Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Cells and Cell Lines
2.2. Reagents
2.3. Immunofluorescence
2.4. Proliferation and Viability Assay
2.5. Scratch Assay
2.6. Transwell-Migration Assay
2.7. Flow Cytometry
2.8. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR
2.9. LEGENDplex Multiplex Assay and ELISA
2.10. Endothelial-Tube-Formation Assay
2.11. Statistical Analysis
3. Results
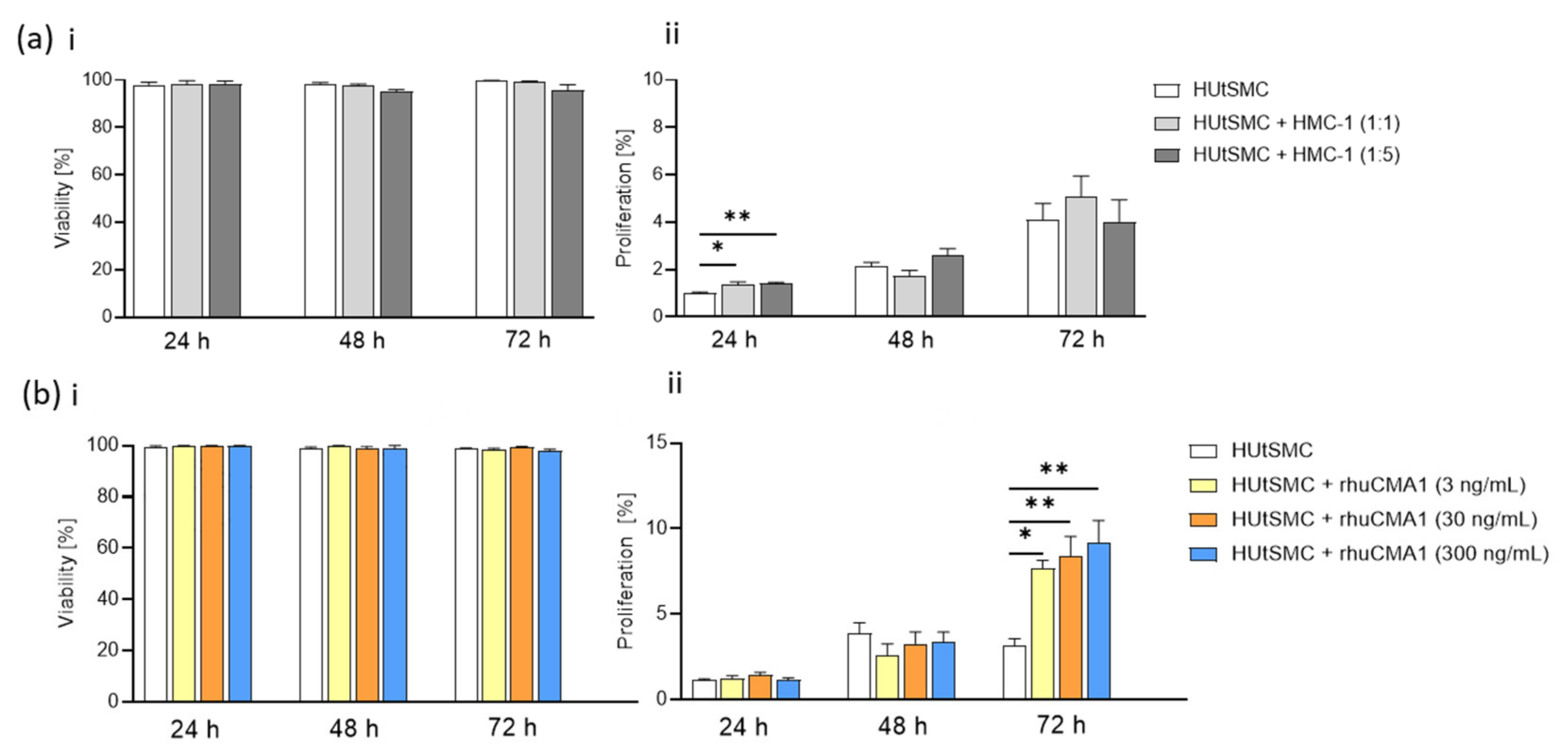
3.1. Phenotypic Switch of HUtSMCs in Response to HMC-1 or rhuCMA1
3.2. HMC-1 and rhuCMA1 Induce Proliferation of HUtSMCs
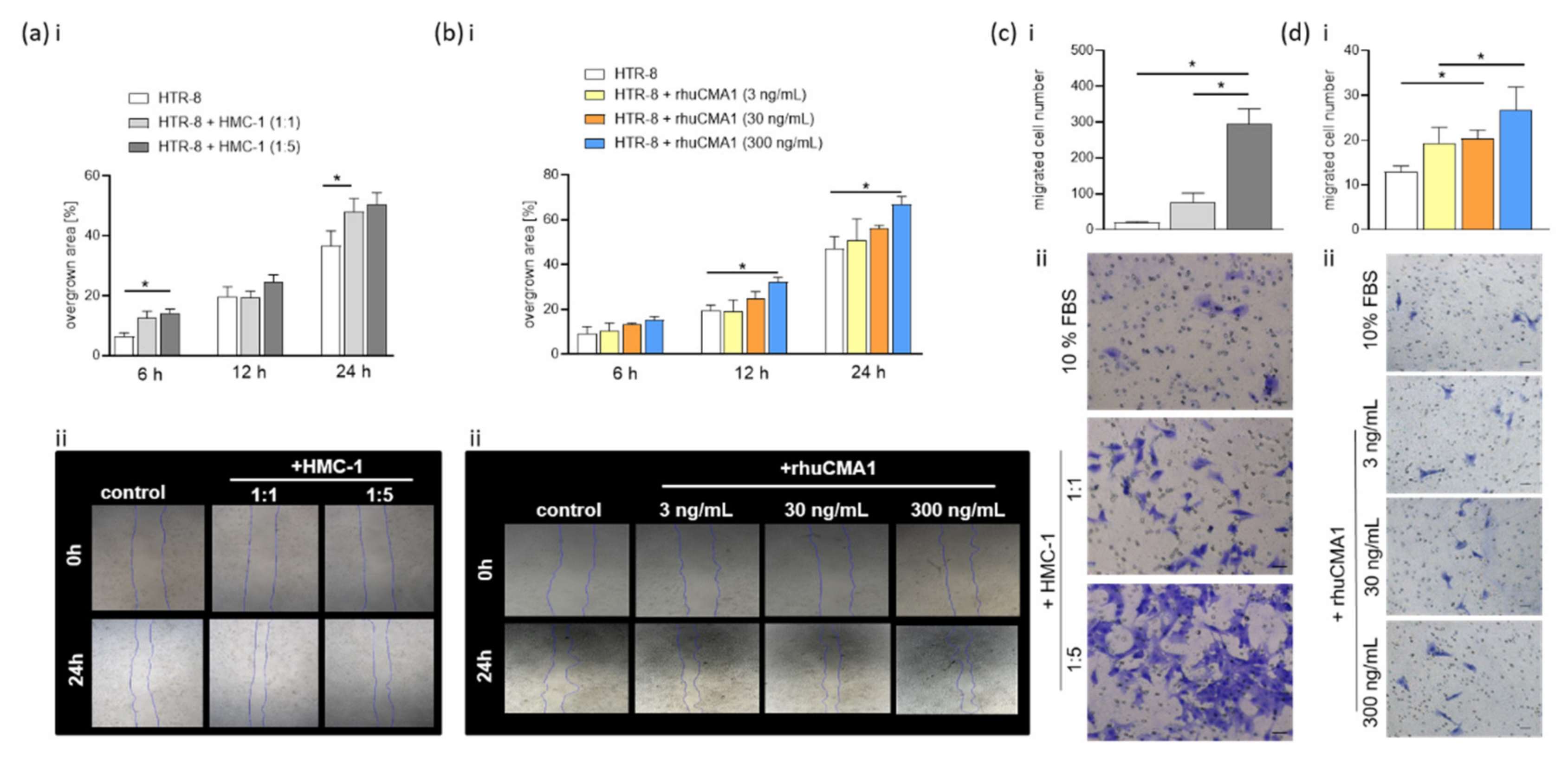
3.3. HMC-1 and rhuCMA1 Attract HUtSMCs in Transwell-Migration Assays
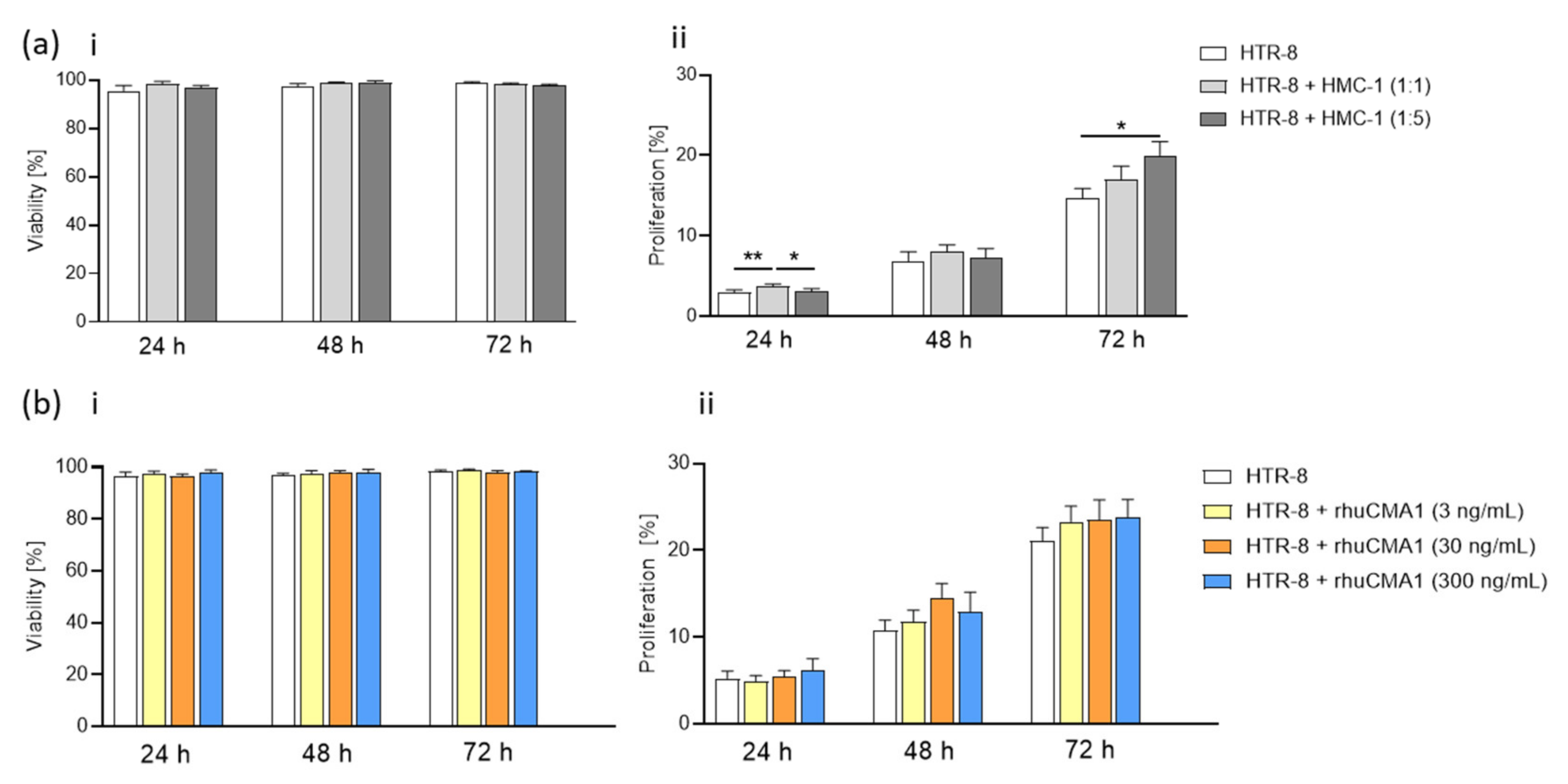
3.4. HMC-1 Supernatant, but Not rhuCMA1, Increases the Proliferation of HTR-8/SVneo Cells
3.5. HMC-1 and rhuCMA1 Induce HTR-8/SVneo Migration
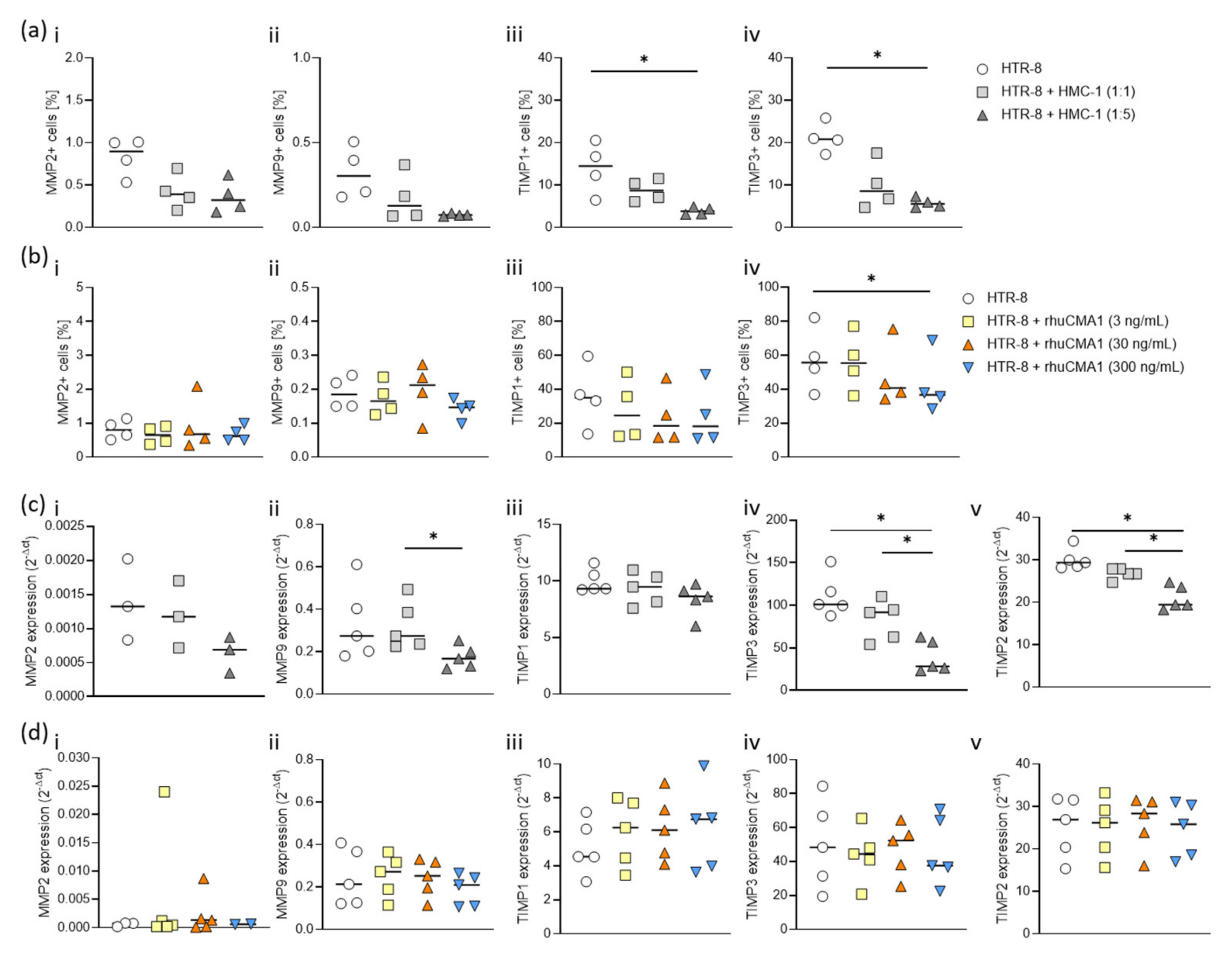
3.6. HMC-1 and rhuCMA1 Downregulate TIMP Expression in HTR-8/SVneo Cells
3.7. rhuCMA1 Induces HUVEC Endothelial-Tube Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brosens, I.; Robertson, W.B.; Dixon, H.G. The physiological response of the vessels of the placental bed to normal pregnancy. J. Pathol. Bacteriol. 1967, 93, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.C.; Hemberger, M.; Lu, Y.; Nozaki, T.; Whiteley, K.; Masutani, M.; Adamson, S.L. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol. Cell. Endocrinol. 2002, 187, 207–212. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Boss, A.L.; Chamley, L.W.; James, J. Placental formation in early pregnancy: How is the centre of the placenta made? Hum. Reprod. Updat. 2018, 24, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Woods, A.; Jauniaux, E.; Kingdom, J. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Tessier, D.R.; Yockell-Lelièvre, J.; Gruslin, A. Uterine Spiral Artery Remodeling: The Role of Uterine Natural Killer Cells and Extravillous Trophoblasts in Normal and High-Risk Human Pregnancies. Am. J. Reprod. Immunol. 2015, 74, 1–11. [Google Scholar] [CrossRef]
- Chang, R.-Q.; Zhou, W.-J.; Li, D.-J.; Li, M.-Q. Innate Lymphoid Cells at the Maternal-Fetal Interface in Human Pregnancy. Int. J. Biol. Sci. 2020, 16, 957–969. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]
- El-Azzamy, H.; Dambaeva, S.V.; Katukurundage, D.; Garcia, M.D.S.; Skariah, A.; Hussein, Y.; Germain, A.; Fernandez, E.; Gilman-Sachs, A.; Beaman, K.D.; et al. Dysregulated uterine natural killer cells and vascular remodeling in women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 2018, 80, e13024. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries In Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Innes, B.A.; Levey, J.; Robson, S.C.; Lash, G.E. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012, 26, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.; Lash, G.E.; Innes, B.A.; Zhang, J.Y.; Robson, S.C.; Bulmer, J.N. Uterine spiral artery muscle dedifferentiation. Hum. Reprod. 2019, 34, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Owens, G.K. Molecular Control of Vascular Smooth Muscle Cell Differentiation and Phenotypic Plasticity. Novartis Found Symp. 2007, 283, 174–193. [Google Scholar] [CrossRef]
- Hao, H.; Gabbiani, G.; Bochaton-Piallat, M.L. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arter. Thromb. Vasc. Biol. 2003, 23, 1510–1520. [Google Scholar] [CrossRef]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Zenclussen, A. Immune Cells in the Uterine Remodeling: Are They the Target of Endocrine Disrupting Chemicals? Front. Immunol. 2020, 11, 246. [Google Scholar] [CrossRef]
- Shimonovitz, S.; Hurwitz, A.; Dushnik, M.; Anteby, E.; Geva-Eldar, T.; Yagel, S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: A possible mechanism for control of trophoblast invasion. Am. J. Obstet. Gynecol. 1994, 171, 832–838. [Google Scholar] [CrossRef]
- Kokkinos, M.; Murthi, P.; Wafai, R.; Thompson, E.W.; Newgreen, D. Cadherins in the human placenta—Epithelial–mesenchymal transition (EMT) and placental development. Placenta 2010, 31, 747–755. [Google Scholar] [CrossRef]
- Nardo, L.G.; Nikas, G.; Makrigiannakis, A. Molecules in blastocyst implantation. Role of matrix metalloproteinases, cytokines and growth factors. J. Reprod. Med. 2003, 48, 137–147. [Google Scholar] [PubMed]
- Campbell, S.; Rowe, J.; Jackson, C.; Gallery, E.D.M. In vitro Migration of Cytotrophoblasts Through a Decidual Endothelial Cell Monolayer: The Role of Matrix Metalloproteinases. Placenta 2003, 24, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kassiri, Z. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and Its Therapeutic Implications in Cardiovascular Pathology. Front. Physiol. 2020, 11, 661. [Google Scholar] [CrossRef]
- Ruiz-Gomez, G.; Vogel, S.; Möller, S.; Pisabarro, T.M.; Hempel, U. Glycosaminoglycans influence enzyme activity of MMP2 and MMP2/TIMP3 complex formation—Insights at cellular and molecular level. Sci. Rep. 2019, 9, 4905. [Google Scholar] [CrossRef]
- Chen, J.; Khalil, R.A. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar] [PubMed] [Green Version]
- Zenclussen, A.C.; Hämmerling, G.J. Cellular Regulation of the Uterine Microenvironment That Enables Embryo Implantation. Front. Immunol. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlebacher, A. Immunology of the Maternal-Fetal Interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Matson, B.C.; Caron, K.M. Uterine natural killer cells as modulators of the maternal-fetal vasculature. Int. J. Dev. Biol. 2014, 58, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.; Barrette, V.F.; Gravel, J.; Carter, A.L.I.; Hatta, K.; Zhang, J.; Chen, Z.; Leno-Durán, E.; Bianco, J.; Leonard, S.; et al. Uterine NK Cells, Spiral Artery Modification and the Regulation of Blood Pressure During Mouse Pregnancy. Am. J. Reprod. Immunol. 2010, 63, 472–481. [Google Scholar] [CrossRef]
- Woidacki, K.; Jensen, F.; Zenclussen, A.C. Mast cells as novel mediators of reproductive processes. Front. Immunol. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Woidacki, K.; Popovic, M.; Metz, M.; Schumacher, A.; Linzke, N.; Teles, A.; Poirier, F.; Fest, S.; Jensen, F.; Rabinovich, G.A.; et al. Mast cells rescue implantation defects caused by c-kit deficiency. Cell Death Dis. 2013, 4, e462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.S.; Jose, M.A. Anti-implantation activity of H2receptor blockers and meloxicam, a COX-inhibitor, in albino Wistar rats. Eur. J. Contracept. Reprod. Health Care 2009, 14, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.M.; Raval, P.J. Interaction of estrogen and histamine during ovum implantation in the rat. Eur. J. Pharmacol. 1979, 57, 171–177. [Google Scholar] [CrossRef]
- Meyer, N.; Woidacki, K.; Knöfler, M.; Meinhardt, G.; Nowak, D.; Velicky, P.; Pollheimer, J.; Zenclussen, A.C. Chymase-producing cells of the innate immune system are required for decidual vascular remodeling and fetal growth. Sci. Rep. 2017, 7, srep45106. [Google Scholar] [CrossRef]
- Meyer, N.; Woidacki, K.; Maurer, M.; Zenclussen, A.C. Safeguarding of Fetal Growth by Mast Cells and Natural Killer Cells: Deficiency of One Is Counterbalanced by the Other. Front. Immunol. 2017, 8, 711. [Google Scholar] [CrossRef]
- Lunghi, L.; Ferretti, M.E.; Medici, S.; Biondi, C.; Vesce, F. Control of human trophoblast function. Reprod. Biol. Endocrinol. 2007, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ogoyama, M.; Ohkuchi, A.; Takahashi, H.; Zhao, D.; Matsubara, S.; Takizawa, T. LncRNA H19-Derived miR-675-5p Accelerates the Invasion of Extravillous Trophoblast Cells by Inhibiting GATA2 and Subsequently Activating Matrix Metalloproteinases. Int. J. Mol. Sci. 2021, 22, 1237. [Google Scholar] [CrossRef]
- Tapia, A.; Salamonsen, L.A.; Manuelpillai, U.; Dimitriadis, E. Leukemia inhibitory factor promotes human first trimester extravillous trophoblast adhesion to extracellular matrix and secretion of tissue inhibitor of metalloproteinases-1 and -2. Hum. Reprod. 2008, 23, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Salamonsen, L.A. Matrix metalloproteinases and endometrial remodelling. Cell Biol. Int. 1994, 18, 1139–1144. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Karumanchi, S.A. Angiogenic Factors in Preeclampsia and Related Disorders. Cold Spring Harb. Perspect. Med. 2012, 2, a006585. [Google Scholar] [CrossRef] [Green Version]
- Demir, R.; Seval, Y.; Huppertz, B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007, 109, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, S.B.; D’Errico, J.N.; Stapleton, P.A. Uterine Vascular Control Preconception and During Pregnancy. Compr. Physiol. 2021, 11, 1871–1893. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Muramatsu, M.; Katada, J.; Hattori, M.; Hayashi, I.; Majima, M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur. J. Pharmacol. 2000, 402, 181–191. [Google Scholar] [CrossRef]
- He, A.; Shi, G.P. Mast cell chymase and tryptase as targets for cardiovascular and metabolic diseases. Curr. Pharm. Des. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Li, M.; Liu, K.; Michalicek, J.; Angus, J.; Hunt, J.E.; Dell’Italia, L.J.; Feneley, M.P.; Graham, R.M.; Husain, A. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J. Clin. Investig. 2004, 114, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Halper, J. Structural Proteins | Major Proteins of the Extracellular Matrix. In Encyclopedia of Biological Chemistry, 3rd ed.; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 611–624. [Google Scholar]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.-Z.; Wang, H.; Takahahashi, K.; Jin, J.-P. Double deletion of calponin 1 and calponin 2 in mice decreases systemic blood pressure with blunted length-tension response of aortic smooth muscle. J. Mol. Cell. Cardiol. 2019, 129, 49–57. [Google Scholar] [CrossRef]
- Hirst, S.J.; Twort, C.H.C.; Lee, T.H. Differential Effects of Extracellular Matrix Proteins on Human Airway Smooth Muscle Cell Proliferation and Phenotype. Am. J. Respir. Cell Mol. Biol. 2000, 23, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Mao, C.; Jia, Y.; Fu, Y.; Kong, W. Extracellular matrix dynamics in vascular remodeling. Am. J. Physiol. Physiol. 2020, 319, C481–C499. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.; Lee, M.Y.; Lemmon, J.A.; Yurdagul, A.; Gomez, M.F.; Bortz, P.D.S.; Wamhoff, B.R. Molecular Mechanisms of Collagen Isotype-Specific Modulation of Smooth Muscle Cell Phenotype. Arter. Thromb. Vasc. Biol. 2009, 29, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Morla, A.O.; Mogford, J.E. Control of Smooth Muscle Cell Proliferation and Phenotype by Integrin Signaling through Focal Adhesion Kinase. Biochem. Biophys. Res. Commun. 2000, 272, 298–302. [Google Scholar] [CrossRef]
- Walker, H.A.; Whitelock, J.; Garl, P.J.; Nemenoff, R.A.; Stenmark, K.R.; Weiser-Evans, M.C. Perlecan Up-Regulation of FRNK Suppresses Smooth Muscle Cell Proliferation via Inhibition of FAK Signaling. Mol. Biol. Cell 2003, 14, 1941–1952. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, J.; Fu, W.; Guo, D.; Jiang, J.; Wang, Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J. Vasc. Surg. 2012, 56, 1698–1709.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazaar, A.L.; Plotnick, M.I.; Kucich, U.; Crichton, I.; Lotfi, S.; Das, S.K.P.; Kane, S.; Rosenbloom, J.; Panettieri, R.A.; Schechter, N.M.; et al. Mast Cell Chymase Modifies Cell-Matrix Interactions and Inhibits Mitogen-Induced Proliferation of Human Airway Smooth Muscle Cells. J. Immunol. 2002, 169, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Lonnemann, G.; Shapiro, L.; Engler-Blum, G.; Müller, G.A.; Koch, K.M.; Dinarello, C.A. Cytokines in human renal interstitial fibrosis. I. Interleukin-1 is a paracrine growth factor for cultured fibrosis-derived kidney fibroblasts. Kidney Int. 1995, 47, 837–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef] [Green Version]
- Pérez, L.; Muñoz-Durango, N.; Riedel, C.; Echeverría, C.; Kalergis, A.; Cabello-Verrugio, C.; Simon, F. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017, 33, 41–54. [Google Scholar] [CrossRef]
- Lee, H.S.; Yun, S.J.; Ha, J.M.; Jin, S.Y.; Ha, H.K.; Song, S.H.; Kim, C.D.; Bae, S.S. Prostaglandin D2 stimulates phenotypic changes in vascular smooth muscle cells. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell. Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Muramatsu, M.; Jin, D.; Furubayashi, K.; Takai, S.; Tamai, H.; Miyazaki, M. The Effects of Chymase on Matrix Metalloproteinase-2 Activation in Neointimal Hyperplasia after Balloon Injury in Dogs. Hypertens. Res. 2007, 30, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, G.; Szewczyk, A.; Pyzlak, M.; Klimkiewicz, J.; Smiertka, W.; Szukiewicz, D. Mast cells, histamine and development of the placental vascular network in pregnancies complicated by preeclampsia and intrauterine growth retardation. Ginekol. Polska 2005, 76, 727–734. [Google Scholar]
- Szewczyk, G.; Pyzlak, M.; Klimkiewicz, J.; Śmiertka, W.; Miedzińska, M.; Szukiewicz, D. Mast Cells and Histamine: Do They Influence Placental Vascular Network and Development in Preeclampsia? Mediat. Inflamm. 2012, 2012, 307189. [Google Scholar] [CrossRef] [Green Version]
- Mitani, R.; Maeda, K.; Fukui, R.; Endo, S.; Saijo, Y.; Shinohara, K.; Kamada, M.; Irahara, M.; Yamano, S.; Nakaya, Y.; et al. Production of human mast cell chymase in human myometrium and placenta in cases of normal pregnancy and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 155–160. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; De Vos, P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef]
- Graham, C.H.; Hawley, T.S.; Hawley, R.G.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef]
- Weber, M.; Weber, M.; Knoefler, I.; Schleussner, E.; Markert, U.R.; Fitzgerald, J.S. HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of “stemness-” associated transcription factors. Biomed. Res. Int. 2013, 2013, 243649. [Google Scholar] [CrossRef] [Green Version]
- Terakawa, M.; Tomimori, Y.; Goto, M.; Hayashi, Y.; Oikawa, S.; Fukuda, Y. Eosinophil migration induced by mast cell chymase is mediated by extracellular signal-regulated kinase pathway. Biochem. Biophys. Res. Commun. 2005, 332, 969–975. [Google Scholar] [CrossRef]
- Tani, K.; Ogushi, F.; Kido, H.; Kawano, T.; Kunori, Y.; Kamimura, T.; Cui, P.; Sone, S. Chymase is a potent chemoattractant for human monocytes and neutrophils. J. Leukoc. Biol. 2000, 67, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Wang, L.; Zhan, M.; Li, S.; Fang, Z.; Xu, C.; Zheng, Y.; He, S. Induction of mast cell accumulation by chymase via an enzymatic activity- and intercellular adhesion molecule-1-dependent mechanism. Br. J. Pharm. 2018, 175, 678–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, N.; Funaki, T.; Murakami, A.; Takai, S.; Miyazaki, M. Mast Cell Chymase Decreases the Barrier Function and Inhibits the Migration of Corneal Epithelial Cells. Curr. Eye Res. 2005, 30, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Madjene, L.C.; Danelli, L.; Dahdah, A.; Vibhushan, S.; Bex-Coudrat, J.; Pacreau, E.; Vaugier, C.; Claver, J.; Rolas, L.; Pons, M.; et al. Mast cell chymase protects against acute ischemic kidney injury by limiting neutrophil hyperactivation and recruitment. Kidney Int. 2019, 97, 516–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruse, K.; Lash, G.E.; Innes, B.A.; Otun, H.A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Localization of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitors for MMPs (TIMPs) in uterine natural killer cells in early human pregnancy. Hum. Reprod. 2008, 24, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.X.; Wang, Y.L.; Qin, L.; Xiao, Z.J.; Herva, R.; Piao, Y.S. Dynamic expression of matrix metalloproteinases (MMP-2, -9 and -14) and the tissue inhibitors of MMPs (TIMP-1, -2 and -3) at the implantation site during tubal pregnancy. Reproduction 2005, 129, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Pang, Z.-J.; Yu, Y.-H. Regulation of trophoblast invasion: The role of matrix metalloproteinases. Rev. Obstet. Gynecol. 2012, 5, e137-43. [Google Scholar]
- Bischoff, P.; Meisser, A.; Campana, A. Paracrine and Autocrine Regulators of Trophoblast Invasion—A Review. Placenta 2000, 21, S55–S60. [Google Scholar] [CrossRef]
- Li, M.Q.; Hou, F.-X.; Shao, J.; Tang, C.-L.; Li, D.-J. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol Reprod. 2010, 82, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Diaconu, N.-C.; Kaminska, R.; Naukkarinen, A.; Harvima, R.; Harvima, I. The increase in tryptase-and chymase-positive mast cells is associated with partial inactivation of chymase and increase in protease inhibitors in basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Crivellato, E. Cross talk between natural killer cells and mast cells in tumor angiogenesis. Agents Actions 2019, 68, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Nico, B.; Crivellato, E.; Roncali, L.; Dammacco, F. The role of mast cells in tumour angiogenesis. Br. J. Haematol. 2001, 115, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Katada, J.; Muramatsu, M.; Hayashi, I.; Tsutsumi, M.; Konishi, Y.; Majima, M. Significance of Vascular Endothelial Cell Growth Factor Up-Regulation Mediated via a Chymase-Angiotensin-Dependent Pathway during Angiogenesis in Hamster Sponge Granulomas. J. Pharmacol. Exp. Ther. 2002, 302, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Dilution | Company |
|---|---|---|
| FVD-eFluor 506 | 1:500 | Invitrogen, Karlsruhe, Germany |
| Cytokeratin 7-APC | 1:100 | R&D Systems, Wiesbaden, Germany |
| MMP2-PE | 1:200 | R&D Systems, Wiesbaden, Germany |
| MMP9-FITC | 1:200 | R&D Systems, Wiesbaden, Germany |
| TIMP1-AF405 | 1:200 | R&D Systems, Wiesbaden, Germany |
| TIMP3-AF750 | 1:200 | R&D Systems, Wiesbaden, Germany |
| Gene | Forward Primer | Reserve Primer | UPL Number |
|---|---|---|---|
| GUSB | cgccctgcctatctgtattc | tccccacagggagtgtgtag | 57 |
| MMP2 | cggttttctcgaatccatga | gagtccgtccttaccgtcaa | 34 |
| MMP9 | tcttccctggagacctgaga | gagtgtaaccatagcggtacagg | 27 |
| TIMP1 | ctgttgttgctgtggctgat | aacttggccctgatgacg | 3 |
| TIMP2 | gaagagcctgaaccacaggt | cggggaggagatgtagcac | 43 |
| TIMP3 | ctgtgcaacttcgtggagag | ggcaggtagtagcaggacttg | 14 |
| Analyte | Detection Range (pg/mL) | Sensitivity (pg/mL) |
|---|---|---|
| MMP2 | 158.20–1400,000 | |
| MMP9 | 87.80–300,000 | |
| TIMP1 | 77.00–800,000 | |
| TIMP3 | 156–10,000 | <2 |
| Chymase | 156–10,000 | <39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Schumacher, A.; Fink, B.; Bauer, M.; Zenclussen, A.C.; Meyer, N. Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells. Cells 2022, 11, 1158. https://doi.org/10.3390/cells11071158
Zhang N, Schumacher A, Fink B, Bauer M, Zenclussen AC, Meyer N. Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells. Cells. 2022; 11(7):1158. https://doi.org/10.3390/cells11071158
Chicago/Turabian StyleZhang, Ningjuan, Anne Schumacher, Beate Fink, Mario Bauer, Ana Claudia Zenclussen, and Nicole Meyer. 2022. "Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells" Cells 11, no. 7: 1158. https://doi.org/10.3390/cells11071158
APA StyleZhang, N., Schumacher, A., Fink, B., Bauer, M., Zenclussen, A. C., & Meyer, N. (2022). Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells. Cells, 11(7), 1158. https://doi.org/10.3390/cells11071158







