Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis
Abstract
:1. Osteoarthritis
2. Inflammation—Role in the Pathogenesis of Osteoarthritis
3. Osteoarthritis—The Role of Cytokines and Other Inflammatory Mediators
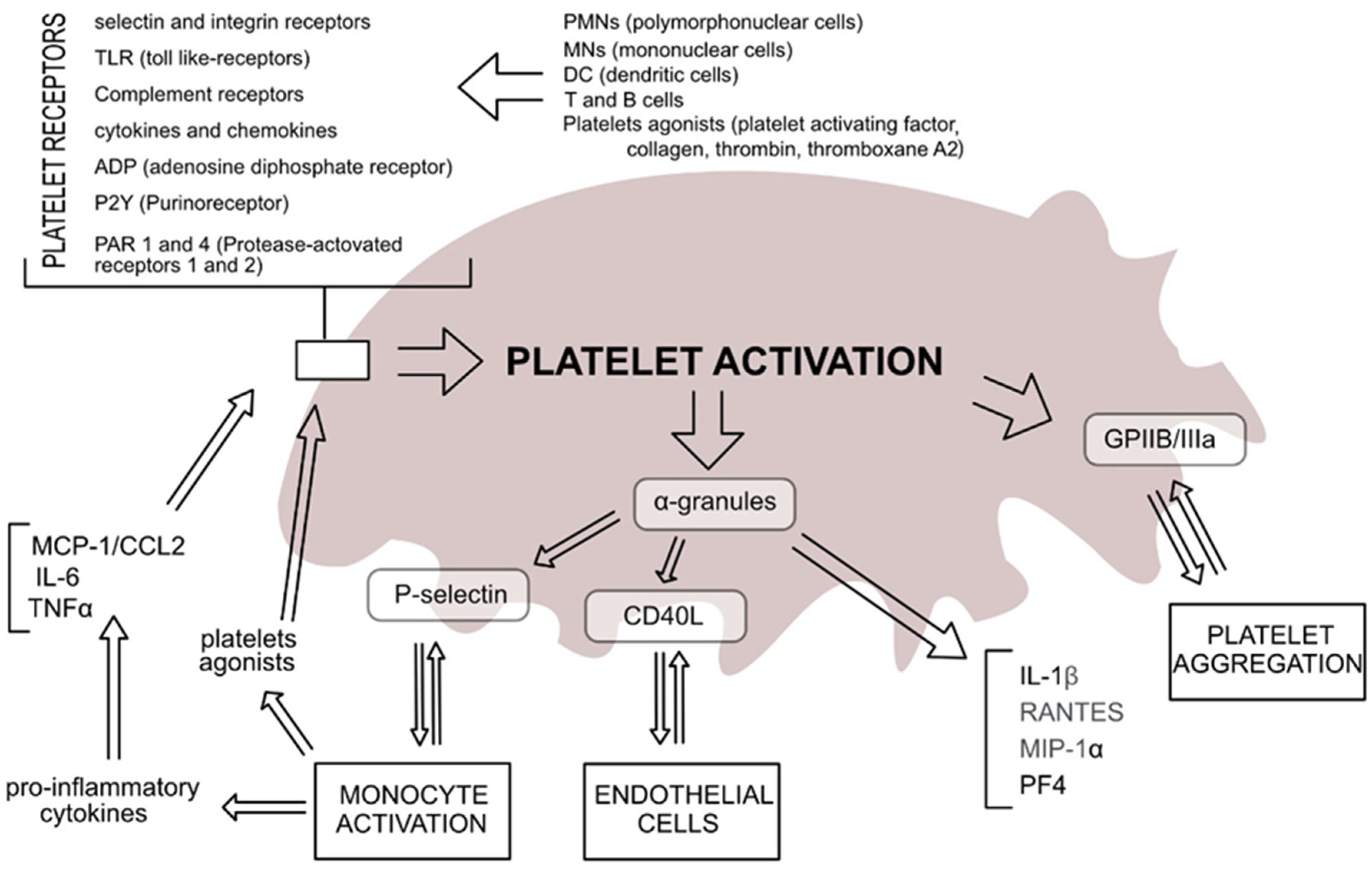
4. Platelets
5. Platelets in OA
6. Platelets as a Treatment for OA
7. Platelets in OA—An Updated Meta-Analysis
8. Materials and Methods
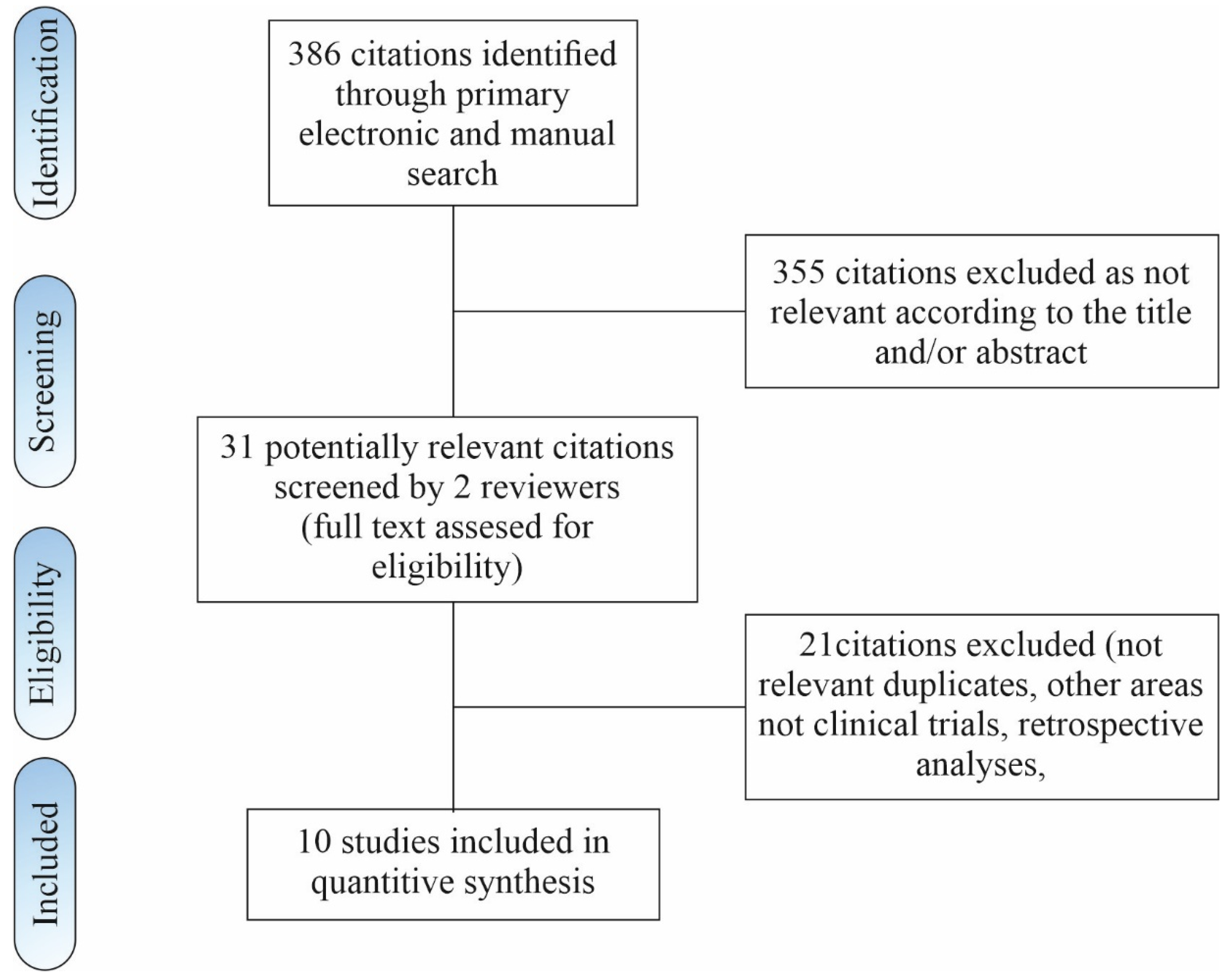
8.1. Search Strategy
8.2. Inclusion and Exclusion Criteria
8.3. Types of Interventions
- -
- Placebo injection (low-volume saline injection, matching the PRP volume);
- -
- High-volume saline image-guided injection;
- -
- Local steroidal injection;
- -
- Hyaluronic acid injection;
- -
- Exercise and other physical therapies (e.g., low-dose radiation therapy, eccentric loading program, dry needling);
- -
- Any other medications administered locally or systemically aimed at treating pain; and
- -
- Combinations of the active interventions listed above.
8.4. Outcomes
- -
- Pain as measured by standard validated pain scale, such as visual analogue score (VAS), EQ-VAS, or numerical rating scale (NRS);
- -
- Functional measurement by any standard validated scale, such as the International Knee Documentation Committee (IKDC), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee Society Score (KSS), Victorian Institute of Sport Assessment (VISA), 36-Item Short Form Survey (SF-36), Knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm Knee Scoring Scale, Tegner Activity Score, and Ikeuchi grade knee rating scale.
8.5. Data Collection and Analysis
8.6. Risk of Bias Assessment
- -
- “Low risk” if all items were scored as “low risk”;
- -
- “Moderate risk” if up to two items were classified as “high risk” or “unclear risk”;
- -
- “High risk” if more than two items were scored as “high risk”.
8.7. Statistical Analysis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, A.E. Osteoarthritis Year in Review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis: Literature Update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the Epidemiology, Risk Factors and Disease Outcomes of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Hurley, M.V.; Scott, D.L.; Rees, J.; Newham, D.J. Sensorimotor Changes and Functional Performance in Patients with Knee Osteoarthritis. Ann. Rheum. Dis. 1997, 56, 641–648. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and Its Management—Epidemiology, Nutritional Aspects and Environmental Factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef]
- Pigeolet, M.; Jayaram, A.; Park, K.B.; Meara, J.G. Osteoarthritis in 2020 and Beyond. Lancet 2021, 397, 1059–1060. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Taruc-Uy, R.L.; Lynch, S.A. Diagnosis and Treatment of Osteoarthritis. Prim. Care Clin. Off. Pract. 2013, 40, 821–836. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.J. Osteoarthritis Year in Review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Tonge, D.P.; Pearson, M.J.; Jones, S.W. The Hallmarks of Osteoarthritis and the Potential to Develop Personalised Disease-Modifying Pharmacological Therapeutics. Osteoarthr. Cartil. 2014, 22, 609–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. JEP 2021, 13, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Yoo, J.; Kim, H. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 30674. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Kraus, V.B. Inflammatory Biomarkers in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, M.H.J.; van Lent, P.L.E.M.; van der Kraan, P.M. Identifying Effector Molecules, Cells, and Cytokines of Innate Immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the Innate Inflammatory Network in Osteoarthritis Progression. Curr. Rheumatol. Rep. 2013, 15, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma Proteins Present in Osteoarthritic Synovial Fluid Can Stimulate Cytokine Production via Toll-like Receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a Central Role for Complement in Osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silawal, S.; Triebel, J.; Bertsch, T.; Schulze-Tanzil, G. Osteoarthritis and the Complement Cascade. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 117954411775143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofat, N. Analysing the Role of Endogenous Matrix Molecules in the Development of Osteoarthritis. Int. J. Exp. Pathol. 2009, 90, 463–479. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Troeberg, L.; Nagase, H. Proteases Involved in Cartilage Matrix Degradation in Osteoarthritis. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2012, 1824, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F. Osteoarthritis Year in Review 2013: Biology. Osteoarthr. Cartil. 2013, 21, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Luyten, F.P.; Tylzanowski, P.; Lories, R.J. Wnt Signaling and Osteoarthritis. Bone 2009, 44, 522–527. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Vitters, E.L.; Bennink, M.B.; van Lent, P.L.E.M.; van Caam, A.P.M.; Blom, A.B.; van den Berg, W.B.; van de Loo, F.A.J.; van der Kraan, P.M. Inducible Chondrocyte-Specific Overexpression of BMP2 in Young Mice Results in Severe Aggravation of Osteophyte Formation in Experimental OA without Altering Cartilage Damage. Ann. Rheum. Dis. 2015, 74, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; Brockbank, S.M.; van Lent, P.L.; van Beuningen, H.M.; Geurts, J.; Takahashi, N.; van der Kraan, P.M.; van de Loo, F.A.; Schreurs, B.W.; Clements, K.; et al. Involvement of the Wnt Signaling Pathway in Experimental and Human Osteoarthritis: Prominent Role of Wnt-Induced Signaling Protein 1. Arthritis Rheum. 2009, 60, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Sanchez, C. Epigenetics, Sirtuins and Osteoarthritis. Jt. Bone Spine 2012, 79, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Tsezou, A. Osteoarthritis Year in Review 2014: Genetics and Genomics. Osteoarthr. Cartil. 2014, 22, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and Epigenetic Regulation in Osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Storey, R. The Role of Platelets in Inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Nurden, A.T. The Biology of the Platelet with Special Reference to Inflammation Wound Healing and Immunity. Front. Biosci. 2018, 23, 726–751. [Google Scholar] [CrossRef] [Green Version]
- Repsold, L.; Joubert, A.M. Platelet Function, Role in Thrombosis, Inflammation, and Consequences in Chronic Myeloproliferative Disorders. Cells 2021, 10, 3034. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed. Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.; Dunbar, M. Platelets and Intravascular Immunity: Guardians of the Vascular Space During Bloodstream Infections and Sepsis. Front. Immunol. 2019, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Platelets in Inflammation and Infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Xiang, S.C.; Rondina, M.T. Platelet Secretion in Inflammatory and Infectious Diseases. Platelets 2017, 28, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Sonmez, M. Role of Platelets in Immune System and Inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Duerschmied, D.; Bode, C.; Ahrens, I. Immune Functions of Platelets. Thromb. Haemost. 2014, 112, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging Roles for Platelets as Immune and Inflammatory Cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [Green Version]
- Herter, J.M.; Rossaint, J.; Zarbock, A. Platelets in Inflammation and Immunity. J. Thromb. Haemost. 2014, 12, 1764–1775. [Google Scholar] [CrossRef]
- Nurden, A. Platelets, Inflammation and Tissue Regeneration. Thromb. Haemost. 2011, 105, S13–S33. [Google Scholar] [CrossRef]
- Andia, I.; Sánchez, M.; Maffulli, N. Joint Pathology and Platelet-Rich Plasma Therapies. Expert Opin. Biol. Ther. 2012, 12, 7–22. [Google Scholar] [CrossRef]
- Alunno, A.; Falcinelli, E.; Luccioli, F.; Petito, E.; Bartoloni, E.; Momi, S.; Mirabelli, G.; Mancini, G.; Gerli, R.; Gresele, P. Platelets Contribute to the Accumulation of Matrix Metalloproteinase Type 2 in Synovial Fluid in Osteoarthritis. Thromb. Haemost. 2017, 117, 2116–2124. [Google Scholar] [CrossRef] [Green Version]
- Gaertner, F.; Massberg, S. Patrolling the Vascular Borders: Platelets in Immunity to Infection and Cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, G.; Bartoloni, E.; Alunno, A.; Bistoni, O.; Cipriani, S.; Topini, F.; Gerli, R. A Platelet’s Guide to Synovitis. Isr. Med. Assoc. J. 2019, 21, 454–459. [Google Scholar] [PubMed]
- Tokarz-Deptuła, B.; Palma, J.; Baraniecki, Ł.; Stosik, M.; Kołacz, R.; Deptuła, W. What Function Do Platelets Play in Inflammation and Bacterial and Viral Infections? Front. Immunol. 2021, 12, 770436. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Koh, I.-H.; Chung, K.; Lee, Y.-J.; Kim, H.-S. Association between Platelet Count and Osteoarthritis in Women Older than 50 Years. Ther. Adv. Musculoskelet. 2020, 12, 1759720X2091286. [Google Scholar] [CrossRef]
- Hall, A.J.; Stubbs, B.; Mamas, M.A.; Myint, P.K.; Smith, T.O. Association between Osteoarthritis and Cardiovascular Disease: Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2016, 23, 938–946. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Taşoğlu, Ö.; Şahin, A.; Karataş, G.; Koyuncu, E.; Taşoğlu, İ.; Tecimel, O.; Özgirgin, N. Blood Mean Platelet Volume and Platelet Lymphocyte Ratio as New Predictors of Hip Osteoarthritis Severity. Medicine 2017, 96, e6073. [Google Scholar] [CrossRef]
- Mabey, T. Cytokines as Biochemical Markers for Knee Osteoarthritis. WJO 2015, 6, 95. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Maślanka, K. Udział Płytek Krwi w Procesach Zapalnych. J. Transfus. Med. 2014, 7, 102–109. [Google Scholar]
- Boilard, E.; Blanco, P.; Nigrovic, P.A. Platelets: Active Players in the Pathogenesis of Arthritis and SLE. Nat. Rev. Rheumatol. 2012, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Harifi, G.; Sibilia, J. Pathogenic Role of Platelets in Rheumatoid Arthritis and Systemic Autoimmune Diseases: Perspectives and Therapeutic Aspects. SMJ 2016, 37, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Olumuyiwa-Akeredolu, O.; Page, M.J.; Soma, P.; Pretorius, E. Platelets: Emerging Facilitators of Cellular Crosstalk in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2019, 15, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Stavropoulos-Kalinoglou, A.; Mikhailidis, D.P.; Douglas, K.M.J.; Kitas, G.D. Platelet Function in Rheumatoid Arthritis: Arthritic and Cardiovascular Implications. Rheumatol. Int. 2011, 31, 153–164. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic Nanoparticles for Inflammation Targeting. Acta Pharm. Sin. B 2018, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.M.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Tunjungputri, R.; Li, Y.; de Groot, P.; Dinarello, C.; Smeekens, S.; Jaeger, M.; Doppenberg-Oosting, M.; Cruijsen, M.; Lemmers, H.; Toenhake-Dijkstra, H.; et al. The Inter-Relationship of Platelets with Interleukin-1β-Mediated Inflammation in Humans. Thromb. Haemost. 2018, 118, 2112–2125. [Google Scholar] [CrossRef]
- Kehrel, B. Platelet-Collagen Interactions. Semin. Thromb. Hemost. 1995, 21, 123–129. [Google Scholar] [CrossRef]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of Platelet Activation and Thrombus Formation by Reactive Oxygen Species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef]
- Riyazi, N.; Slagboom, E.; de Craen, A.J.M.; Meulenbelt, I.; Houwing-Duistermaat, J.J.; Kroon, H.M.; van Schaardenburg, D.; Rosendaal, F.R.; Breedveld, F.C.; Huizinga, T.W.J.; et al. Association of the Risk of Osteoarthritis with High Innate Production of Interleukin-1β and Low Innate Production of Interleukin-10 Ex Vivo, upon Lipopolysaccharide Stimulation. Arthritis Rheum. 2005, 52, 1443–1450. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Fu, Z.; Liu, M. Chondroprotective Effects of Alpha-Lipoic Acid in a Rat Model of Osteoarthritis. Free Radic. Res. 2016, 50, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Characterization of the L-Arginine: Nitric Oxide Pathway in Human Platelets. Br. J. Pharmacol. 1990, 101, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, L.; Ishijima, M.; Kaneko, H.; Kurihara, H.; Arikawa-Hirasawa, E.; Kubota, M.; Liu, L.; Xu, Z.; Futami, I.; Yusup, A.; et al. Correlations between Both the Expression Levels of Inflammatory Mediators and Growth Factor in Medial Perimeniscal Synovial Tissue and the Severity of Medial Knee Osteoarthritis. Int. Orthop. 2011, 35, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Percival, S.S.; Conrad, B.P.; Seay, A.N.; Montero, C.; Vincent, K.R. Hyaluronic Acid (HA) Viscosupplementation on Synovial Fluid Inflammation in Knee Osteoarthritis: A Pilot Study. TOORTHJ 2013, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on Erythrocytes, Platelets and Clot Viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 Trans- and IL-6 Classic Signalling Is Determined by the Ratio of the IL-6 Receptor α to Gp130 Expression: Fusing Experimental Insights and Dynamic Modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senchenkova, E.Y.; Komoto, S.; Russell, J.; Almeida-Paula, L.D.; Yan, L.-S.; Zhang, S.; Granger, D.N. Interleukin-6 Mediates the Platelet Abnormalities and Thrombogenesis Associated with Experimental Colitis. Am. J. Pathol. 2013, 183, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.; Bertoncello, I.; Jackson, H.; Arnold, J.; Kavnoudias, H. The Role of Interleukin 6 in Megakaryocyte Formation, Megakaryocyte Development and Platelet Production. In Novartis Foundation Symposia; Bock, G.R., Marsh, J., Widdows, K., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 160–173. ISBN 978-0-470-51426-9. [Google Scholar]
- Porée, B.; Kypriotou, M.; Chadjichristos, C.; Beauchef, G.; Renard, E.; Legendre, F.; Melin, M.; Gueret, S.; Hartmann, D.-J.; Malléin-Gerin, F.; et al. Interleukin-6 (IL-6) and/or Soluble IL-6 Receptor Down-Regulation of Human Type II Collagen Gene Expression in Articular Chondrocytes Requires a Decrease of Sp1·Sp3 Ratio and of the Binding Activity of Both Factors to the COL2A1 Promoter. J. Biol. Chem. 2008, 283, 4850–4865. [Google Scholar] [CrossRef] [Green Version]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 Is a Significant Predictor of Radiographic Knee Osteoarthritis: The Chingford Study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [Green Version]
- Balbaloglu, O.; Korkmaz, M.; Yolcu, S.; Karaaslan, F.; Beceren, N.G.Ç. Evaluation of Mean Platelet Volume (MPV) Levels in Patients with Synovitis Associated with Knee Osteoarthritis. Platelets 2014, 25, 81–85. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Abbas, H.; Ambrosius, W.; Nicklas, B.J.; Davis, C.; Messier, S.P.; Pahor, M. Inflammatory Markers and Physical Function among Older Adults with Knee Osteoarthritis. J. Rheumatol. 2004, 31, 2027–2031. [Google Scholar] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet Quantification and Growth Factor Analysis from Platelet-Rich Plasma: Implications for Wound Healing. Plast. Reconstr. Surg. 2004, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Evert, P.A. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. CPB 2012, 13, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: An Expert Opinion and Proposal for a Novel Classification and Coding System. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Wanstrath, A.W.; Hettlich, B.F.; Su, L.; Smith, A.; Zekas, L.J.; Allen, M.J.; Bertone, A.L. Evaluation of a Single Intra-Articular Injection of Autologous Protein Solution for Treatment of Osteoarthritis in a Canine Population: Autologous Protein Solution for Treatment of Canine Osteoarthritis. Vet. Surg. 2016, 45, 764–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterman, C.; McCarthy, M.B.R.; Cote, M.P.; Beitzel, K.; Bradley, J.; Polkowski, G.; Mazzocca, A.D. Platelet-Rich Plasma Increases Anti-Inflammatory Markers in a Human Coculture Model for Osteoarthritis. Am. J. Sports Med. 2015, 43, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Joshi Jubert, N.; Rodríguez, L.; Reverté-Vinaixa, M.M.; Navarro, A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop. J. Sports Med. 2017, 5, 232596711668938. [Google Scholar] [CrossRef]
- Re’em, T.; Kaminer-Israeli, Y.; Ruvinov, E.; Cohen, S. Chondrogenesis of HMSC in Affinity-Bound TGF-Beta Scaffolds. Biomaterials 2012, 33, 751–761. [Google Scholar] [CrossRef]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A Review of the Effects of Insulin-like Growth Factor and Platelet Derived Growth Factor on in Vivo Cartilage Healing and Repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 Enhances the Mitotic and Chondrogenic Potentials of Human Adult Bone Marrow-Derived Mesenchymal Stem Cells. J. Cell. Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef]
- Garbin, L.C.; Olver, C.S. Platelet-Rich Products and Their Application to Osteoarthritis. J. Equine Vet. Sci. 2020, 86, 102820. [Google Scholar] [CrossRef] [PubMed]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The Anti-Inflammatory and Matrix Restorative Mechanisms of Platelet-Rich Plasma in Osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lacko, M.; Harvanová, D.; Slovinská, L.; Matuška, M.; Balog, M.; Lacková, A.; Špaková, T.; Rosocha, J. Effect of Intra-Articular Injection of Platelet-Rich Plasma on the Serum Levels of Osteoarthritic Biomarkers in Patients with Unilateral Knee Osteoarthritis. JCM 2021, 10, 5801. [Google Scholar] [CrossRef]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef]
- Trams, E.; Kulinski, K.; Kozar-Kaminska, K.; Pomianowski, S.; Kaminski, R. The Clinical Use of Platelet-Rich Plasma in Knee Disorders and Surgery—A Systematic Review and Meta-Analysis. Life 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, M.; Vílchez-Cavazos, J.F.; Peña-Martínez, V.M.; Said-Fernández, S.; Lara-Arias, J.; Martínez-Rodríguez, H.G. Leukocyte-Poor Platelet-Rich Plasma Is More Effective than the Conventional Therapy with Acetaminophen for the Treatment of Early Knee Osteoarthritis. Arch. Orthop. Trauma Surg. 2016, 136, 1723–1732. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Clinical Outcomes of Knee Osteoarthritis Treated With an Autologous Protein Solution Injection: A 1-Year Pilot Double-Blinded Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 171–180. [Google Scholar] [CrossRef]
- Elik, H.; Doğu, B.; Yılmaz, F.; Begoğlu, F.A.; Kuran, B. The Efficiency of Platelet-Rich Plasma Treatment in Patients with Knee Osteoarthritis. J. Back Musculoskelet. Rehabil. 2020, 33, 127–138. [Google Scholar] [CrossRef]
- Uslu Güvendi, E.; Aşkin, A.; Güvendi, G.; Koçyiğit, H. Comparison of Efficiency Between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients with Knee Osteoarthritis. Arch. Rheumatol. 2018, 33, 273–281. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Di Martino, A.; Merli, M.L.; Cenacchi, A.; Fornasari, P.; Marcacci, M.; Kon, E. Platelet-Rich Plasma Intra-Articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1575–1582. [Google Scholar] [CrossRef]
- Paterson, K.L.; Nicholls, M.; Bennell, K.L.; Bates, D. Intra-Articular Injection of Photo-Activated Platelet-Rich Plasma in Patients with Knee Osteoarthritis: A Double-Blind, Randomized Controlled Pilot Study. BMC Musculoskelet. Disord. 2016, 17, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duymus, T.M.; Mutlu, S.; Dernek, B.; Komur, B.; Aydogmus, S.; Kesiktas, F.N. Choice of Intra-Articular Injection in Treatment of Knee Osteoarthritis: Platelet-Rich Plasma, Hyaluronic Acid or Ozone Options. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-Articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rayegani, S.M.; Raeissadat, S.A.; Sanei Taheri, M.; Babaee, M.; Bahrami, M.H.; Eliaspour, D.; Ghorbani, E. Does Intra Articular Platelet Rich Plasma Injection Improve Function, Pain and Quality of Life in Patients with Osteoarthritis of the Knee? A Randomized Clinical Trial. Orthop. Rev. 2014, 6, 5405. [Google Scholar] [CrossRef] [Green Version]
- Louis, M.L.; Magalon, J.; Jouve, E.; Bornet, C.E.; Mattei, J.C.; Chagnaud, C.; Rochwerger, A.; Veran, J.; Sabatier, F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared With Viscosupplementation. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1530–1540.e2. [Google Scholar] [CrossRef]
- Ahmad, H.S.; Farrag, S.E.; Okasha, A.E.; Kadry, A.O.; Ata, T.B.; Monir, A.A.; Shady, I. Clinical Outcomes Are Associated with Changes in Ultrasonographic Structural Appearance after Platelet-Rich Plasma Treatment for Knee Osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 960–966. [Google Scholar] [CrossRef]
- Su, K.; Bai, Y.; Wang, J.; Zhang, H.; Liu, H.; Ma, S. Comparison of Hyaluronic Acid and PRP Intra-Articular Injection with Combined Intra-Articular and Intraosseous PRP Injections to Treat Patients with Knee Osteoarthritis. Clin. Rheumatol. 2018, 37, 1341–1350. [Google Scholar] [CrossRef]
- Lisi, C.; Perotti, C.; Scudeller, L.; Sammarchi, L.; Dametti, F.; Musella, V.; Di Natali, G. Treatment of Knee Osteoarthritis: Platelet-Derived Growth Factors vs. Hyaluronic Acid. A Randomized Controlled Trial. Clin. Rehabil. 2018, 32, 330–339. [Google Scholar] [CrossRef]
- Buendía-López, D.; Medina-Quirós, M.; Fernández-Villacañas Marín, M.Á. Clinical and Radiographic Comparison of a Single LP-PRP Injection, a Single Hyaluronic Acid Injection and Daily NSAID Administration with a 52-Week Follow-up: A Randomized Controlled Trial. J. Orthop. Traumatol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Rahimzadeh, P.; Imani, F.; Faiz, S.H.R.; Entezary, S.R.; Narimani Zamanabadi, M.; Alebouyeh, M.R. The Effects of Injecting Intra-Articular Platelet-Rich Plasma or Prolotherapy on Pain Score and Function in Knee Osteoarthritis. CIA 2018, 13, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Montañez-Heredia, E.; Irízar, S.; Huertas, P.; Otero, E.; del Valle, M.; Prat, I.; Díaz-Gallardo, M.; Perán, M.; Marchal, J.; Hernandez-Lamas, M. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int. J. Mol. Sci. 2016, 17, 71064. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich Plasma Shows Beneficial Effects for Patients with Knee Osteoarthritis by Suppressing Inflammatory Factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardo, G.; Kon, E.; Di Martino, A.; Di Matteo, B.; Merli, M.L.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-Rich Plasma vs Hyaluronic Acid to Treat Knee Degenerative Pathology: Study Design and Preliminary Results of a Randomized Controlled Trial. BMC Musculoskelet. Disord. 2012, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeissadat, S.A.; Rayegani, S.M.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) versus Hyaluronic Acid (A One-Year Randomized Clinical Trial). Clin. Med. Insights Arthritis Musculoskelet Disord. 2015, 8, CMAMD.S17894. [Google Scholar] [CrossRef] [PubMed]
- Spaková, T.; Rosocha, J.; Lacko, M.; Harvanová, D.; Gharaibeh, A. Treatment of Knee Joint Osteoarthritis with Autologous Platelet-Rich Plasma in Comparison with Hyaluronic Acid. Am. J. Phys. Med. Rehabil. 2012, 91, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment With Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Janmohammadi, N.; Hosseini, A.; Khafri, S.; Esmaeilnejad-Ganji, S.M. Single- and Double-Dose of Platelet-Rich Plasma versus Hyaluronic Acid for Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. WJO 2019, 10, 310–326. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Hsu, K.-C.; Li, T.-Y.; Chang, C.-K.; Chen, L.-C. Effects of Platelet-Rich Plasma on Pain and Muscle Strength in Patients With Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2018, 97, 248–254. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Yang, C.-C.; Hsu, C.-J.; Yeh, M.-L.; Renn, J.-H. Intra-Articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 106–117. [Google Scholar] [CrossRef]
- Yu, W.; Xu, P.; Huang, G.; Liu, L. Clinical Therapy of Hyaluronic Acid Combined with Platelet-rich Plasma for the Treatment of Knee Osteoarthritis. Exp. Ther. Med. 2018, 16, 2119–2125. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; He, Z.; Shu, L.; Li, X.; Ma, M.; Ye, C. Intra-Articular Platelet-Rich Plasma Combined With Hyaluronic Acid Injection for Knee Osteoarthritis Is Superior to Platelet-Rich Plasma or Hyaluronic Acid Alone in Inhibiting Inflammation and Improving Pain and Function. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Goetz, L.L.; Duncan, M.B.; Gilman, J.B.; Elmore, L.W.; Sell, S.A.; McClure, M.J.; Quagliano, P.V.; Martin, C.C. Randomized, Placebo-Controlled Analysis of the Knee Synovial Environment Following Platelet-Rich Plasma Treatment for Knee Osteoarthritis. PM&R 2021, 13, 707–719. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Gharooee Ahangar, A.; Rayegani, S.M.; Minator Sajjadi, M.; Ebrahimpour, A.; Yavari, P. Platelet-Rich Plasma-Derived Growth Factor vs Hyaluronic Acid Injection in the Individuals with Knee Osteoarthritis: A One Year Randomized Clinical Trial. JPR 2020, 13, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-B.; Kim, J.-H.; Ha, C.-W.; Lee, D.-H. Clinical Efficacy of Platelet-Rich Plasma Injection and Its Association With Growth Factors in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized Double-Blind Controlled Clinical Trial As Compared With Hyaluronic Acid. Am. J. Sports Med. 2021, 49, 487–496. [Google Scholar] [CrossRef]
- Dulic, O.; Rasovic, P.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Maric, D.; Miskulin, M.; Bumbasirevic, M. Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Medicina 2021, 57, 1193. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Ghazi Hosseini, P.; Bahrami, M.H.; Salman Roghani, R.; Fathi, M.; Gharooee Ahangar, A.; Darvish, M. The Comparison Effects of Intra-Articular Injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and Ozone in Knee Osteoarthritis; a One Year Randomized Clinical Trial. BMC Musculoskelet. Disord. 2021, 22, 134. [Google Scholar] [CrossRef]
- Elksniņš-Finogejevs, A.; Vidal, L.; Peredistijs, A. Intra-Articular Platelet-Rich Plasma vs Corticosteroids in the Treatment of Moderate Knee Osteoarthritis: A Single-Center Prospective Randomized Controlled Study with a 1-Year Follow Up. J. Orthop. Surg. Res. 2020, 15, 257. [Google Scholar] [CrossRef]
- Pishgahi, A.; Abolhasan, R.; Shakouri, S.K.; Soltani-Zangbar, M.S.; Dareshiri, S.; Ranjbar Kiyakalayeh, S.; Khoeilar, A.; Zamani, M.; Motavalli Khiavi, F.; Pourabbas Kheiraddin, B.; et al. Effect of Dextrose Prolotherapy, Platelet Rich Plasma and Autologous Conditioned Serum on Knee Osteoarthritis: A Randomized Clinical Trial. IJAAI 2020. [Google Scholar] [CrossRef]
- Dório, M.; Pereira, R.M.R.; Luz, A.G.B.; Deveza, L.A.; de Oliveira, R.M.; Fuller, R. Efficacy of Platelet-Rich Plasma and Plasma for Symptomatic Treatment of Knee Osteoarthritis: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. BMC Musculoskelet. Disord. 2021, 22, 822. [Google Scholar] [CrossRef]
- Kesiktas, F.N.; Dernek, B.; Sen, E.I.; Albayrak, H.N.; Aydin, T.; Yildiz, M. Comparison of the Short-Term Results of Single-Dose Intra-Articular Peptide with Hyaluronic Acid and Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Randomized Study. Clin. Rheumatol. 2020, 39, 3057–3064. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee. J. Stem. Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.J.F.C.; Schott, V.; Balduino, A.; Bastos, R.; Miguel Oliveira, J.; Reis, R.L.; Rodeo, S.; et al. Intra-Articular Injection of Culture-Expanded Mesenchymal Stem Cells with or without Addition of Platelet-Rich Plasma Is Effective in Decreasing Pain and Symptoms in Knee Osteoarthritis: A Controlled, Double-Blind Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-Articular Injections of Expanded Mesenchymal Stem Cells with and without Addition of Platelet-Rich Plasma Are Safe and Effective for Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef]
- Görmeli, G.; Görmeli, C.A.; Ataoglu, B.; Çolak, C.; Aslantürk, O.; Ertem, K. Multiple PRP Injections Are More Effective than Single Injections and Hyaluronic Acid in Knees with Early Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kavadar, G.; Demircioglu, D.T.; Celik, M.Y.; Emre, T.Y. Effectiveness of Platelet-Rich Plasma in the Treatment of Moderate Knee Osteoarthritis: A Randomized Prospective Study. J. Phys. Ther. Sci. 2015, 27, 3863–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simental-Mendía, M.; Acosta-Olivo, C.A.; Hernández-Rodríguez, A.N.; Santos-Santos, O.R.; de la Garza-Castro, S.; Peña-Martínez, V.M.; Vilchez-Cavazos, F. Intraarticular Injection of Platelet-Rich Plasma in Knee Osteoarthritis: Single versus Triple Application Approach. Pilot Study. Acta Reum. Port. 2019, 44, 138–144. [Google Scholar]
- Raeissadat, S.A.; Rayegani, S.M.; Ahangar, A.G.; Abadi, P.H.; Mojgani, P.; Ahangar, O.G. Efficacy of Intra-Articular Injection of a Newly Developed Plasma Rich in Growth Factor (PRGF) Versus Hyaluronic Acid on Pain and Function of Patients with Knee Osteoarthritis: A Single-Blinded Randomized Clinical Trial. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2017, 10, 117954411773345. [Google Scholar] [CrossRef]
- Vaquerizo, V.; Plasencia, M.Á.; Arribas, I.; Seijas, R.; Padilla, S.; Orive, G.; Anitua, E. Comparison of Intra-Articular Injections of Plasma Rich in Growth Factors (PRGF-Endoret) Versus Durolane Hyaluronic Acid in the Treatment of Patients with Symptomatic Osteoarthritis: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1635–1643. [Google Scholar] [CrossRef]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Aduriz Recalde, E.; Garcia Gutierrez, A.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1070–1078. [Google Scholar] [CrossRef]
- Smith, P.A. Intra-Articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Sports Med. 2016, 44, 884–891. [Google Scholar] [CrossRef]
- Cerza, F.; Carnì, S.; Carcangiu, A.; Di Vavo, I.; Schiavilla, V.; Pecora, A.; De Biasi, G.; Ciuffreda, M. Comparison Between Hyaluronic Acid and Platelet-Rich Plasma, Intra-Articular Infiltration in the Treatment of Gonarthrosis. Am. J. Sports Med. 2012, 40, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Available online: www.training.cochrane.org/handbook (accessed on 10 January 2022).
- R Core Team A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 10 January 2022).
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).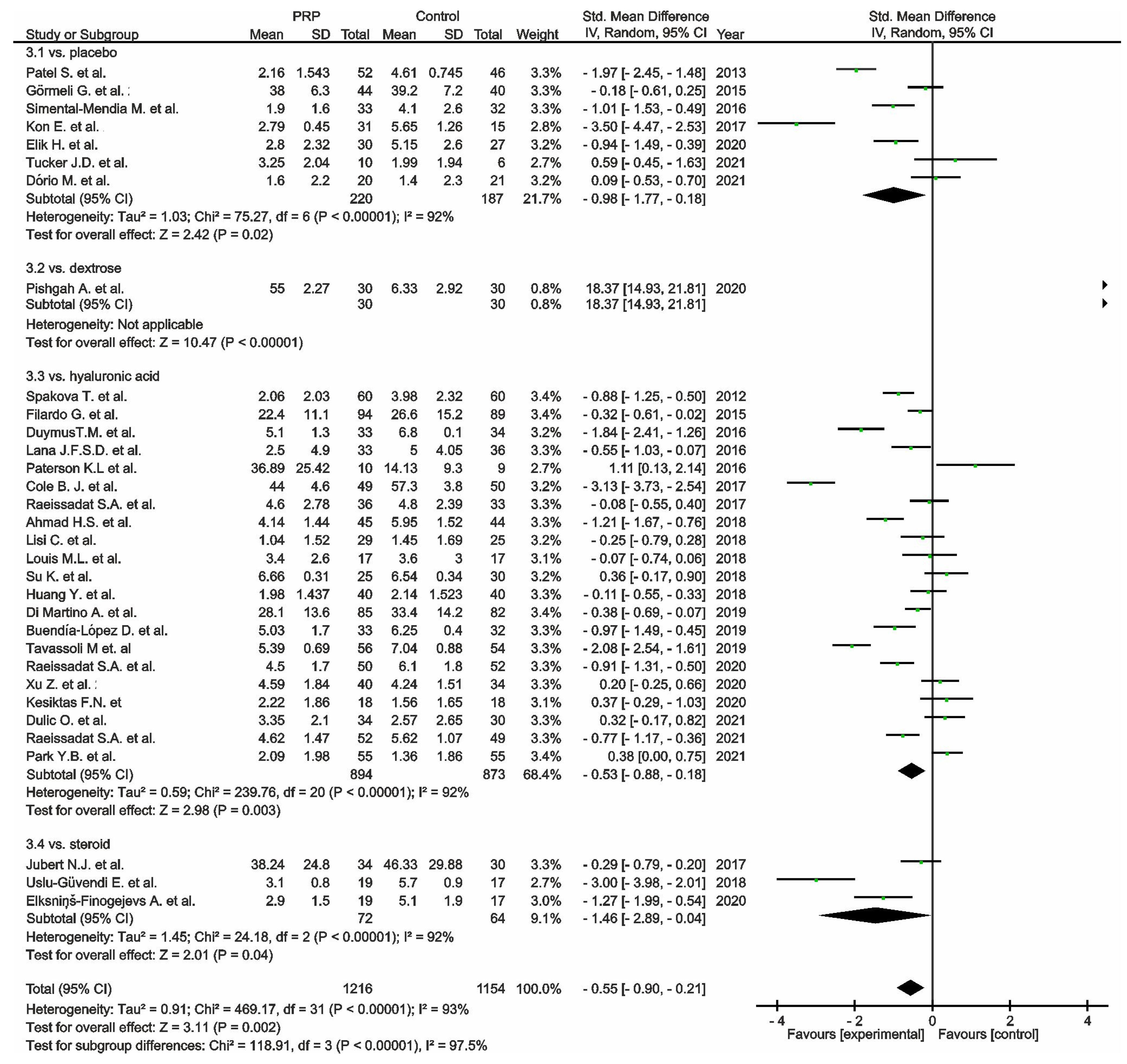
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).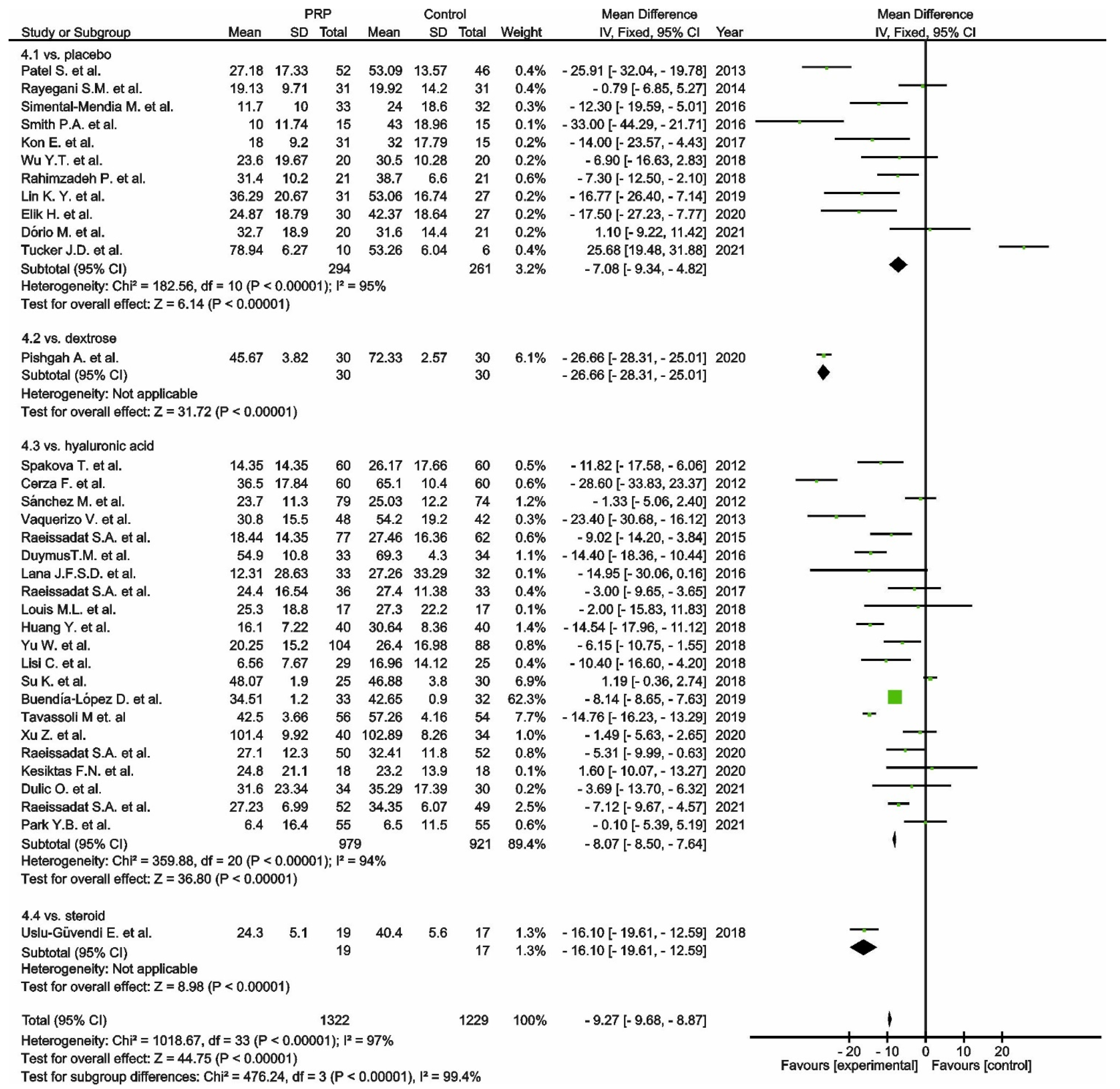
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).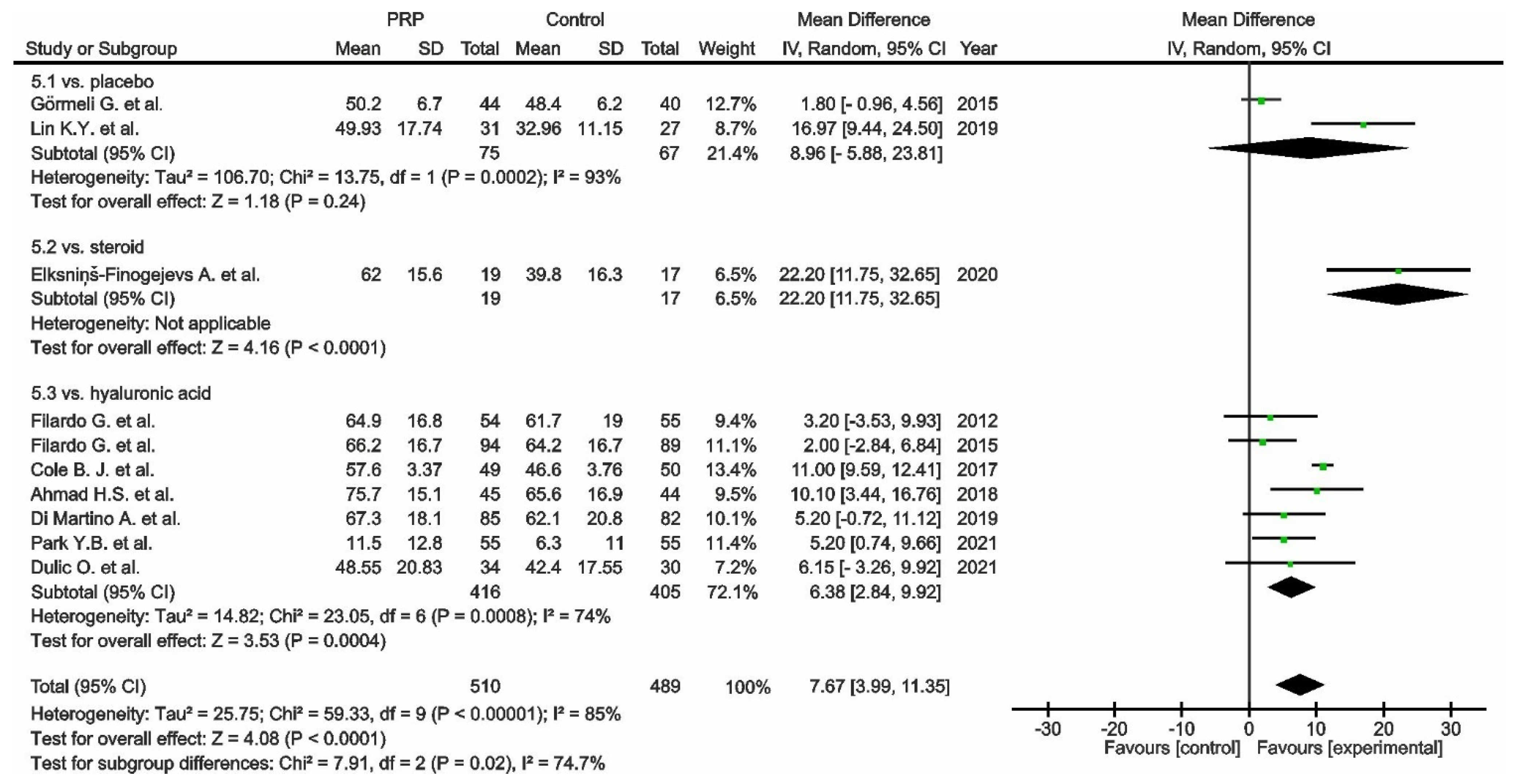
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
, mean with 95% confidence interval for source data).
 , mean with 95% confidence interval for total and subtotals;
, mean with 95% confidence interval for total and subtotals;  , mean with 95% confidence interval for source data).
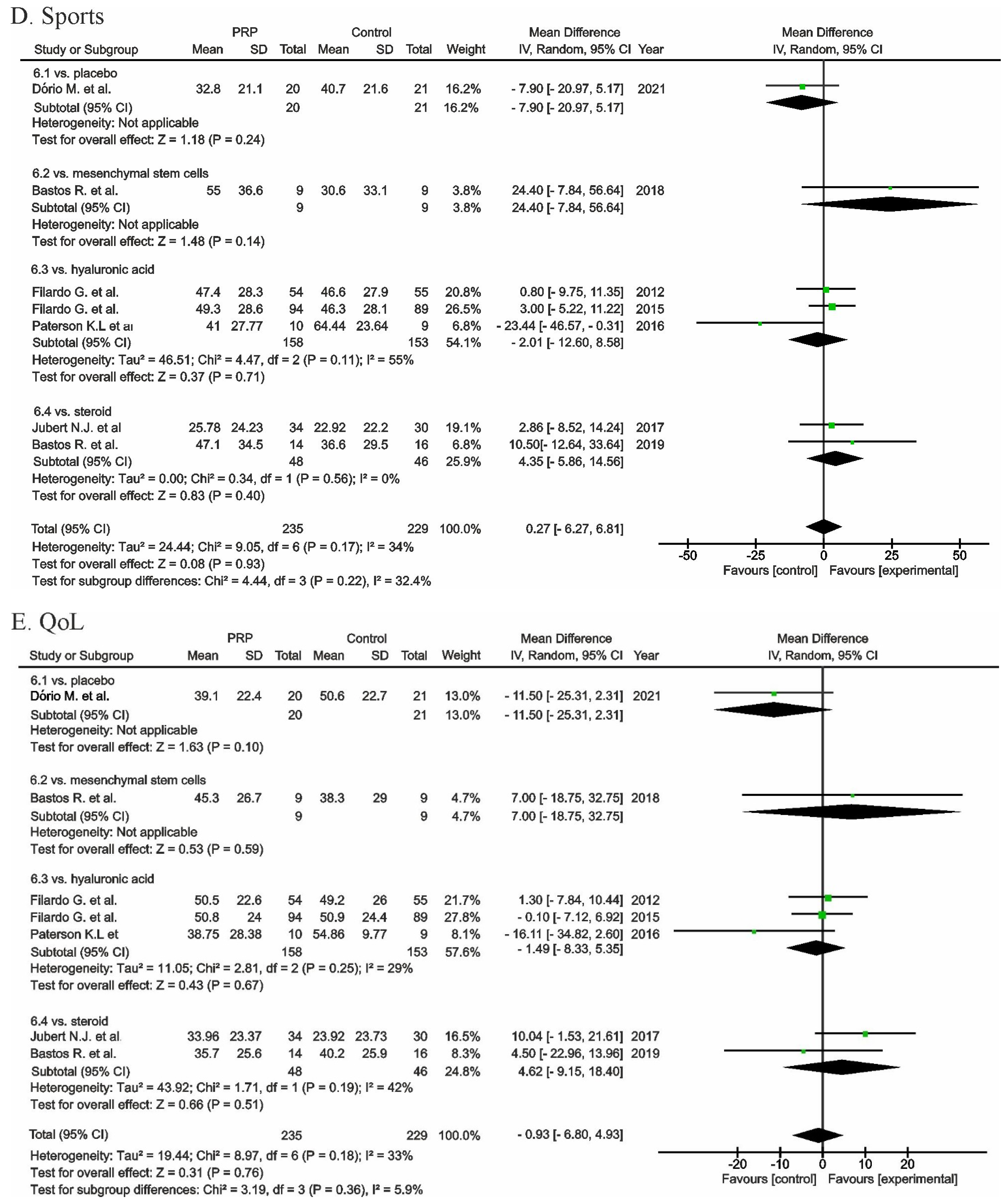
, mean with 95% confidence interval for source data).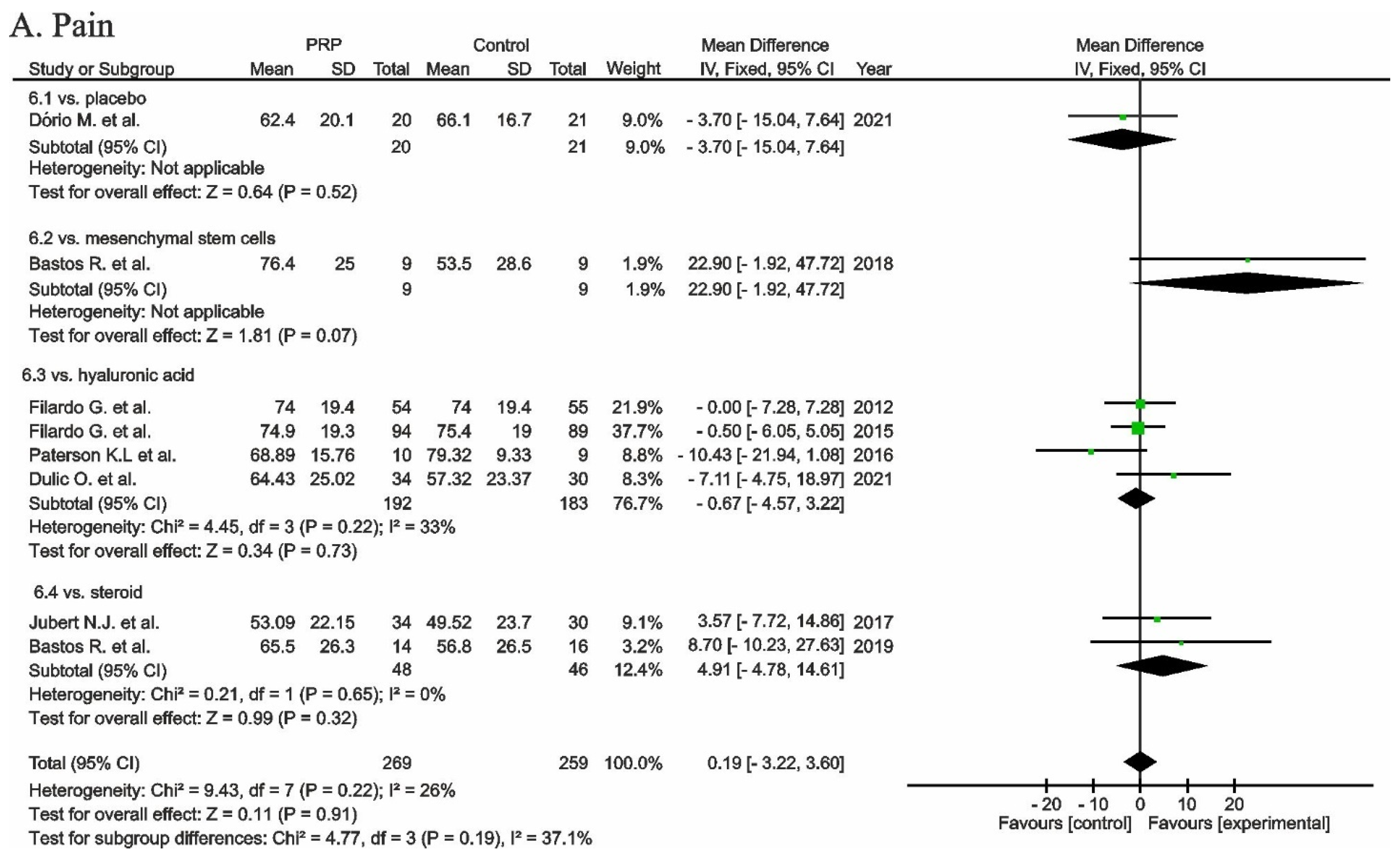

 , odds ratio with 95% confidence interval for total and subtotals;
, odds ratio with 95% confidence interval for total and subtotals;  , odds ratio with 95% confidence interval for source data).
, odds ratio with 95% confidence interval for source data).
 , odds ratio with 95% confidence interval for total and subtotals;
, odds ratio with 95% confidence interval for total and subtotals;  , odds ratio with 95% confidence interval for source data).
, odds ratio with 95% confidence interval for source data).
 , low risk of bias;
, low risk of bias;  , unclear risk of bias;
, unclear risk of bias;  , high risk of bias).
, high risk of bias).
 , low risk of bias;
, low risk of bias;  , unclear risk of bias;
, unclear risk of bias;  , high risk of bias).
, high risk of bias).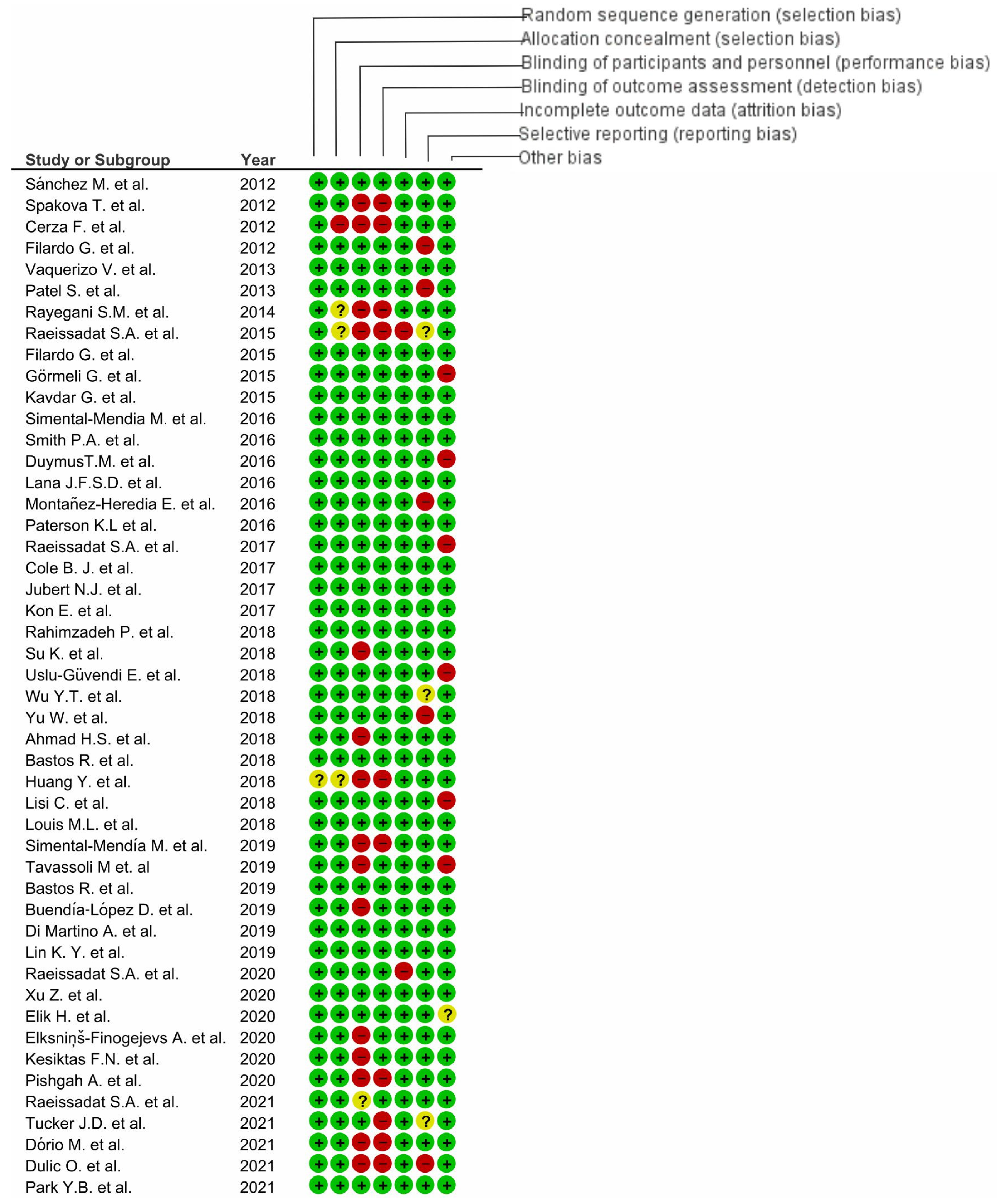
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramś, E.; Malesa, K.; Pomianowski, S.; Kamiński, R. Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis. Cells 2022, 11, 1080. https://doi.org/10.3390/cells11071080
Tramś E, Malesa K, Pomianowski S, Kamiński R. Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis. Cells. 2022; 11(7):1080. https://doi.org/10.3390/cells11071080
Chicago/Turabian StyleTramś, Ewa, Kamila Malesa, Stanisław Pomianowski, and Rafał Kamiński. 2022. "Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis" Cells 11, no. 7: 1080. https://doi.org/10.3390/cells11071080
APA StyleTramś, E., Malesa, K., Pomianowski, S., & Kamiński, R. (2022). Role of Platelets in Osteoarthritis—Updated Systematic Review and Meta-Analysis on the Role of Platelet-Rich Plasma in Osteoarthritis. Cells, 11(7), 1080. https://doi.org/10.3390/cells11071080







