichtnekert, J.; Anders, H.J.; Mulay, S.R. The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is “Inflammation” Always Inflammation? Med. Inflamm. 2016, 2016, 2856213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales, J.J.; Orfao, A.; Miralles, J.M.; López-Berges, M.C.; García, L.C.; González, M.; Mories, M.T.; San Miguel, J. Immunological features of sporadic multinodular goiter. Clin. Investig. 1993, 71, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Toba, H.; Tomankova, T.; Wang, Y.; Bai, X.; Cho, H.R.; Guan, Z.; Adeyi, O.A.; Tian, F.; Keshavjee, S.; Liu, M. XB130 deficiency enhances lipopolysaccharide-induced septic response and acute lung injury. Oncotarget 2016, 7, 25420–25431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.R.; Wang, Y.; Bai, X.; Xiang, Y.Y.; Lu, C.; Post, A.; Al Habeeb, A.; Liu, M. XB130 deficiency enhances carcinogen-induced skin tumorigenesis. Carcinogenesis 2019, 40, 1363–1375. [Google Scholar] [CrossRef] [PubMed]





| Week 2 | ||
| Lipid catabolic process | Fatty acid metabolism | Coenzyme metabolic process |
| Electron transport chain and ATP metabolic process | Tricarboxylic and dicarboxylic acid metabolic process | Pyruvate metabolism and TCA cycle |
| Cellular response to interferon-beta | Triglyceride metabolic process | |
| Signal unattached Mad2 | Generation messenger molecules | |
| Week 4 | ||
| Coenzyme metabolic process | Oxidative phosphorylation | TCA cycle and electron transport chain |
| Mitochondrial translation | tRNA aminoacylation process | Intracellular transmembrane protein |
| Collagen metabolic process | Cell junction assembly | Integrin–cell-surface interaction |
| HS-GAG biosynthesis | PLK1 signaling | Blood coagulation and wound healing |
| Negative adaptive immune | ||
| Week 12 | ||
| Negative regulation of immune response | Antigen receptor-mediated signaling | Regulation of adaptive immune response |
| Regulation of inflammatory response | Activation of T cell and leukocyte | Proliferation of T cell and leukocyte |
| Leukocyte mediated immunity | IFN-γ production | IL-1 production |
| Regulation of cytokine production | Leukocyte neutrophil migration | Cell chemotaxis |
| Exogenous peptide antigen | FC-epsilon receptor signaling | Integrin-linked kinase signaling |
| Integrin–cell-surface interactions | Integrin 2 pathway | Organization of extracellular structure |
| Integrin 5 pathway | TGF beta | Organization of extracellular matrix |
| Integrin 3 pathway | Cell junction assembly | Elastic fiber formation |
| O-linked glycosylation | Collagen formation | Collagen biosynthesis |
| uPA signaling | HS-GAG diseases metabolism | Vasculature development |
| Blood vessel morphogenesis | Wound healing response | Cell–cell adhesion |
| FOXM1 pathway | Aurora A signaling | Aurora B signaling |
| Chromosome segregation | E2F pathway | DNA replication |
| Mitosis | FRA1/2 transcription factor | Chromatid separation |
| PLK1 signaling | Semaphorin interactions | ATR pathway |
| TCR signaling | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugihara, J.; Wong, A.; Shimizu, H.; Zhao, J.; Cho, H.-R.; Wang, Y.; Refetoff, S.; Arvan, P.; Liu, M. Thyroidal Transcriptomic Profiles of Pathoadaptive Responses to Congenital Hypothyroidism in XB130 Knockout Mice. Cells 2022, 11, 975. https://doi.org/10.3390/cells11060975
Sugihara J, Wong A, Shimizu H, Zhao J, Cho H-R, Wang Y, Refetoff S, Arvan P, Liu M. Thyroidal Transcriptomic Profiles of Pathoadaptive Responses to Congenital Hypothyroidism in XB130 Knockout Mice. Cells. 2022; 11(6):975. https://doi.org/10.3390/cells11060975
Chicago/Turabian StyleSugihara, Junichi, Aaron Wong, Hiroki Shimizu, Jinbo Zhao, Hae-Ra Cho, Yingchun Wang, Samuel Refetoff, Peter Arvan, and Mingyao Liu. 2022. "Thyroidal Transcriptomic Profiles of Pathoadaptive Responses to Congenital Hypothyroidism in XB130 Knockout Mice" Cells 11, no. 6: 975. https://doi.org/10.3390/cells11060975






