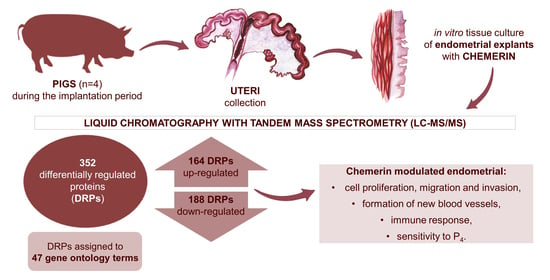
Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Tissue Cultures
2.3. Protein Extraction
2.4. Protein’s Digestion
2.5. Reversed-Phase Peptide Fractionation at High pH
2.6. Mass Spectrometry
2.7. Data Analysis
2.8. Network and Functional Analysis
2.9. Validation of LC-MS/MS Results by Western Blot
3. Results
3.1. Differentially Regulated Proteins and Functional Annotations (GO and KEGG)
3.2. Western Blot
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazer, F.W.; Johnson, G.A.; Wu, G. Amino Acids and Conceptus Development During the Peri-Implantation Period of Pregnancy. Adv. Exp. Med. Biol. 2015, 843, 23–52. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, C.; Seo, H.; Wu, G.; Johnson, G.A.; Bazer, F.W. Insights into the Regulation of Implantation and Placentation in Humans, Rodents, Sheep, and Pigs. Adv. Exp. Med. Biol. 2022, 1354, 25–48. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst-uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, D.; Baldini, E.; Massimiani, M.; Camaioni, A.; Salustri, A.; Bernardini, R.; Centanni, M.; Ulisse, S.; Moretti, C.; Campagnolo, L. Thyroid hormone regulates protease expression and activation of Notch signaling in implantation and embryo development. J. Endocrinol. 2018, 236, 1–12. [Google Scholar] [CrossRef]
- Aghajanova, L.; Stavreus-Evers, A.; Lindeberg, M.; Landgren, B.M.; Sparre, L.S.; Hovatta, O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil. Steril. 2011, 95, 230–237e.2. [Google Scholar] [CrossRef]
- Castellucci, M.; De Matteis, R.; Meisser, A.; Cancello, R.; Monsurrò, V.; Islami, D.; Sarzani, R.; Marzioni, D.; Cinti, S.; Bischof, P. Leptin modulates extracellular matrix molecules and metalloproteinases: Possible implications for trophoblast invasion. Mol. Hum. Reprod. 2000, 6, 951–958. [Google Scholar] [CrossRef]
- Gudelska, M.; Dobrzyn, K.; Kiezun, M.; Kisielewska, K.; Rytelewska, E.; Kaminski, T.; Smolinska, N. Chemerin affects P4 and E2 synthesis in the porcine endometrium during early pregnancy. Int. J. Mol. Sci. 2022, 23, 945. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [Green Version]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Rytelewska, E.; Kiezun, M.; Kisielewska, K.; Gudelska, M.; Dobrzyn, K.; Kaminska, B.; Kaminski, T.; Smolinska, N. Chemerin as a modulator of ovarian steroidogenesis in pigs: An in vitro study. Theriogenology 2021, 160, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kim, J.Y.; Xue, K.; Liu, J.Y.; Leader, A.; Tsang, B.K. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology 2012, 153, 5600–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverchon, M.; Bertoldo, M.J.; Ramé, C.; Froment, P.; Dupont, J. CHEMERIN (RARRES2) decreases in vitro granulosa cell steroidogenesis and blocks oocyte meiotic progression in bovine species. Biol. Reprod. 2014, 90, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Zaobidna, E.; Bogus-Nowakowska, K.; Wyrebek, J.; Bors, K.; et al. Expression of chemerin and its receptors in the porcine hypothalamus and plasma chemerin levels during the oestrous cycle and early pregnancy. Int. J. Mol. Sci. 2019, 20, 3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisielewska, K.; Rytelewska, E.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Bogus-Nowakowska, K.; Kaminska, B.; Smolinska, N.; Kaminski, T. Expression of chemerin receptors CMKLR1, GPR1 and CCRL2 in the porcine pituitary during the oestrous cycle and early pregnancy and the effect of chemerin on MAPK/Erk1/2, Akt and AMPK signalling pathways. Theriogenology 2020, 157, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, K.; Rytelewska, E.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Bogus-Nowakowska, K.; Kaminska, B.; Smolinska, N.; Kaminski, T. Relative abundance of chemerin mRNA transcript and protein in pituitaries of pigs during the estrous cycle and early pregnancy and associations with LH and FSH secretion during the estrous cycle. Anim. Reprod. Sci. 2020, 219, 106532. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, E.; Kisielewska, K.; Kiezun, M.; Dobrzyn, K.; Gudelska, M.; Rak, A.; Dupont, J.J.; Kaminska, B.; Kaminski, T.; Smolinska, N. Expression of chemerin and its receptors in the ovaries of prepubertal and mature gilts. Mol. Reprod. Dev. 2020, 87, 739–762. [Google Scholar] [CrossRef]
- Gudelska, M.; Dobrzyn, K.; Kiezun, M.; Rytelewska, E.; Kisielewska, K.; Kaminska, B.; Kaminski, T.; Smolinska, N. The expression of chemerin and its receptors (CMKLR1, GPR1, CCRL2) in the porcine uterus during the oestrous cycle and early pregnancy and in trophoblasts and conceptuses. Animal 2020, 14, 2116–2128. [Google Scholar] [CrossRef]
- Yang, X.; Yao, J.; Wei, Q.; Ye, J.; Yin, X.; Quan, X.; Lan, Y.; Xing, H. Role of chemerin/CMKLR1 in the maintenance of early pregnancy. Front. Med. 2018, 12, 525–532. [Google Scholar] [CrossRef]
- Pierzchała, D.; Liput, K.; Korwin-Kossakowska, A.; Ogłuszka, M.; Poławska, E.; Nawrocka, A.; Urbański, P.; Ciepłoch, A.; Juszczuk-Kubiak, E.; Lepczyński, A.; et al. Molecular characterisation of uterine endometrial proteins during early stages of pregnancy in pigs by MALDI TOF/TOF. Int. J. Mol. Sci. 2021, 22, 6720. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, N.; Dobrzyn, K.; Kiezun, M.; Szeszko, K.; Maleszka, A.; Kaminski, T. Effect of adiponectin on the steroidogenic acute regulatory protein, P450 side chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase gene expression, progesterone and androstenedione production by the porcine uterus during early pregnancy. J. Physiol. Pharmacol. 2016, 67, 443–456. [Google Scholar] [PubMed]
- Dobrzyn, K.; Kiezun, M.; Szeszko, K.; Kisielewska, K.; Rytelewska, E.; Gudelska, M.; Zaobidna, E.; Bors, K.; Kopij, G.; Szymanska, K.; et al. Orexin B affects the transcriptome of incubated in vitro porcine endometrial explants from the early-implantation period. Reprod. Domest. Anim. 2021, 56, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Plubell, D.L.; Wilmarth, P.A.; Zhao, Y.; Fenton, A.M.; Minnier, J.; Reddy, A.P.; Klimek, J.; Yang, X.; David, L.L.; Pamir, N. Extended multiplexing of Tandem Mass Tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol. Cell. Proteom. 2017, 16, 873–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.D.; Clarke, C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar] [CrossRef] [Green Version]
- Garg, D.; Ng, S.S.M.; Baig, K.M.; Driggers, P.; Segars, J. Progesterone-mediated non-classical signaling. Trends Endocrinol. Metab. 2017, 28, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kanda, Y.; Roberts, D.J.; Ecker, J.L.; Losel, R.; Wehling, M.; Peluso, J.J.; Pru, J.K. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol. Cell. Endocrinol. 2008, 287, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.K.; Slonina, D.; Rekawiecki, R.; Kotwica, J. Expression of progesterone receptor membrane component (PGRMC) 1 and 2, serpine mRNA binding protein 1 (SERBP1) and nuclear progesterone receptor (PGR) in the bovine endometrium during the estrous cycle and the first trimester of pregnancy. Reprod. Biol. 2013, 13, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lyzikova, Y.A.; Zinovkin, D.A.; Pranjol, M.Z.I. Increase in FoxP3, CD56 immune cells and decrease in glands PGRMC1 expression in the endometrium are associated with recurrent miscarriages. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 121–126. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bayless, K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol. Hum. Reprod. 2010, 16, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Dunlap, K.A.; Kim, J.; Bailey, D.W.; Spencer, T.E.; Burghardt, R.C.; Wagner, G.F.; Johnson, G.A.; Bazer, F.W. Stanniocalcin 1 is a luminal epithelial marker for implantation in pigs regulated by progesterone and estradiol. Endocrinology 2009, 150, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Bishop, A.; Cartwright, J.E.; Whitley, G.S. Stanniocalcin-1 in the female reproductive system and pregnancy. Hum. Reprod. Update 2021, 27, 1098–1114. [Google Scholar] [CrossRef]
- Allegra, A.; Marino, A.; Coffaro, F.; Lama, A.; Rizza, G.; Scaglione, P.; Sammartano, F.; Santoro, A.; Volpes, A. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle? Hum. Reprod. 2009, 24, 2549–2557. [Google Scholar] [CrossRef] [Green Version]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Ledgard, A.M.; Lee, R.S.-F.; Peterson, A.J.; Ralph Gwatkin Editor in Chief, B. Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol. Reprod. Dev. 2009, 76, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, S.E.; Meyer, S.U.; Zitta, K.; Hiendleder, S.; Sinowatz, F.; Bauersachs, S.; Büttner, M.; Fröhlich, T.; Arnold, G.J.; Reichenbach, H.-D.; et al. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol. Cell. Endocrinol. 2010, 332, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzusch, K.; Ruck, P.; Dietl, J.A.; Horny, H.P.; Kaiserling, E. Immunohistochemical localization of tissue inhibitor of metalloproteinases-2 (TIMP-2) in first trimester human placental decidua. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 68, 105–107. [Google Scholar] [CrossRef]
- Ruck, P.; Marzusch, B.K.; Horny, H.P.; Dietl, J.; Kaiserling, E. The distribution of tissue inhibitor of metalloproteinases-2 (TIMP-2) in the human placenta. Placenta 1996, 17, 263–266. [Google Scholar] [CrossRef]
- Seval, Y.; Akkoyunlu, G.; Demir, R.; Asar, M. Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy. Acta Histochem. 2004, 106, 353–362. [Google Scholar] [CrossRef]
- Paule, S.; Li, Y.; Nie, G. Cytoskeletal remodelling proteins identified in fetal-maternal interface in pregnant women and rhesus monkeys. J. Mol. Histol. 2011, 42, 161–166. [Google Scholar] [CrossRef]
- Gupta, A.; Ing, N.H.; Bazer, F.W.; Bustamante, L.S.; Jaeger, L.A. Beta transforming growth factors (TGFss) at the porcine conceptus-maternal interface. Part I: Expression of TGFbeta1, TGFbeta2, and TGFbeta3 messenger ribonucleic acids. Biol. Reprod. 1998, 59, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Dekaney, C.M.; Bazer, F.W.; Madrigal, M.M.; Jaeger, L.A. Beta transforming growth factors (TGFbeta) at the porcine conceptus-maternal interface. Part II: Uterine TGFbeta bioactivity and expression of immunoreactive TGFbetas (TGFbeta1, TGFbeta2, and TGFbeta3) and their receptors (type I and type II). Biol. Reprod. 1998, 59, 911–917. [Google Scholar] [CrossRef]
- Moussad, E.E.-D.A.; Rageh, M.A.E.; Wilson, A.K.; Geisert, R.D. Temporal and spatial expression of connective tissue growth factor (CCN2; CTGF) and transforming growth factor β type 1 (TGF-β1) at the utero-placental interface during early pregnancy in the pig. J. Clin. Pathol. Mol. Pathol. 2002, 55, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Massuto, D.A.; Kneese, E.C.; Johnson, G.A.; Burghardt, R.C.; Hooper, R.N.; Ing, N.H.; Jaeger, L.A. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: Proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 2010, 139, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Bazer, F.W.; Jaeger, L.A. Differential expression of beta transforming growth factors (TGFβ1, TGFβ2, and TGFβ3) and their receptors (Type I and Type II) in peri-implantation porcine conceptuses. Biol. Reprod. 1996, 55, 796–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Lim, H.; Wang, J.; Paria, B.C.; BazDresch, M.; Dey, S.K. Inappropriate expression of human transforming growth factor (TGF)-α in the uterus of transgenic mouse causes downregulation of TGF-β receptors and delays the blastocyst-attachment reaction. J. Mol. Endocrinol. 1997, 18, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Tamada, H.; McMaster, M.T.; Flanders, K.C.; Andrews, G.K.; Dey, S.K. Cell type-specific expression of transforming growth factor-beta 1 in the mouse uterus during the periimplantation period. Mol. Endocrinol. 1990, 4, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Segerson, E.C. Immunosuppressive activity of a porcine high-molecular weight uterine macromolecule is associated with transforming growth factor-β. J. Reprod. Immunol. 1995, 29, 47–60. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Kumar, V.; Maurya, V.K.; Soni, U.K.; Jha, R.K. Endoglin (CD105) coordinates the process of endometrial receptivity for embryo implantation. Mol. Cell. Endocrinol. 2016, 425, 69–83. [Google Scholar] [CrossRef]
- Dantzer, V.; Leiser, R. Initial vascularisation in the pig placenta: I. Demonstration of nonglandular areas by histology and corrosion casts. Anat. Rec. 1994, 238, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kieckbusch, J.; Gaynor, L.M.; Moffett, A.; Colucci, F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat. Commun. 2014, 5, 3359. [Google Scholar] [CrossRef]
- Carlino, C.; Trotta, E.; Stabile, H.; Morrone, S.; Bulla, R.; Soriani, A.; Iannitto, M.L.; Agostinis, C.; Mocci, C.; Minozzi, M.; et al. Chemerin regulates NK cell accumulation and endothelial cell morphogenesis in the decidua during early pregnancy. J. Clin. Endocrinol. Metab. 2012, 97, 3603–3612. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dunk, C.E.; Shynlova, O.; Caniggia, I.; Lye, S.J. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia. EBioMedicine 2019, 39, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Münz, M.; Kieu, C.; Mack, B.; Schmitt, B.; Zeidler, R.; Gires, O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 2004, 23, 5748–5758. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.J.; De Leij, L.M.F.H.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ma, W.Y.; Xu, S.C.; Liang, Y.; Fu, Y.B.; Pang, B.; Xin, T.; Fan, H.T.; Zhang, R.; Luo, J.G.; et al. The overexpression of epithelial cell adhesion molecule (EpCAM) in glioma. J. Neurooncol. 2014, 119, 39–47. [Google Scholar] [CrossRef]
- Martowicz, A.; Spizzo, G.; Gastl, G.; Untergasser, G. Phenotype-dependent effects of EpCAM expression on growth and invasion of human breast cancer cell lines. BMC Cancer 2012, 12, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankpal, N.V.; Fleming, T.P.; Gillanders, W.E. EpCAM modulates NF-κB signaling and interleukin-8 expression in breast cancer. Mol. Cancer Res. 2013, 11, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzaman, K.; Samadi, M.; Moradi-kalbolandi, S.; Majidzadeh, A.K.; Salehi, M.; Jalili, N.; hadi Jazayeri, M.; Khorammi, S.; Darvishi, B.; Siavashi, V.; et al. Development of a recombinant anti-VEGFR2-EPCAM bispecific antibody to improve antiangiogenic efficiency. Exp. Cell Res. 2021, 405, 112685. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.S.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.M.; Cheng, D.; Schafenacker, A.M.; Paik, D.Y.; Goldstein, A.S.; Witte, O.N.; Jaroszewicz, A.; Pellegrini, M.; Memarzadeh, S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells 2013, 31, 808–822. [Google Scholar] [CrossRef] [Green Version]
- Tarmann, T.; Dohr, G.; Schiechl, H.; Barth, S.; Hartmann, M. Immunohistochemical Detection of an Epithelial Membrane Protein in Rat Embryos at Different Stages of Development. Cells Tissues Organs 1990, 137, 141–145. [Google Scholar] [CrossRef]
- Hirata, K.I.; Ishida, T.; Penta, K.; Rezaee, M.; Yang, E.; Wohlgemuth, J.; Quertermous, T. Cloning of an immunoglobulin family adhesion molecule selectively expressed by endothelial cells. J. Biol. Chem. 2001, 276, 16223–16231. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Kundu, R.K.; Yang, E.; Hirata, K.I.; Ho, Y.D.; Quertermous, T. Targeted disruption of endothelial cell-selective adhesion molecule inhibits angiogenic processes in vitro and in vivo. J. Biol. Chem. 2003, 278, 34598–34604. [Google Scholar] [CrossRef] [Green Version]
- Muller, W.A.; Weigl, S.A.; Deng, X.; Phillips, D.M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993, 178, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.J.; Berndt, M.C.; Gorski, J.; White, G.C.; Lyman, S.; Paddock, C.; Muller, W.A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990, 247, 1219–1222. [Google Scholar] [CrossRef]
- Fogler, W.E.; Volker, K.; McCormick, K.L.; Watanabe, M.; Ortaldo, J.R.; Wiltrout, R.H. NK cell infiltration into lung, liver, and subcutaneous B16 melanoma is mediated by VCAM-1/VLA-4 interaction. J. Immunol. 1996, 156, 4707–4714. [Google Scholar] [PubMed]
- Sibai, B.M. Diagnosis and management of gestational hypertension and preeclampsia. Obstet. Gynecol. 2003, 102, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, Z.; Sun, F.; Zhou, Z.; Zhang, B.; Wang, B.; Chen, J.; Li, M.; Xiao, T.; Neuman, R.I.; et al. Placental trophoblast-specific overexpression of chemerin induces preeclampsia-like symptoms. Clin. Sci. 2022, 136, 257–272. [Google Scholar] [CrossRef]
- Farzadnia, M.; Ayatollahi, H.; Hasan-zade, M.; Rahimi, H.R. A comparative study of serum level of vascular cell adhesion molecule-1 (sVCAM-1), intercellular adhesion molecule-1(ICAM-1) and high sensitive C—Reactive protein (hs-CRP) in normal and pre-eclamptic pregnancies. Iran. J. Basic Med. Sci. 2013, 16, 689. [Google Scholar] [CrossRef]
- Krauss, T.; Azab, H.; Dietrich, M.; Augustin, H.G. Fetal plasma levels of circulating endothelial cell adhesion molecules in normal and preeclamptic pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 78, 41–45. [Google Scholar] [CrossRef]
- Higgins, J.R.; Papayianni, A.; Brady, H.R.; Darling, M.R.N.; Walshe, J.J. Circulating vascular cell adhesion molecule–1 in pre-eclampsia, gestational hypertension, and normal pregnancy: Evidence of selective dysregulation of vascular cell adhesion molecule–1 homeostasis in pre-eclampsia. Am. J. Obstet. Gynecol. 1998, 179, 464–469. [Google Scholar] [CrossRef]
- Madazli, R.; Budak, E.; Calay, Z.; Aksu, M.F. Correlation between placental bed biopsy findings, vascular cell adhesion molecule and fibronectin levels in pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 514–518. [Google Scholar] [CrossRef]
- Sakurai, M.; Sato, Y.; Mukai, K.; Suematsu, M.; Fukui, E.; Yoshizawa, M.; Tanemura, K.; Hoshino, Y.; Matsumoto, H.; Sato, E. Distribution of tubulointerstitial nephritis antigen-like 1 and structural matrix proteins in mouse embryos during preimplantation development in vivo and in vitro. Zygote 2012, 22, 259–265. [Google Scholar] [CrossRef]
- Igarashi, T.; Tajiri, Y.; Sakurai, M.; Sato, E.; Li, D.; Mukai, K.; Suematsu, M.; Fukui, E.; Yoshizawa, M.; Matsumoto, H. Tubulointerstitial nephritis antigen-like 1 is expressed in the uterus and binds with integrins in decidualized endometrium during postimplantation in mice. Biol. Reprod. 2010, 82, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, T.; Tajiri, Y.; Sakurai, M.; Sato, E.; Li, D.; Mukai, K.; Suematsu, M.; Fukui, E.; Yoshizawa, M.; Matsumoto, H. Tubulointerstitial Nephritis Antigen-Like 1 is expressed in extraembryonic tissues and interacts with laminin 1 in the reichert membrane at postimplantation in the mouse. Biol. Reprod. 2009, 81, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Mary, S.; Kulkarni, M.J.; Mehendale, S.S.; Joshi, S.R.; Giri, A.P. Tubulointerstitial nephritis antigen-like 1 protein is downregulated in the placenta of pre-eclamptic women. Clin. Proteom. 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.P.; Roten, L.T.; Dyer, T.D.; East, C.E.; Forsmo, S.; Blangero, J.; Brennecke, S.P.; Austgulen, R.; Moses, E.K. The ERAP2 gene is associated with preeclampsia in Australian and Norwegian populations. Hum. Genet. 2009, 126, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seamon, K.; Kurlak, L.O.; Warthan, M.; Stratikos, E.; Strauss, J.F.; Mistry, H.D.; Lee, E.D. The differential expression of ERAP1/ERAP2 and immune cell activation in pre-eclampsia. Front. Immunol. 2020, 11, 396. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Ding, Y.; Zhong, Y.; Yu, L.; Peng, M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine 2015, 48, 299–308. [Google Scholar] [CrossRef]
- Stepan, H.; Philipp, A.; Roth, I.; Kralisch, S.; Jank, A.; Schaarschmidt, W.; Lössner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; et al. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6months after pregnancy. Regul. Pept. 2011, 168, 69–72. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzechowska, K.; Dobrzyń, K.; Kieżun, M.; Malinowska, A.; Świderska, B.; Kamiński, T.; Smolińska, N. Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis. Cells 2022, 11, 1161. https://doi.org/10.3390/cells11071161
Orzechowska K, Dobrzyń K, Kieżun M, Malinowska A, Świderska B, Kamiński T, Smolińska N. Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis. Cells. 2022; 11(7):1161. https://doi.org/10.3390/cells11071161
Chicago/Turabian StyleOrzechowska, Kinga, Kamil Dobrzyń, Marta Kieżun, Agata Malinowska, Bianka Świderska, Tadeusz Kamiński, and Nina Smolińska. 2022. "Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis" Cells 11, no. 7: 1161. https://doi.org/10.3390/cells11071161
APA StyleOrzechowska, K., Dobrzyń, K., Kieżun, M., Malinowska, A., Świderska, B., Kamiński, T., & Smolińska, N. (2022). Chemerin Effect on the Endometrial Proteome of the Domestic Pig during Implantation Obtained by LC-MS/MS Analysis. Cells, 11(7), 1161. https://doi.org/10.3390/cells11071161