Regulation of Aging and Longevity by Ion Channels and Transporters
Abstract
1. Introduction
2. Regulation of Aging and Longevity by Ion Channels and Transporters Related to Ca2+ Homeostasis and Electrical Excitability
2.1. Regulation of Aging by Ca2+ Channels in the Plasma Membrane
2.2. Regulation of Aging and Longevity by Ca2+ Channels in the Endoplasmic Reticulum (ER)
2.3. Transcriptional Control of Excitability and Longevity
3. Regulation of Aging and Longevity by Mitochondrial Ion Channels and Transporters
3.1. Mitochondrial Ca2+ Uniporter
3.2. Other Mitochondrial Channels and Transporters
4. Mitochondrial Uncoupling Proteins and Longevity
Role of UCP Proteins in the Response to Oxidative Stress
5. Regulation of Lifespan by Ion Channels Involved in Autophagy and Lysosomal Proteostasis
5.1. Involvement of Vesicular Ion Channels in Endolysosomal Function and Lifespan
5.2. Transcriptional Regulation of Endolysosomal Function
6. Regulation of Ion Channel Activity by the Longevity Factor, Klotho
Regulation of Ion Channel Activity by Klotho
7. Roles for Ion Channels in Regulation of Longevity by Temperature
7.1. Thermosensitive Channels and Lifespan
7.2. Heat-shock Response and Lifespan
8. Regulation of Longevity by Channels Involved in Other Sensory Modalities
9. Closing Remarks and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From Discoveries in Ageing Research to Therapeutics for Healthy Ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans Mutant That Lives Twice as Long as Wild Type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef]
- Friedman, D.B.; Johnson, T.E. A Mutation in the Age-1 Gene in Caenorhabditis Elegans Lengthens Life and Reduces Hermaphrodite Fertility. Genetics 1988, 118, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork Head Transcription Factor DAF-16 Transduces Insulin-like Metabolic and Longevity Signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium Signalling Remodelling and Disease. Biochem. Soc. Trans. 2012, 40, 297–309. [Google Scholar] [CrossRef]
- Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol Trisphosphate and Calcium Signalling Mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Zullo, J.M.; Drake, D.; Aron, L.; O’Hern, P.; Dhamne, S.C.; Davidsohn, N.; Mao, C.-A.; Klein, W.H.; Rotenberg, A.; Bennett, D.A.; et al. Regulation of Lifespan by Neural Excitation and REST. Nature 2019, 574, 359–364. [Google Scholar] [CrossRef]
- Fergestad, T.; Ganetzky, B.; Palladino, M.J. Neuropathology in Drosophila Membrane Excitability Mutants. Genetics 2006, 172, 1031. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-O.; Karagas, N.E.; Jung, J.; Wang, Q.; Rousseau, M.A.; Chao, Y.; Insolera, R.; Soppina, P.; Collins, C.A.; Zhou, Y.; et al. Regulation of Longevity by Depolarization-Induced Activation of PLC-β-IP3R Signaling in Neurons. Proc. Natl. Acad. Sci. USA 2021, 118, e2004253118. [Google Scholar] [CrossRef]
- Reynolds, E.R. Shortened Lifespan and Other Age-Related Defects in Bang Sensitive Mutants of Drosophila Melanogaster. G3 Genes Genomes Genet. 2018, 8, 3953–3960. [Google Scholar] [CrossRef]
- Garber, G.; Smith, L.A.; Reenan, R.A.; Rogina, B. Effect of Sodium Channel Abundance on Drosophila Development, Reproductive Capacity and Aging. Fly 2012, 6, 57–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cirelli, C.; Bushey, D.; Hill, S.; Huber, R.; Kreber, R.; Ganetzky, B.; Tononi, G. Reduced Sleep in Drosophila Shaker Mutants. Nature 2005, 434, 1087–1092. [Google Scholar] [CrossRef]
- Palladino, M.J.; Bower, J.E.; Kreber, R.; Ganetzky, B. Neural Dysfunction and Neurodegeneration in Drosophila Na+/K+ ATPase Alpha Subunit Mutants. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 1276–1286. [Google Scholar] [CrossRef]
- Andries, M.; van Damme, P.; Robberecht, W.; van den Bosch, L. Ivermectin Inhibits AMPA Receptor-Mediated Excitotoxicity in Cultured Motor Neurons and Extends the Life Span of a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2007, 25, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Warnier, M.; Flaman, J.-M.; Chouabe, C.; Wiel, C.; Gras, B.; Griveau, A.; Blanc, E.; Foy, J.-P.; Mathot, P.; Saintigny, P.; et al. The SCN9A Channel and Plasma Membrane Depolarization Promote Cellular Senescence through Rb Pathway. Aging Cell 2018, 17, e12736. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, H.; Mao, Z.; Zhang, L.; Bao, K.; Jiang, B.; Xia, C.; Li, W.; Hu, Z.; Li, J. Verapamil Extends Lifespan in Caenorhabditis Elegans by Inhibiting Calcineurin Activity and Promoting Autophagy. Aging 2020, 12, 5300–5317. [Google Scholar] [CrossRef]
- Sutphin, G.L.; Backer, G.; Sheehan, S.; Bean, S.; Corban, C.; Liu, T.; Peters, M.J.; van Meurs, J.B.J.; Murabito, J.M.; Johnson, A.D.; et al. Caenorhabditis Elegans Orthologs of Human Genes Differentially Expressed with Age Are Enriched for Determinants of Longevity. Aging Cell 2017, 16, 672–682. [Google Scholar] [CrossRef]
- Moore, S.J.; Murphy, G.G. The Role of L-Type Calcium Channels in Neuronal Excitability and Aging. Neurobiol. Learn. Mem. 2020, 173, 107230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Lu, K.; Wang, F.; Deng, J.; Xu, Z.; Wang, X.; Zhou, Q.; Le, W.; Zhao, Y. Verapamil Ameliorates Motor Neuron Degeneration and Improves Lifespan in the SOD1 G93A Mouse Model of ALS by Enhancing Autophagic Flux. Aging Dis. 2019, 10, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Huntula, S.; Saegusa, H.; Wang, X.; Zong, S.; Tanabe, T. Involvement of N-Type Ca2+ Channel in Microglial Activation and Its Implications to Aging-Induced Exaggerated Cytokine Response. Cell Calcium 2019, 82, 102059. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamaguchi, T.; Sakakibara, Y.; Taguchi, K.; Maeda, M.; Kuzuya, M.; Hattori, Y. ENOS-Dependent Antisenscence Effect of a Calcium Channel Blocker in Human Endothelial Cells. PLoS ONE 2014, 9, e88391. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, G.; Wang, H.; Si, X.; Zhou, G.; Xiong, Y.; Li, S.; Dai, R.; Yang, C. TRPC5 Channel Modulates Endothelial Cells Senescence. Eur. J. Pharmacol. 2017, 802, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Belrose, J.C.; Xie, Y.-F.; Gierszewski, L.J.; MacDonald, J.F.; Jackson, M.F. Loss of Glutathione Homeostasis Associated with Neuronal Senescence Facilitates TRPM2 Channel Activation in Cultured Hippocampal Pyramidal Neurons. Mol. Brain 2012, 5, 11. [Google Scholar] [CrossRef]
- Yee, N.S.; Brown, R.D.; Lee, M.S.; Zhou, W.; Jensen, C.; Gerke, H.; Yee, R.K. TRPM8 Ion Channel Is Aberrantly Expressed and Required for Preventing Replicative Senescence in Pancreatic Adenocarcinoma: Potential Role of TRPM8 as a Biomarker and Target. Cancer Biol. Ther. 2012, 13, 592–599. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Lee, M. Transient Receptor Potential Channel TRPM8 Is Over-Expressed and Required for Cellular Proliferation in Pancreatic Adenocarcinoma. Cancer Lett. 2010, 297, 49–55. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Lee, M.; Yee, R.K. Targeted Silencing of TRPM7 Ion Channel Induces Replicative Senescence and Produces Enhanced Cytotoxicity with Gemcitabine in Pancreatic Adenocarcinoma. Cancer Lett. 2012, 318, 99–105. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, Ischemia and Excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Iizuka, A.; Nakamura, K.; Hirai, H. Long-Term Oral Administration of the NMDA Receptor Antagonist Memantine Extends Life Span in Spinocerebellar Ataxia Type 1 Knock-in Mice. Neurosci. Lett. 2015, 592, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, J. C9orf72 Dipeptide Repeats Cause Selective Neurodegeneration and Cell-Autonomous Excitotoxicity in Drosophila Glutamatergic Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 7741–7752. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L.; Barnes, C.A. Regional Changes in the Hippocampal Density of AMPA and NMDA Receptors across the Lifespan of the Rat. Brain Res. 2000, 885, 1–5. [Google Scholar] [CrossRef]
- Adams, M.M.; Shi, L.; Linville, M.C.; Forbes, M.E.; Long, A.B.; Bennett, C.; Newton, I.G.; Carter, C.S.; Sonntag, W.E.; Riddle, D.R.; et al. Caloric Restriction and Age Affect Synaptic Proteins in Hippocampal CA3 and Spatial Learning Ability. Exp. Neurol. 2008, 211, 141–149. [Google Scholar] [CrossRef]
- Dubal, D.B.; Zhu, L.; Sanchez, P.E.; Worden, K.; Broestl, L.; Johnson, E.; Ho, K.; Yu, G.Q.; Kim, D.; Betourne, A.; et al. Life Extension Factor Klotho Prevents Mortality and Enhances Cognition in HAPP Transgenic Mice. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 2358–2371. [Google Scholar] [CrossRef]
- Papadia, S.; Soriano, F.X.; Léveillé, F.; Martel, M.A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA Receptor Activity Boosts Intrinsic Antioxidant Defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Caccamo, A.; Medina, D.X.; Benavides, A.D.; Javors, M.A.; Kraig, E.; Strong, R.; Richardson, A.; Oddo, S. Lifelong Rapamycin Administration Ameliorates Age-Dependent Cognitive Deficits by Reducing IL-1β and Enhancing NMDA Signaling. Aging Cell 2012, 11, 326–335. [Google Scholar] [CrossRef]
- Shi, L.; Adams, M.M.; Linville, M.C.; Newton, I.G.; Forbes, M.E.; Long, A.B.; Riddle, D.R.; Brunso-Bechtold, J.K. Caloric Restriction Eliminates the Aging-Related Decline in NMDA and AMPA Receptor Subunits in the Rat Hippocampus and Induces Homeostasis. Exp. Neurol. 2007, 206, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Vural, H.; Yilmaz, M.; Sutcu, R.; Sirmali, R.; Hicyilmaz, H.; Delibas, N. Calorie Restriction Modulates Hippocampal NMDA Receptors in Diet-Induced Obese Rats. J. Recept. Signal Transduct. Res. 2011, 31, 214–219. [Google Scholar] [CrossRef]
- Woll, K.A.; van Petegem, F. Calcium-Release Channels: Structure and Function of IP 3 Receptors and Ryanodine Receptors. Physiol. Rev. 2022, 102, 209–268. [Google Scholar] [CrossRef]
- Van de Leemput, J.; Chandran, J.; Knight, M.A.; Holtzclaw, L.A.; Scholz, S.; Cookson, M.R.; Houlden, H.; Gwinn-Hardy, K.; Fung, H.-C.; Lin, X.; et al. Deletion at ITPR1 Underlies Ataxia in Mice and Spinocerebellar Ataxia 15 in Humans. PLoS Genet. 2007, 3, e108. [Google Scholar] [CrossRef] [PubMed]
- Klar, J.; Ali, Z.; Farooq, M.; Khan, K.; Wikström, J.; Iqbal, M.; Zulfiqar, S.; Faryal, S.; Baig, S.M.; Dahl, N. A Missense Variant in ITPR1 Provides Evidence for Autosomal Recessive SCA29 with Asymptomatic Cerebellar Hypoplasia in Carriers. Eur. J. Hum. Genet. 2017, 25, 848–853. [Google Scholar] [CrossRef]
- Sasaki, M.; Ohba, C.; Iai, M.; Hirabayashi, S.; Osaka, H.; Hiraide, T.; Saitsu, H.; Matsumoto, N. Sporadic Infantile-Onset Spinocerebellar Ataxia Caused by Missense Mutations of the Inositol 1,4,5-Triphosphate Receptor Type 1 Gene. J. Neurol. 2015, 262, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chardon, J.W.; Carter, M.T.; Friend, K.L.; Dudding, T.E.; Schwartzentruber, J.; Zou, R.; Schofield, P.W.; Douglas, S.; Bulman, D.E.; et al. Missense Mutations in ITPR1 Cause Autosomal Dominant Congenital Nonprogressive Spinocerebellar Ataxia. Orphanet J. Rare Dis. 2012, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Alzayady, K.J.; Burglen, L.; Brémond-Gignac, D.; Marchesin, V.; Roche, O.; Rio, M.; Funalot, B.; Calmon, R.; Durr, A.; et al. Recessive and Dominant De Novo ITPR1 Mutations Cause Gillespie Syndrome. Am. J. Hum. Genet. 2016, 98, 971–980. [Google Scholar] [CrossRef] [PubMed]
- McEntagart, M.; Williamson, K.A.; Rainger, J.K.; Wheeler, A.; Seawright, A.; De Baere, E.; Verdin, H.; Bergendahl, L.T.; Quigley, A.; Rainger, J.; et al. A Restricted Repertoire of De Novo Mutations in ITPR1 Cause Gillespie Syndrome with Evidence for Dominant-Negative Effect. Am. J. Hum. Genet. 2016, 98, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Nakagawa, T.; Inoue, T.; Nagata, E.; Tanaka, K.; Takano, H.; Kuno, J.; Sakakibara, S.; Yamada, M.; Yoneshima, H.; et al. Ataxia and Epileptic Seizures in Mice Lacking Type 1 Inositol 1,4,5-Trisphosphate Receptor. Nature 1996, 379, 168–171. [Google Scholar] [CrossRef]
- Higo, T.; Hamada, K.; Hisatsune, C.; Nukina, N.; Hashikawa, T.; Hattori, M.; Nakamura, T.; Mikoshiba, K. Mechanism of ER Stress-Induced Brain Damage by IP(3) Receptor. Neuron 2010, 68, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Kasumu, A.W.; Liang, X.; Egorova, P.; Vorontsova, D.; Bezprozvanny, I. Chronic Suppression of Inositol 1,4,5-Triphosphate Receptor-Mediated Calcium Signaling in Cerebellar Purkinje Cells Alleviates Pathological Phenotype in Spinocerebellar Ataxia 2 Mice. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 12786–12796. [Google Scholar] [CrossRef]
- Chen, X.; Tang, T.-S.; Tu, H.; Nelson, O.; Pook, M.; Hammer, R.; Nukina, N.; Bezprozvanny, I. Deranged Calcium Signaling and Neurodegeneration in Spinocerebellar Ataxia Type 3. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 12713–12724. [Google Scholar] [CrossRef]
- Tang, T.-S.; Tu, H.; Chan, E.Y.W.; Maximov, A.; Wang, Z.; Wellington, C.L.; Hayden, M.R.; Bezprozvanny, I. Huntingtin and Huntingtin-Associated Protein 1 Influence Neuronal Calcium Signaling Mediated by Inositol-(1,4,5) Triphosphate Receptor Type 1. Neuron 2003, 39, 227–239. [Google Scholar] [CrossRef]
- Tiscione, S.A.; Casas, M.; Horvath, J.D.; Lam, V.; Hino, K.; Ory, D.S.; Santana, L.F.; Simó, S.; Dixon, R.E.; Dickson, E.J. IP3R-Driven Increases in Mitochondrial Ca2+ Promote Neuronal Death in NPC Disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2110629118. [Google Scholar] [CrossRef] [PubMed]
- Staats, K.A.; Humblet-Baron, S.; Bento-Abreu, A.; Scheveneels, W.; Nikolaou, A.; Deckers, K.; Lemmens, R.; Goris, A.; van Ginderachter, J.A.; van Damme, P.; et al. Genetic Ablation of IP3 Receptor 2 Increases Cytokines and Decreases Survival of SOD1G93A Mice. Hum. Mol. Genet. 2016, 25, 3491–3499. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Vindrieux, D.; Goehrig, D.; Jaber, S.; Collin, G.; Griveau, A.; Wiel, C.; Bendridi, N.; Djebali, S.; Farfariello, V.; et al. Calcium Channel ITPR2 and Mitochondria-ER Contacts Promote Cellular Senescence and Aging. Nat. Commun. 2021, 12, 720. [Google Scholar] [CrossRef]
- Bartok, A.; Weaver, D.; Golenár, T.; Nichtova, Z.; Katona, M.; Bánsághi, S.; Alzayady, K.J.; Thomas, V.K.; Ando, H.; Mikoshiba, K.; et al. IP3 Receptor Isoforms Differently Regulate ER-Mitochondrial Contacts and Local Calcium Transfer. Nat. Commun. 2019, 10, 3726. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Lallet-Daher, H.; Gitenay, D.; Gras, B.; le Calvé, B.; Augert, A.; Ferrand, M.; Prevarskaya, N.; Simonnet, H.; Vindrieux, D.; et al. Endoplasmic Reticulum Calcium Release through ITPR2 Channels Leads to Mitochondrial Calcium Accumulation and Senescence. Nat. Commun. 2014, 5, 3792. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Warnier, M.; Raynard, C.; Ferrand, M.; Kirsh, O.; Defossez, P.-A.; Martin, N.; Bernard, D. The Nuclear Receptor RXRA Controls Cellular Senescence by Regulating Calcium Signaling. Aging Cell 2018, 17, e12831. [Google Scholar] [CrossRef]
- Kim, S.H.; Zhan, L.; Hanson, K.A.; Tibbetts, R.S. High-Content RNAi Screening Identifies the Type 1 Inositol Triphosphate Receptor as a Modifier of TDP-43 Localization and Neurotoxicity. Hum. Mol. Genet. 2012, 21, 4845–4856. [Google Scholar] [CrossRef]
- Chun, L.; Gong, J.; Yuan, F.; Zhang, B.; Liu, H.; Zheng, T.; Yu, T.; Xu, X.Z.S.; Liu, J. Metabotropic GABA Signalling Modulates Longevity in C. elegans. Nat. Commun. 2015, 6, 8828. [Google Scholar] [CrossRef] [PubMed]
- Kawli, T.; Wu, C.; Tan, M.-W. Systemic and Cell Intrinsic Roles of Gqalpha Signaling in the Regulation of Innate Immunity, Oxidative Stress, and Longevity in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. USA 2010, 107, 13788–13793. [Google Scholar] [CrossRef] [PubMed]
- Burkewitz, K.; Feng, G.; Dutta, S.; Kelley, C.A.; Steinbaugh, M.; Cram, E.J.; Mair, W.B. Atf-6 Regulates Lifespan through ER-Mitochondrial Calcium Homeostasis. Cell Rep. 2020, 32, 108125. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, C.J.; Anderson, D.J. The Neuron-Restrictive Silencer Factor (NRSF): A Coordinate Repressor of Multiple Neuron-Specific Genes. Science 1995, 267, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and Its Corepressors Mediate Plasticity of Neuronal Gene Chromatin throughout Neurogenesis. Cell 2005, 121, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.A.; Tapia-Ramirez, J.; Kim, S.; Toledo-Aral, J.J.; Zheng, Y.; Boutros, M.C.; Altshuller, Y.M.; Frohman, M.A.; Kraner, S.D.; Mandel, G. REST: A Mammalian Silencer Protein That Restricts Sodium Channel Gene Expression to Neurons. Cell 1995, 80, 949–957. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Zukin, R.S. REST, a Master Transcriptional Regulator in Neurodegenerative Disease. Curr. Opin. Neurobiol. 2018, 48, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.-H.; Kim, H.-M.; Drake, D.; Liu, X.S.; et al. REST and Stress Resistance in Ageing and Alzheimer’s Disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Feldman, H.M.; Lu, T.; Drake, D.; Lim, E.T.; Ling, K.-H.; Bishop, N.A.; Pan, Y.; Seo, J.; Lin, Y.-T.; et al. REST and Neural Gene Network Dysregulation in IPSC Models of Alzheimer’s Disease. Cell Rep. 2019, 26, 1112–1127.e9. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Tartari, M.; Crotti, A.; Goffredo, D.; Valenza, M.; Conti, L.; Cataudella, T.; Leavitt, B.R.; Hayden, M.R.; Timmusk, T.; et al. Huntingtin Interacts with REST/NRSF to Modulate the Transcription of NRSE-Controlled Neuronal Genes. Nat. Genet. 2003, 35, 76–83. [Google Scholar] [CrossRef]
- Zuccato, C.; Belyaev, N.; Conforti, P.; Ooi, L.; Tartari, M.; Papadimou, E.; MacDonald, M.; Fossale, E.; Zeitlin, S.; Buckley, N.; et al. Widespread Disruption of Repressor Element-1 Silencing Transcription Factor/Neuron-Restrictive Silencer Factor Occupancy at Its Target Genes in Huntington’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 6972–6983. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A Forty-Kilodalton Protein of the Inner Membrane Is the Mitochondrial Calcium Uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Perocchi, F.; Gohil, V.M.; Girgis, H.S.; Bao, X.R.; McCombs, J.E.; Palmer, A.E.; Mootha, V.K. MICU1 Encodes a Mitochondrial EF Hand Protein Required for Ca2+ Uptake. Nature 2010, 467, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Plovanich, M.; Bogorad, R.L.; Sancak, Y.; Kamer, K.J.; Strittmatter, L.; Li, A.A.; Girgis, H.S.; Kuchimanchi, S.; de Groot, J.; Speciner, L.; et al. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PLoS ONE 2013, 8, e55785. [Google Scholar] [CrossRef]
- Mallilankaraman, K.; Cárdenas, C.; Doonan, P.J.; Chandramoorthy, H.C.; Irrinki, K.M.; Golenár, T.; Csordás, G.; Madireddi, P.; Yang, J.; Müller, M.; et al. MCUR1 Is an Essential Component of Mitochondrial Ca2+ Uptake That Regulates Cellular Metabolism. Nat. Cell Biol. 2012, 14, 1336–1343. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Yang, W.; Liao, Z.-Y.; Wu, Y.-X.; Fan, Z.; Guo, A.; Yu, J.; Chen, Q.-N.; Wu, J.-H.; Zhou, J.; et al. MICU3 Regulates Mitochondrial Ca2+-Dependent Antioxidant Response in Skeletal Muscle Aging. Cell Death Dis. 2021, 12, 1115. [Google Scholar] [CrossRef]
- Qiu, J.; Tan, Y.-W.; Hagenston, A.M.; Martel, M.-A.; Kneisel, N.; Skehel, P.A.; Wyllie, D.J.A.; Bading, H.; Hardingham, G.E. Mitochondrial Calcium Uniporter Mcu Controls Excitotoxicity and Is Transcriptionally Repressed by Neuroprotective Nuclear Calcium Signals. Nat. Commun. 2013, 4, 2034. [Google Scholar] [CrossRef]
- König, T.; Tröder, S.E.; Bakka, K.; Korwitz, A.; Richter-Dennerlein, R.; Lampe, P.A.; Patron, M.; Mühlmeister, M.; Guerrero-Castillo, S.; Brandt, U.; et al. The M-AAA Protease Associated with Neurodegeneration Limits MCU Activity in Mitochondria. Mol. Cell 2016, 64, 148–162. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Lu, X.; Correll, R.N.; Schwanekamp, J.A.; Vagnozzi, R.J.; Sargent, M.A.; York, A.J.; Zhang, J.; Bers, D.M.; Molkentin, J.D. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Pan, X.; Liu, J.C.; Menazza, S.; Liu, J.; Nguyen, T.T.; Pan, H.; Parks, R.J.; Anderson, S.; Noguchi, A.; et al. Assessment of Cardiac Function in Mice Lacking the Mitochondrial Calcium Uniporter. J. Mol. Cell. Cardiol. 2015, 85, 178–182. [Google Scholar] [CrossRef]
- Dong, Z.; Shanmughapriya, S.; Tomar, D.; Siddiqui, N.; Lynch, S.; Nemani, N.; Breves, S.L.; Zhang, X.; Tripathi, A.; Palaniappan, P.; et al. Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor That Augments MCU Channel Activity. Mol. Cell 2017, 65, 1014–1028.e7. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Quan, X.; Bang, S.; Yoo, H.; Kim, J.; Park, J.; Park, K.-S.; Chung, J. Mitochondrial Calcium Uniporter in Drosophila Transfers Calcium between the Endoplasmic Reticulum and Mitochondria in Oxidative Stress-Induced Cell Death. J. Biol. Chem. 2017, 292, 14473–14485. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Fujii, M.; Hartman, P.S.; Tsuda, M.; Yasuda, K.; Senoo-Matsuda, N.; Yanase, S.; Ayusawa, D.; Suzuki, K. A Mutation in Succinate Dehydrogenase Cytochrome b Causes Oxidative Stress and Ageing in Nematodes. Nature 1998, 394, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Senoo-Matsuda, N.; Yasuda, K.; Tsuda, M.; Ohkubo, T.; Yoshimura, S.; Nakazawa, H.; Hartman, P.S.; Ishii, N. A Defect in the Cytochrome b Large Subunit in Complex II Causes Both Superoxide Anion Overproduction and Abnormal Energy Metabolism in Caenorhabditis Elegans. J. Biol. Chem. 2001, 276, 41553–41558. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, S.Y.; Canbakis Cecen, F.S.; Cho, Y.; Kwon, S.-K. Dysfunction of Mitochondrial Ca2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 599792. [Google Scholar] [CrossRef] [PubMed]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX Is an Essential Component of Mitochondrial Na+/Ca2+ Exchange. Proc. Natl. Acad. Sci. USA 2010, 107, 436–441. [Google Scholar] [CrossRef]
- Luongo, T.S.; Lambert, J.P.; Gross, P.; Nwokedi, M.; Lombardi, A.A.; Shanmughapriya, S.; Carpenter, A.C.; Kolmetzky, D.; Gao, E.; van Berlo, J.H.; et al. The Mitochondrial Na+/Ca2+ Exchanger Is Essential for Ca2+ Homeostasis and Viability. Nature 2017, 545, 93–97. [Google Scholar] [CrossRef]
- De Marchi, U.; Santo-Domingo, J.; Castelbou, C.; Sekler, I.; Wiederkehr, A.; Demaurex, N. NCLX Protein, but Not LETM1, Mediates Mitochondrial Ca2+ Extrusion, Thereby Limiting Ca2+-Induced NAD(P)H Production and Modulating Matrix Redox State. J. Biol. Chem. 2014, 289, 20377–20385. [Google Scholar] [CrossRef]
- Natarajan, G.K.; Glait, L.; Mishra, J.; Stowe, D.F.; Camara, A.K.S.; Kwok, W.-M. Total Matrix Ca2+ Modulates Ca2+ Efflux via the Ca2+/H+ Exchanger in Cardiac Mitochondria. Front. Physiol. 2020, 11, 510600. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, L.; Clapham, D.E. Genome-Wide RNAi Screen Identifies Letm1 as a Mitochondrial Ca2+/H+ Antiporter. Science 2009, 326, 144–147. [Google Scholar] [CrossRef]
- Hagenston, A.M.; Yan, J.; Bas-Orth, C.; Tan, Y.; Sekler, I.; Bading, H. Disrupted Expression of Mitochondrial NCLX Sensitizes Neuroglial Networks to Excitotoxic Stimuli and Renders Synaptic Activity Toxic. J. Biol. Chem. 2021, 298, 101508. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired Mitochondrial Calcium Efflux Contributes to Disease Progression in Models of Alzheimer’s Disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef] [PubMed]
- Ludtmann, M.H.R.; Kostic, M.; Horne, A.; Gandhi, S.; Sekler, I.; Abramov, A.Y. LRRK2 Deficiency Induced Mitochondrial Ca2+ Efflux Inhibition Can Be Rescued by Na+/Ca2+/Li+ Exchanger Upregulation. Cell Death Dis. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Callio, J.; Otero, P.A.; Sekler, I.; Wills, Z.P.; Chu, C.T. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 11151–11165. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.; Ludtmann, M.H.R.; Bading, H.; Hershfinkel, M.; Steer, E.; Chu, C.T.; Abramov, A.Y.; Sekler, I. PKA Phosphorylation of NCLX Reverses Mitochondrial Calcium Overload and Depolarization, Promoting Survival of PINK1-Deficient Dopaminergic Neurons. Cell Rep. 2015, 13, 376–386. [Google Scholar] [CrossRef]
- Dolga, A.M.; Netter, M.F.; Perocchi, F.; Doti, N.; Meissner, L.; Tobaben, S.; Grohm, J.; Zischka, H.; Plesnila, N.; Decher, N.; et al. Mitochondrial Small Conductance SK2 Channels Prevent Glutamate-Induced Oxytosis and Mitochondrial Dysfunction. J. Biol. Chem. 2013, 288, 10792–10804. [Google Scholar] [CrossRef]
- Krabbendam, I.E.; Honrath, B.; Dilberger, B.; Iannetti, E.F.; Branicky, R.S.; Meyer, T.; Evers, B.; Dekker, F.J.; Koopman, W.J.H.; Beyrath, J.; et al. SK Channel-Mediated Metabolic Escape to Glycolysis Inhibits Ferroptosis and Supports Stress Resistance in C. elegans. Cell Death Dis. 2020, 11, 263. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Wang, S.; McDonald, T.; van Eyk, J.E.; Sidor, A.; O’Rourke, B. Cytoprotective Role of Ca2+-Activated K+ Channels in the Cardiac Inner Mitochondrial Membrane. Science 2002, 298, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Gururaja Rao, S.; Bednarczyk, P.; Towheed, A.; Shah, K.; Karekar, P.; Ponnalagu, D.; Jensen, H.N.; Addya, S.; Reyes, B.A.S.; van Bockstaele, E.J.; et al. BKCa (Slo) Channel Regulates Mitochondrial Function and Lifespan in Drosophila Melanogaster. Cells 2019, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Zhang, C.Y.; Lowell, B.B. The Mitochondrial Uncoupling-Protein Homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Horvath, T.L. The Role of Mitochondrial Uncoupling Proteins in Lifespan. Pflug. Arch. Eur. J. Physiol. 2010, 459, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive but Not Obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling Protein-2: A Novel Gene Linked to Obesity and Hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.P. Uncoupling Protein-3: A New Member of the Mitochondrial Carrier Family with Tissue-Specific Expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Plerre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Arsenijevic, D.; Onuma, H.; Pecqueur, C.; Raimbault, S.; Manning, B.S.; Miroux, B.; Couplan, E.; Alves-Guerra, M.C.; Goubern, M.; Surwit, R.; et al. Disruption of the Uncoupling Protein-2 Gene in Mice Reveals a Role in Immunity and Reactive Oxygen Species Production. Nat. Genet. 2000, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.J.; Grujic, D.; Zhang, C.Y.; Hagen, T.; Boss, O.; Ido, Y.; Szczepanik, A.; Wade, J.; Mootha, V.; Cortright, R.; et al. Energy Metabolism in Uncoupling Protein 3 Gene Knockout Mice. J. Biol. Chem. 2000, 275, 16258–16266. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schneitler, C.; Oberkofler, H.; Ebenbichler, C.; Paulweber, B.; Sandhofer, F.; Ladurner, G.; Hell, E.; Strosberg, A.D.; Patsch, J.R.; et al. A Common Polymorphism in the Promoter of UCP2 Is Associated with Decreased Risk of Obesity in Middle-Aged Humans. Nat. Genet. 2001, 28, 178–183. [Google Scholar] [CrossRef]
- Wolkow, C.A.; Iser, W.B. Uncoupling Protein Homologs May Provide a Link between Mitochondria, Metabolism and Lifespan. Ageing Res. Rev. 2006, 5, 196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klingenberg, M.; Appel, M. The Uncoupling Protein Dimer Can Form a Disulfide Cross-Link between the Mobile C-Terminal SH Groups. Eur. J. Biochem. 1989, 180, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Fatty Acid Circuit as a Physiological Mechanism of Uncoupling of Oxidative Phosphorylation. FEBS Lett. 1991, 294, 158–162. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High Protonic Potential Actuates a Mechanism of Production of Reactive Oxygen Species in Mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Duval, C.; Nègre-Salvayre, A.; Doglio, A.; Salvayre, R.; Pénicaud, L.; Casteilla, L. Increased Reactive Oxygen Species Production with Antisense Oligonucleotides Directed against Uncoupling Protein 2 in Murine Endothelial Cells. Biochem. Cell Biol. 2002, 80, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Nègre-Salvayre, A.; Hirtz, C.; Carrera, G.; Cazenave, R.; Troly, M.; Salvayre, R.; Pénicaud, L.; Casteilla, L. A Role for Uncoupling Protein-2 as a Regulator of Mitochondrial Hydrogen Peroxide Generation. FASEB J. 1997, 11, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Bussière, F.; Hekimi, S. Mitochondrial Electron Transport Is a Key Determinant of Life Span in Caenorhabditis Elegans. Dev. Cell 2001, 1, 633–644. [Google Scholar] [CrossRef]
- Holzenberger, M.; Dupont, J.; Ducos, B.; Leneuve, P.; Géloën, A.; Even, P.C.; Cervera, P.; le Bouc, Y. IGF-1 Receptor Regulates Lifespan and Resistance to Oxidative Stress in Mice. Nature 2003, 421, 182–187. [Google Scholar] [CrossRef]
- Fridell, Y.W.C.; Sánchez-Blanco, A.; Silvia, B.A.; Helfand, S.L. Targeted Expression of the Human Uncoupling Protein 2 (HUCP2) to Adult Neurons Extends Life Span in the Fly. Cell Metab. 2005, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, R.Y.N.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Ruvkun, G. A Systematic RNAi Screen Identifies a Critical Role for Mitochondria in C. elegans Longevity. Nat. Genet. 2003, 33, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dillin, A.; Hsu, A.L.; Arantes-Oliveira, N.; Lehrer-Graiwer, J.; Hsin, H.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Kenyon, C. Rates of Behavior and Aging Specified by Mitochondrial Function during Development. Science 2002, 298, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Talbot, D.A.; Selman, C.; Snart, S.; McLaren, J.S.; Redman, P.; Krol, E.; Jackson, D.M.; Johnson, M.S.; Brand, M.D. Uncoupled and Surviving: Individual Mice with High Metabolism Have Greater Mitochondrial Uncoupling and Live Longer. Aging Cell 2004, 3, 87–95. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Horvath, T.L. Uncoupling Protein-2 Regulates Lifespan in Mice. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E621–E627. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Sanchez-Alavez, M.; Winsky-Sommerer, R.; Morale, M.C.; Lucero, J.; Brownell, S.; Fabre, V.; Huitron-Resendiz, S.; Henriksen, S.; Zorrilla, E.P.; et al. Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Science 2006, 314, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Fridell, Y.W.C.; Hoh, M.; Kréneisz, O.; Hosier, S.; Chang, C.; Scantling, D.; Mulkey, D.K.; Helfand, S.L. Increased Uncoupling Protein (UCP) Activity in Drosophila Insulin-Producing Neurons Attenuates Insulin Signaling and Extends Lifespan. Aging 2009, 1, 699–713. [Google Scholar] [CrossRef][Green Version]
- Humphrey, D.M.; Toivonen, J.M.; Giannakou, M.; Partridge, L.; Brand, M.D. Expression of Human Uncoupling Protein-3 in Drosophila Insulin-Producing Cells Increases Insulin-like Peptide (DILP) Levels and Shortens Lifespan. Exp. Gerontol. 2009, 44, 316–327. [Google Scholar] [CrossRef]
- Bevilacqua, L.; Ramsey, J.J.; Hagopian, K.; Weindruch, R.; Harper, M.E. Effects of Short- and Medium-Term Calorie Restriction on Muscle Mitochondrial Proton Leak and Reactive Oxygen Species Production. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E852–E861. [Google Scholar] [CrossRef]
- McDonald, R.B.; Walker, K.M.; Warman, D.B.; Griffey, S.M.; Warden, C.H.; Ramsey, J.J.; Horwitz, B.A. Characterization of Survival and Phenotype throughout the Life Span in UCP2/UCP3 Genetically Altered Mice. Exp. Gerontol. 2008, 43, 1061–1068. [Google Scholar] [CrossRef]
- Brand, M.D. Uncoupling to Survive? The Role of Mitochondrial Inefficiency in Ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]
- Gates, A.C.; Bernal-Mizrachi, C.; Chinault, S.L.; Feng, C.; Schneider, J.G.; Coleman, T.; Malone, J.P.; Townsend, R.R.; Chakravarthy, M.V.; Semenkovich, C.F. Respiratory Uncoupling in Skeletal Muscle Delays Death and Diminishes Age-Related Disease. Cell Metab. 2007, 6, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Crocco, P.; D’Aquila, P.; Montesanto, A.; Bellizzi, D.; Passarino, G. Two Variants Located in the Upstream Enhancer Region of Human UCP1 Gene Affect Gene Expression and Are Correlated with Human Longevity. Exp. Gerontol. 2011, 46, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Kontani, Y.; Wang, Y.; Kimura, K.; Inokuma, K.I.; Saito, M.; Suzuki-Miura, T.; Wang, Z.; Sato, Y.; Mori, N.; Yamashita, H. UCP1 Deficiency Increases Susceptibility to Diet-Induced Obesity with Age. Aging Cell 2005, 4, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Vellai, T.; Takács-Vellai, K.; Sass, M.; Klionsky, D.J. The Regulation of Aging: Does Autophagy Underlie Longevity? Trends Cell Biol. 2009, 19, 487–494. [Google Scholar] [CrossRef]
- Nakamura, S.; Yoshimori, T. Autophagy and Longevity. Mol. Cells 2018, 41, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Tazi, M.; Caution, K.; Ahmed, A.; Kanneganti, A.; Assani, K.; Kopp, B.; Marsh, C.; Dakhlallah, D.; Amer, A.O. Aging Is Associated with Hypermethylation of Autophagy Genes in Macrophages. Epigenetics 2016, 11, 381–388. [Google Scholar] [CrossRef]
- Liu, Y.; Palanivel, R.; Rai, E.; Park, M.; Gabor, T.V.; Scheid, M.P.; Xu, A.; Sweeney, G. Adiponectin Stimulates Autophagy and Reduces Oxidative Stress to Enhance Insulin Sensitivity during High-Fat Diet Feeding in Mice. Diabetes 2015, 64, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Scaravilli, F.; Easton, D.F.; Duden, R.; O’Kane, C.J.; et al. Inhibition of MTOR Induces Autophagy and Reduces Toxicity of Polyglutamine Expansions in Fly and Mouse Models of Huntington Disease. Nat. Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in Mice Activates Autophagy and Extends Lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Bjedov, I.; Cochemé, H.M.; Foley, A.; Wieser, D.; Woodling, N.S.; Castillo-Quan, J.I.; Norvaisas, P.; Lujan, C.; Regan, J.; Toivonen, J.M.; et al. Fine-Tuning Autophagy Maximises Lifespan and Is Associated with Changes in Mitochondrial Gene Expression in Drosophila. PLoS Genet. 2020, 16, e1009083. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A Role for Autophagy in the Extension of Lifespan by Dietary Restriction in C. elegans. PLoS Genet. 2008, 4, e24. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Zhao, D.; Li, X.; Yang, C.; Wang, X. Lysosome Activity Is Modulated by Multiple Longevity Pathways and Is Important for Lifespan Extension in C. elegans. eLife 2020, 9, e55745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cuervo, A.M. Restoration of Chaperone-Mediated Autophagy in Aging Liver Improves Cellular Maintenance and Hepatic Function. Nat. Med. 2008, 14, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Kiffin, R.; Christian, C.; Knecht, E.; Cuervo, A.M. Activation of Chaperone-Mediated Autophagy during Oxidative Stress. Mol. Biol. Cell 2004, 15, 4829–4840. [Google Scholar] [CrossRef] [PubMed]
- Bouderlique, T.; Vuppalapati, K.K.; Newton, P.T.; Li, L.; Barenius, B.; Chagin, A.S. Targeted Deletion of Atg5 in Chondrocytes Promotes Age-Related Osteoarthritis. Ann. Rheum. Dis. 2016, 75, 627–631. [Google Scholar] [CrossRef]
- Wu, J.J.; Quijano, C.; Chen, E.; Liu, H.; Cao, L.; Fergusson, M.M.; Rovira, I.I.; Gutkind, S.; Daniels, M.P.; Komatsu, M.; et al. Mitochondrial Dysfunction and Oxidative Stress Mediate the Physiological Impairment Induced by the Disruption of Autophagy. Aging 2009, 1, 425–437. [Google Scholar] [CrossRef]
- Juhász, G.; Érdi, B.; Sass, M.; Neufeld, T.P. Atg7-Dependent Autophagy Promotes Neuronal Health, Stress Tolerance, and Longevity but Is Dispensable for Metamorphosis in Drosophila. Genes Dev. 2007, 21, 3061–3066. [Google Scholar] [CrossRef]
- Inoue, K.; Rispoli, J.; Kaphzan, H.; Klann, E.; Chen, E.I.; Kim, J.; Komatsu, M.; Abeliovich, A. Macroautophagy Deficiency Mediates Age-Dependent Neurodegeneration through a Phospho-Tau Pathway. Mol. Neurodegener. 2012, 7, 48. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of Basal Autophagy in Neural Cells Causes Neurodegenerative Disease in Mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Wong, C.-O.; Zhu, M.X. The Role of TRPMLs in Endolysosomal Trafficking and Function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-O.; Li, R.; Montell, C.; Venkatachalam, K. Drosophila TRPML Is Required for TORC1 Activation. Curr. Biol. 2012, 22, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Wong, C.-O.; Montell, C. Feast or Famine. Autophagy 2013, 9, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Bargal, R.; Avidan, N.; Ben-Asher, E.; Olender, Z.; Zeigler, M.; Frumkin, A.; Raas-Rothschild, A.; Glusman, G.; Lancet, D.; Bach, G. Identification of the Gene Causing Mucolipidosis Type IV. Nat. Genet. 2000, 26, 118–121. [Google Scholar] [CrossRef]
- Bassi, M.T.; Manzoni, M.; Monti, E.; Pizzo, M.T.; Ballabio, A.; Borsani, G. Cloning of the Gene Encoding a Novel Integral Membrane Protein, Mucolipidin-and Identification of the Two Major Founder Mutations Causing Mucolipidosis Type IV. Am. J. Hum. Genet. 2000, 67, 1110–1120. [Google Scholar] [CrossRef]
- Sun, M.; Goldin, E.; Stahl, S.; Falardeau, J.L.; Kennedy, J.C.; Acierno, J.S.; Bove, C.; Kaneski, C.R.; Nagle, J.; Bromley, M.C.; et al. Mucolipidosis Type IV Is Caused by Mutations in a Gene Encoding a Novel Transient Receptor Potential Channel. Hum. Mol. Genet. 2000, 9, 2471–2478. [Google Scholar] [CrossRef]
- Vergarajauregui, S.; Connelly, P.S.; Daniels, M.P.; Puertollano, R. Autophagic Dysfunction in Mucolipidosis Type IV Patients. Hum. Mol. Genet. 2008, 17, 2723–2737. [Google Scholar] [CrossRef]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets MTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef]
- Xu, M.; Almasi, S.; Yang, Y.; Yan, C.; Sterea, A.M.; Rizvi Syeda, A.K.; Shen, B.; Richard Derek, C.; Huang, P.; Gujar, S.; et al. The Lysosomal TRPML1 Channel Regulates Triple Negative Breast Cancer Development by Promoting MTORC1 and Purinergic Signaling Pathways. Cell Calcium 2019, 79, 80–88. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Zhu, X.; Yao, J.; Shen, B.; Dong, X.P. Lysosomal Ca2+ Release Channel TRPML1 Regulates Lysosome Size by Promoting MTORC1 Activity. Eur. J. Cell Biol. 2019, 98, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-J.; Xu, J.; Fu, C.; Zhang, J.; Zheng, Y.G.; Jia, H.; Liu, J.O. Regulation of MTORC1 by Lysosomal Calcium and Calmodulin. eLife 2016, 5, e19360. [Google Scholar] [CrossRef] [PubMed]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The Transcription Factor TFEB Links MTORC1 Signaling to Transcriptional Control of Lysosome Homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 Functions as a Transcriptional Regulator of Autophagy by Preventing Nuclear Transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong-A, L.; Patange, S.; Raben, N.; Puertollano, R. The Nutrient-Responsive Transcription Factor TFE3 Promotes Autophagy, Lysosomal Biogenesis, and Clearance of Cellular Debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Long, A.A.; Elsaesser, R.; Nikolaeva, D.; Broadie, K.; Montell, C. Motor Deficit in a Drosophila Model of Mucolipidosis Type IV Due to Defective Clearance of Apoptotic Cells. Cell 2008, 135, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, B.; Browning, M.F.; Curcio-Morelli, C.; Varro, A.; Michaud, N.; Nanthakumar, N.; Walkley, S.U.; Pickel, J.; Slaugenhaupt, S.A. Neurologic, Gastric, and Opthalmologic Pathologies in a Murine Model of Mucolipidosis Type IV. Am. J. Hum. Genet. 2007, 81, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, D.; Neff, F.; Xie, K. Longevity, Aging and Rapamycin. Cell. Mol. Life Sci. CMLS 2014, 71, 4325–4346. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Gao, Q.; Yang, J.; Yan, X.; Zhao, H.; Su, L.; Yang, M.; Gao, C.; Yao, Y.; et al. Rapamycin Directly Activates Lysosomal Mucolipin TRP Channels Independent of MTOR. PLoS Biol. 2019, 17, e3000252. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.A.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Liu, J.; Chen, E.B.; Wang, J.J.; Cao, L.; Narayan, N.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. Increased Mammalian Lifespan and a Segmental and Tissue-Specific Slowing of Aging after Genetic Reduction of MTOR Expression. Cell Rep. 2013, 4, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Murphy, C.T. Regulation of Reproduction and Longevity by Nutrient-Sensing Pathways. J. Cell Biol. 2018, 217, 93–106. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I.; Tain, L.S.; Kinghorn, K.J.; Li, L.; Grönke, S.; Hinze, Y.; Blackwell, T.K.; Bjedov, I.; Partridge, L. A Triple Drug Combination Targeting Components of the Nutrient-Sensing Network Maximizes Longevity. Proc. Natl. Acad. Sci. USA 2019, 116, 20817–20819. [Google Scholar] [CrossRef]
- Lu, Y.X.; Regan, J.C.; Eßer, J.; Drews, L.F.; Weinseis, T.; Stinn, J.; Hahn, O.; Miller, R.A.; Grönke, S.; Partridge, L. A TORC1-Histone Axis Regulates Chromatin Organisation and Non-Canonical Induction of Autophagy to Ameliorate Ageing. eLife 2021, 10, e62233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; McBrayer, M.K.; Wolfe, D.M.; Haslett, L.J.; Kumar, A.; Sato, Y.; Lie, P.P.Y.; Mohan, P.; Coffey, E.E.; Kompella, U.; et al. Presenilin 1 Maintains Lysosomal Ca2+ Homeostasis via TRPML1 by Regulating VATPase-Mediated Lysosome Acidification. Cell Rep. 2015, 12, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Rietdorf, K.; Arredouani, A.; Davis, L.C.; Lloyd-Evans, E.; Koegel, H.; Funnell, T.M.; Morgan, A.J.; Ward, J.A.; Watanabe, K.; et al. Purified TPC Isoforms Form NAADP Receptors with Distinct Roles for Ca(2+) Signaling and Endolysosomal Trafficking. Curr. Biol. 2010, 20, 703–709. [Google Scholar] [CrossRef]
- Ruas, M.; Davis, L.C.; Chen, C.; Morgan, A.J.; Chuang, K.; Walseth, T.F.; Grimm, C.; Garnham, C.; Powell, T.; Platt, N.; et al. Expression of Ca2+-Permeable Two-Pore Channels Rescues NAADP Signalling in TPC-Deficient Cells. EMBO J. 2015, 34, 1743–1758. [Google Scholar] [CrossRef]
- Gerndt, S.; Chen, C.C.; Chao, Y.K.; Yuan, Y.; Burgstaller, S.; Rosato, A.S.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-Mediated Switching of Ion Selectivity in TPC2 Differentially Promotes Lysosomal Function. eLife 2020, 9, e54712. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dong, X.P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC Proteins Are Phosphoinositide- Activated Sodium-Selective Ion Channels in Endosomes and Lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef]
- Pitt, S.J.; Funnell, T.M.; Sitsapesan, M.; Venturi, E.; Rietdorf, K.; Ruas, M.; Ganesan, A.; Gosain, R.; Churchill, G.C.; Zhu, M.X.; et al. TPC2 Is a Novel NAADP-Sensitive Ca2+ Release Channel, Operating as a Dual Sensor of Luminal PH and Ca2+. J. Biol. Chem. 2010, 285, 35039–35046. [Google Scholar] [CrossRef] [PubMed]
- Ogunbayo, O.A.; Zhu, Y.; Rossi, D.; Sorrentino, V.; Ma, J.; Zhu, M.X.; Evans, A.M. Cyclic Adenosine Diphosphate Ribose Activates Ryanodine Receptors, Whereas NAADP Activates Two-Pore Domain Channels. J. Biol. Chem. 2011, 286, 9136–9140. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP Mobilizes Calcium from Acidic Organelles through Two-Pore Channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Grossmann, S.; Mallmann, R.T.; Rommel, C.; Hein, L.; Klugbauer, N. Two-Pore Channels Affect EGF Receptor Signaling by Receptor Trafficking and Expression. iScience 2021, 24, 102099. [Google Scholar] [CrossRef] [PubMed]
- García-Rúa, V.; Feijóo-Bandín, S.; Rodríguez-Penas, D.; Mosquera-Leal, A.; Abu-Assi, E.; Beiras, A.; María Seoane, L.; Lear, P.; Parrington, J.; Portolés, M.; et al. Endolysosomal Two-Pore Channels Regulate Autophagy in Cardiomyocytes. J. Physiol. 2016, 594, 3061–3077. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Duann, P.; Komazaki, S.; Park, K.H.; Li, H.; Sun, M.; Sermersheim, M.; Gumpper, K.; Parrington, J.; Galione, A.; et al. Lysosomal Two-Pore Channel Subtype 2 (TPC2) Regulates Skeletal Muscle Autophagic Signaling. J. Biol. Chem. 2015, 290, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.J.S.; Antonioli, M.; Hirata, H.; Ureshino, R.P.; Nascimento, A.R.; Bincoletto, C.; Vescovo, T.; Piacentini, M.; Fimia, G.M.; Smaili, S.S. Glutamate Induces Autophagy via the Two-Pore Channels in Neural Cells. Oncotarget 2017, 8, 12730–12740. [Google Scholar] [CrossRef]
- Grimm, C.; Holdt, L.M.; Chen, C.C.; Hassan, S.; Müller, C.; Jörs, S.; Cuny, H.; Kissing, S.; Schröder, B.; Butz, E.; et al. High Susceptibility to Fatty Liver Disease in Two-Pore Channel 2-Deficient Mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Cristina Brailoiu, G.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of Lysosomal Morphology by Pathogenic LRRK2 Is Corrected by TPC2 Inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef]
- Lange, P.F.; Wartosch, L.; Jentsch, T.J.; Fuhrmann, J.C. ClC-7 Requires Ostm1 as a β-Subunit to Support Bone Resorption and Lysosomal Function. Nature 2006, 440, 220–223. [Google Scholar] [CrossRef]
- Kornak, U.; Kasper, D.; Bösl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the ClC-7 Chloride Channel Leads to Osteopetrosis in Mice and Man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef]
- Jentsch, T.J. Chloride and the Endosomal-Lysosomal Pathway: Emerging Roles of CLC Chloride Transporters. J. Physiol. 2007, 578, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; He, H.; Stauber, T. Neurodegeneration Upon Dysfunction of Endosomal/Lysosomal CLC Chloride Transporters. Front. Cell Dev. Biol. 2021, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.; Planells-Cases, R.; Fuhrmann, J.C.; Scheel, O.; Zeitz, O.; Ruether, K.; Schmitt, A.; Poët, M.; Steinfeld, R.; Schweizer, M.; et al. Loss of the Chloride Channel ClC-7 Leads to Lysosomal Storage Disease and Neurodegeneration. EMBO J. 2005, 24, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Pressey, S.N.R.; O’Donnell, K.J.; Stauber, T.; Fuhrmann, J.C.; Tyynelä, J.; Jentsch, T.J.; Cooper, J.D. Distinct Neuropathologic Phenotypes after Disrupting the Chloride Transport Proteins ClC-6 or ClC-7/Ostm1. J. Neuropathol. Exp. Neurol. 2010, 69, 1228–1246. [Google Scholar] [CrossRef]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal Amino Acid Transporter SLC38A9 Signals Arginine Sufficiency to MTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araújo, M.E.G.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 Is a Component of the Lysosomal Amino Acid Sensing Machinery That Controls MTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef]
- Jung, J.; Genau, H.M.; Behrends, C. Amino Acid-Dependent MTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol. Cell. Biol. 2015, 35, 2479–2494. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; vander Heiden, M.G.; Sabatini, D.M. MTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 2017, 171, 642–654.e12. [Google Scholar] [CrossRef]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.N.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal Cholesterol Activates MTORC1 via an SLC38A9-Niemann-Pick C1 Signaling Complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef]
- Cormerais, Y.; Massard, P.A.; Vucetic, M.; Giuliano, S.; Tambutté, E.; Durivault, J.; Vial, V.; Endou, H.; Wempe, M.F.; Parks, S.K.; et al. The Glutamine Transporter ASCT2 (SLC1A5) Promotes Tumor Growth Independently of the Amino Acid Transporter LAT1 (SLC7A5). J. Biol. Chem. 2018, 293, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Elorza, A.; Soro-Arnáiz, I.; Meléndez-Rodríguez, F.; Rodríguez-Vaello, V.; Marsboom, G.; de Cárcer, G.; Acosta-Iborra, B.; Albacete-Albacete, L.; Ordóñez, A.; Serrano-Oviedo, L.; et al. HIF2α Acts as an MTORC1 Activator through the Amino Acid Carrier SLC7A5. Mol. Cell 2012, 48, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates MTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Nakaya, M.; Xiao, Y.; Zhou, X.; Chang, J.H.; Chang, M.; Cheng, X.; Blonska, M.; Lin, X.; Sun, S.C. Inflammatory T Cell Responses Rely on Amino Acid Transporter ASCT2 Facilitation of Glutamine Uptake and MTORC1 Kinase Activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Okamoto, H.; Huang, Z.J.; Anguiano, G.; Chen, S.; Liu, Q.; Cavino, K.; Xin, Y.; Na, E.; Hamid, R.; et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab. 2017, 25, 1348–1361.e8. [Google Scholar] [CrossRef] [PubMed]
- Dato, S.; Hoxha, E.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Amino Acids and Amino Acid Sensing: Implication for Aging and Diseases. Biogerontology 2019, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Crocco, P.; Hoxha, E.; Dato, S.; de Rango, F.; Montesanto, A.; Rose, G.; Passarino, G. Physical Decline and Survival in the Elderly Are Affected by the Genetic Variability of Amino Acid Transporter Genes. Aging 2018, 10, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Najumudeen, A.K.; Ceteci, F.; Fey, S.K.; Hamm, G.; Steven, R.T.; Hall, H.; Nikula, C.J.; Dexter, A.; Murta, T.; Race, A.M.; et al. The Amino Acid Transporter SLC7A5 Is Required for Efficient Growth of KRAS-Mutant Colorectal Cancer. Nat. Genet. 2021, 53, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A Lysosome-to-Nucleus Signalling Mechanism Senses and Regulates the Lysosome via MTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR Network Reveals an Integrated Control of Cellular Clearance Pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A Gene Network Regulating Lysosomal Biogenesis and Function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ploper, D.; Taelman, V.F.; Robert, L.; Perez, B.S.; Titz, B.; Chen, H.-W.; Graeber, T.G.; von Euw, E.; Ribas, A.; de Robertis, E.M. MITF Drives Endolysosomal Biogenesis and Potentiates Wnt Signaling in Melanoma Cells. Proc. Natl. Acad. Sci. USA 2015, 112, E420–E429. [Google Scholar] [CrossRef] [PubMed]
- Bouché, V.; Espinosa, A.P.; Leone, L.; Sardiello, M.; Ballabio, A.; Botas, J. Drosophila Mitf Regulates the V-ATPase and the Lysosomal-Autophagic Pathway. Autophagy 2016, 12, 484–498. [Google Scholar] [CrossRef]
- Song, W.; Wang, F.; Lotfi, P.; Sardiello, M.; Segatori, L. 2-Hydroxypropyl-β-Cyclodextrin Promotes Transcription Factor EB-Mediated Activation of Autophagy. J. Biol. Chem. 2014, 289, 10211–10222. [Google Scholar] [CrossRef]
- Polito, V.A.; Li, H.; Martini-Stoica, H.; Wang, B.; Yang, L.; Xu, Y.; Swartzlander, D.B.; Palmieri, M.; di Ronza, A.; Lee, V.M.-Y.; et al. Selective Clearance of Aberrant Tau Proteins and Rescue of Neurotoxicity by Transcription Factor EB. EMBO Mol. Med. 2014, 6, 1142–1160. [Google Scholar] [CrossRef]
- Palmieri, M.; Pal, R.; Nelvagal, H.R.; Lotfi, P.; Stinnett, G.R.; Seymour, M.L.; Chaudhury, A.; Bajaj, L.; Bondar, V.V.; Bremner, L.; et al. MTORC1-Independent TFEB Activation via Akt Inhibition Promotes Cellular Clearance in Neurodegenerative Storage Diseases. Nat. Commun. 2017, 8, 14338. [Google Scholar] [CrossRef]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Williams, E.G.; Lan, J.; van Weeghel, M.; Schomakers, B.; van der Veen, H.; van der Wel, N.N.; Yao, P.; et al. Mitochondrial Translation and Dynamics Synergistically Extend Lifespan in C. elegans through HLH-30. J. Cell Biol. 2020, 219, e201907067. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.X.; Sen, I.; Janssens, G.E.; Zhou, X.; Fonslow, B.R.; Edgar, D.; Stroustrup, N.; Swoboda, P.; Yates, J.R.; Ruvkun, G.; et al. DAF-16/FOXO and HLH-30/TFEB Function as Combinatorial Transcription Factors to Promote Stress Resistance and Longevity. Nat. Commun. 2018, 9, 4400. [Google Scholar] [CrossRef]
- Wang, C.; Niederstrasser, H.; Douglas, P.M.; Lin, R.; Jaramillo, J.; Li, Y.; Olswald, N.W.; Zhou, A.; McMillan, E.A.; Mendiratta, S.; et al. Small-Molecule TFEB Pathway Agonists That Ameliorate Metabolic Syndrome in Mice and Extend C. elegans Lifespan. Nat. Commun. 2017, 8, 2270. [Google Scholar] [CrossRef]
- Nakamura, S.; Karalay, Ö.; Jäger, P.S.; Horikawa, M.; Klein, C.; Nakamura, K.; Latza, C.; Templer, S.E.; Dieterich, C.; Antebi, A. Mondo Complexes Regulate TFEB via TOR Inhibition to Promote Longevity in Response to Gonadal Signals. Nat. Commun. 2016, 7, 10944. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.R.; de Magalhaes Filho, C.D.; McQuary, P.R.; Chu, C.C.; Visvikis, O.; Chang, J.T.; Gelino, S.; Ong, B.; Davis, A.E.; Irazoqui, J.E.; et al. The TFEB Orthologue HLH-30 Regulates Autophagy and Modulates Longevity in Caenorhabditis Elegans. Nat. Commun. 2013, 4, 2267. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Ruvkun, G. MXL-3 and HLH-30 Transcriptionally Link Lipolysis and Autophagy to Nutrient Availability. Nat. Cell Biol. 2013, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Piao, W.; Takamura, T.; Kori, H.; Miyachi, H.; Kitano, S.; Iwamoto, Y.; Yamada, M.; Imayoshi, I.; Shioda, S.; et al. Enhanced Lysosomal Degradation Maintains the Quiescent State of Neural Stem Cells. Nat. Commun. 2019, 10, 5446. [Google Scholar] [CrossRef] [PubMed]
- Pastore, N.; Vainshtein, A.; Klisch, T.J.; Armani, A.; Huynh, T.; Herz, N.J.; Polishchuk, E.V.; Sandri, M.; Ballabio, A. TFE3 Regulates Whole-Body Energy Metabolism in Cooperation with TFEB. EMBO Mol. Med. 2017, 9, 605–621. [Google Scholar] [CrossRef]
- Jung, J.; Liao, H.; Coker, S.A.; Liang, H.; Hancock, J.F.; Denicourt, C.; Venkatachalam, K. P53 Mitigates the Effects of Oncogenic HRAS in Urothelial Cells via the Repression of MCOLN1. iScience 2021, 24, 102701. [Google Scholar] [CrossRef]
- Jung, J.; Cho, K.-J.; Naji, A.K.; Clemons, K.N.; Wong, C.O.; Villanueva, M.; Gregory, S.; Karagas, N.E.; Tan, L.; Liang, H.; et al. HRAS-Driven Cancer Cells Are Vulnerable to TRPML1 Inhibition. EMBO Rep. 2019, 20, e46685. [Google Scholar] [CrossRef]
- Medina, D.L.; di Paola, S.; Peluso, I.; Armani, A.; de Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal Calcium Signalling Regulates Autophagy through Calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef]
- Kundu, S.T.; Grzeskowiak, C.L.; Fradette, J.J.; Gibson, L.A.; Rodriguez, L.B.; Creighton, C.J.; Scott, K.L.; Gibbons, D.L. TMEM106B Drives Lung Cancer Metastasis by Inducing TFEB-Dependent Lysosome Synthesis and Secretion of Cathepsins. Nat. Commun. 2018, 9, 2731. [Google Scholar] [CrossRef]
- Kauffman, E.C.; Ricketts, C.J.; Rais-Bahrami, S.; Yang, Y.; Merino, M.J.; Bottaro, D.P.; Srinivasan, R.; Linehan, W.M. Molecular Genetics and Cellular Features of TFE3 and TFEB Fusion Kidney Cancers. Nat. Rev. Urol. 2014, 11, 465–475. [Google Scholar] [CrossRef]
- Urbanelli, L.; Magini, A.; Ercolani, L.; Sagini, K.; Polchi, A.; Tancini, B.; Brozzi, A.; Armeni, T.; Principato, G.; Emiliani, C. Oncogenic H-Ras Up-Regulates Acid β-Hexosaminidase by a Mechanism Dependent on the Autophagy Regulator TFEB. PLoS ONE 2014, 9, e89485. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Venkatachalam, K. TRPML1 and RAS-Driven Cancers–Exploring a Link with Great Therapeutic Potential. Channels 2019, 13, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Stoykova, S.; Nicolay, B.N.; Ross, K.N.; Fitamant, J.; Boukhali, M.; Lengrand, J.; Deshpande, V.; Selig, M.K.; Ferrone, C.R.; et al. Transcriptional Control of Autophagy-Lysosome Function Drives Pancreatic Cancer Metabolism. Nature 2015, 524, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Calcagnì, A.; Kors, L.; Verschuren, E.; de Cegli, R.; Zampelli, N.; Nusco, E.; Confalonieri, S.; Bertalot, G.; Pece, S.; Settembre, C.; et al. Modelling TFE Renal Cell Carcinoma in Mice Reveals a Critical Role of WNT Signaling. eLife 2016, 5, e17047. [Google Scholar] [CrossRef]
- Blessing, A.M.; Rajapakshe, K.; Reddy Bollu, L.; Shi, Y.; White, M.A.; Pham, A.H.; Lin, C.; Jonsson, P.; Cortes, C.J.; Cheung, E.; et al. Transcriptional Regulation of Core Autophagy and Lysosomal Genes by the Androgen Receptor Promotes Prostate Cancer Progression. Autophagy 2017, 13, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, C.; Croce, C.M.; Guan, J.L. P62/SQSTM1 Synergizes with Autophagy for Tumor Growth in Vivo. Genes Dev. 2014, 28, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Kalamida, D.; Sivridis, E.; Karagounis, I.V.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Increased Expression of Transcription Factor EB (TFEB) Is Associated with Autophagy, Migratory Phenotype and Poor Prognosis in Non-Small Cell Lung Cancer. Lung Cancer 2015, 90, 98–105. [Google Scholar] [CrossRef]
- Zhao, B.; Dierichs, L.; Gu, J.N.; Trajkovic-Arsic, M.; Axel Hilger, R.; Savvatakis, K.; Vega-Rubin-de-Celis, S.; Liffers, S.T.; Peña-Llopis, S.; Behrens, D.; et al. TFEB-Mediated Lysosomal Biogenesis and Lysosomal Drug Sequestration Confer Resistance to MEK Inhibition in Pancreatic Cancer. Cell Death Discov. 2020, 6, 12. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Cho, Y.R.; Lee, S.Y.; Sung, G.J.; Shin, D.M.; Choi, K.C.; Son, J. TFEB Supports Pancreatic Cancer Growth through the Transcriptional Regulation of Glutaminase. Cancers 2021, 13, 483. [Google Scholar] [CrossRef]
- Li, S.; Song, Y.; Quach, C.; Guo, H.; Jang, G.B.; Maazi, H.; Zhao, S.; Sands, N.A.; Liu, Q.; In, G.K.; et al. Transcriptional Regulation of Autophagy-Lysosomal Function in BRAF-Driven Melanoma Progression and Chemoresistance. Nat. Commun. 2019, 10, 1693. [Google Scholar] [CrossRef]
- Carreira, S.; Goodall, J.; Denat, L.; Rodriguez, M.; Nuciforo, P.; Hoek, K.S.; Testori, A.; Larue, L.; Goding, C.R. Mitf Regulation of Dia1 Controls Melanoma Proliferation and Invasiveness. Genes Dev. 2006, 20, 3426–3439. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y.; Giuliano, S.; Fenouille, N.; Allegra, M.; Hofman, V.; Hofman, P.; Bahadoran, P.; Lacour, J.-P.; Tartare-Deckert, S.; Bertolotto, C.; et al. Hypoxia and MITF Control Metastatic Behaviour in Mouse and Human Melanoma Cells. Oncogene 2012, 31, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Aida, S.; Sonobe, Y.; Tanimura, H.; Oikawa, N.; Yuhki, M.; Sakamoto, H.; Mizuno, T. MITF Suppression Improves the Sensitivity of Melanoma Cells to a BRAF Inhibitor. Cancer Lett. 2017, 409, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative Genomic Analyses Identify MITF as a Lineage Survival Oncogene Amplified in Malignant Melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y.; Guiliano, S.; Botton, T.; Rocchi, S.; Hofman, V.; Hofman, P.; Bahadoran, P.; Bertolotto, C.; Ballotti, R. Mitf Is the Key Molecular Switch between Mouse or Human Melanoma Initiating Cells and Their Differentiated Progeny. Oncogene 2011, 30, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Bourseguin, J.; Bonet, C.; Renaud, E.; Pandiani, C.; Boncompagni, M.; Giuliano, S.; Pawlikowska, P.; Karmous-Benailly, H.; Ballotti, R.; Rosselli, F.; et al. FANCD2 Functions as a Critical Factor Downstream of MiTF to Maintain the Proliferation and Survival of Melanoma Cells. Sci. Rep. 2016, 6, 36539. [Google Scholar] [CrossRef]
- Kasitinon, S.Y.; Eskiocak, U.; Martin, M.; Bezwada, D.; Khivansara, V.; Tasdogan, A.; Zhao, Z.; Mathews, T.; Aurora, A.B.; Morrison, S.J. TRPML1 Promotes Protein Homeostasis in Melanoma Cells by Negatively Regulating MAPK and MTORC1 Signaling. Cell Rep. 2019, 28, 2293–2305. [Google Scholar] [CrossRef]
- Xing, Y.; Wei, X.; Liu, Y.; Wang, M.-M.; Sui, Z.; Wang, X.; Zhu, W.; Wu, M.; Lu, C.; Fei, Y.-H.; et al. Autophagy Inhibition Mediated by MCOLN1/TRPML1 Suppresses Cancer Metastasis via Regulating a ROS-Driven TP53/P53 Pathway. Autophagy 2021, 1–23. [Google Scholar] [CrossRef]
- Nguyen, O.N.P.; Grimm, C.; Schneider, L.S.; Chao, Y.K.; Atzberger, C.; Bartel, K.; Watermann, A.; Ulrich, M.; Mayr, D.; Wahl-Schott, C.; et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017, 77, 1427–1438. [Google Scholar] [CrossRef]
- Faviaa, A.; Desiderib, M.; Gambaraa, G.; D’Alessioa, A.; Ruas, M.; Esposito, B.; del Bufalo, D.; Parrington, J.; Ziparoa, E.; Palombia, F.; et al. VEGF-Induced Neoangiogenesis Is Mediated by NAADP and Two-Pore Channel-2-Dependent Ca2+ Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 4706–4715. [Google Scholar] [CrossRef]
- Favia, A.; Pafumi, I.; Desideri, M.; Padula, F.; Montesano, C.; Passeri, D.; Nicoletti, C.; Orlandi, A.; del Bufalo, D.; Sergi, M.; et al. NAADP-Dependent Ca(2+) Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016, 6, 18925. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, M.; Meng, Z.; Lo, C.-Y.; Chan, F.L.; Jiang, L.; Meng, X.; Yao, X. Endolysosomal Ion Channel MCOLN2 (Mucolipin-2) Promotes Prostate Cancer Progression via IL-1β/NF-ΚB Pathway. Br. J. Cancer 2021, 125, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamada, K.; Kim, H.C.; Kim, Y.S.; Noda, Y.; Imura, A.; Nabeshima, Y.-I.; Nabeshima, T. Cognition Impairment in the Genetic Model of Aging Klotho Gene Mutant Mice: A Role of Oxidative Stress. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 50–52. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Physiology: Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho and Aging. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Dubal, D.B.; Yokoyama, J.S.; Zhu, L.; Broestl, L.; Worden, K.; Wang, D.; Sturm, V.E.; Kim, D.; Klein, E.; Yu, G.Q.; et al. Life Extension Factor Klotho Enhances Cognition. Cell Rep. 2014, 7, 1065–1076. [Google Scholar] [CrossRef]
- Arking, D.E.; Krebsova, A.; Macek, M.; Macek, M.; Arking, A.; Mian, I.S.; Fried, L.; Hamosh, A.; Dey, S.; McIntosh, I.; et al. Association of Human Aging with a Functional Variant of Klotho. Proc. Natl. Acad. Sci. USA 2002, 99, 856–861. [Google Scholar] [CrossRef]
- Arking, D.E.; Becker, D.M.; Yanek, L.R.; Fallin, D.; Judge, D.P.; Moy, T.F.; Becker, L.C.; Dietz, H.C. KLOTHO Allele Status and the Risk of Early-Onset Occult Coronary Artery Disease. Am. J. Hum. Genet. 2003, 72, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Arking, D.E.; Atzmon, G.; Arking, A.; Barzilai, N.; Dietz, H.C. Association between a Functional Variant of the KLOTHO Gene and High-Density Lipoprotein Cholesterol, Blood Pressure, Stroke, and Longevity. Circ. Res. 2005, 96, 412–418. [Google Scholar] [CrossRef]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-O, M.; Nabeshima, Y.I. Identification of the Human Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Klotho Protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-O, M.; Nabeshima, Y.I. Structure of the Mouse Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef]
- Cha, S.K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro-o, M.; Huang, C.L. Removal of Sialic Acid Involving Klotho Causes Cell-Surface Retention of TRPV5 Channel via Binding to Galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin Stimulates the Cleavage and Release of the Extracellular Domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA 2007, 104, 19796–19801. [Google Scholar] [CrossRef]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of Fibroblast Growth Factor-23 Signaling by Klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Hoefs, S.; van der Kemp, A.W.; Topala, C.N.; Bindels, R.J.; Hoenderop, J.G. Cell Signalling: The β-Glucuronidase Klotho Hydrolyzes and Activates the TRPV5 Channel. Science 2005, 310, 490–493. [Google Scholar] [CrossRef]
- Lu, P.; Boros, S.; Chang, Q.; Bindels, R.J.; Hoenderop, J.G. The Beta-Glucuronidase Klotho Exclusively Activates the Epithelial Ca2+ Channels TRPV5 and TRPV6. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2008, 23, 3397–3402. [Google Scholar] [CrossRef]
- Wolf, M.T.F.; An, S.W.; Nie, M.; Bal, M.S.; Huang, C.L. Klotho Up-Regulates Renal Calcium Channel Transient Receptor Potential Vanilloid 5 (TRPV5) by Intra- and Extracellular N-Glycosylation-Dependent Mechanisms. J. Biol. Chem. 2014, 289, 35849–35857. [Google Scholar] [CrossRef]
- Takumida, M.; Ishibashi, T.; Hamamoto, T.; Hirakawa, K.; Anniko, M. Age-Dependent Changes in the Expression of Klotho Protein, TRPV5 and TRPV6 in Mouse Inner Ear. Acta Oto-Laryngol. 2009, 129, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; van Leeuwen, J.P.T.M.; van der Eerden, B.C.J.; Kersten, F.F.J.; van derKemp, A.W.C.M.; Mérillat, A.-M.; Waarsing, J.H.; Rossier, B.C.; Vallon, V.; Hummler, E.; et al. Renal Ca2+ Wasting, Hyperabsorption, and Reduced Bone Thickness in Mice Lacking TRPV5. J. Clin. Investig. 2003, 112, 1906–1914. [Google Scholar] [CrossRef]
- Huang, C.L. Regulation of Ion Channels by Secreted Klotho: Mechanisms and Implications. Kidney Int. 2010, 77, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Imura, A.; Tsuji, Y.; Murata, M.; Maeda, R.; Kubota, K.; Iwano, A.; Obuse, C.; Togashi, K.; Tominaga, M.; Kita, N.; et al. α-Klotho as a Regulator of Calcium Homeostasis. Science 2007, 316, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Almilaji, A.; Pakladok, T.; Munõz, C.; Elvira, B.; Sopjani, M.; Lang, F. Upregulation of KCNQ1/KCNE1 K+ Channels by Klotho. Channels 2014, 8, 222–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, J.; Cha, S.K.; An, S.W.; Kuro-O, M.; Birnbaumer, L.; Huang, C.L. Cardioprotection by Klotho through Downregulation of TRPC6 Channels in the Mouse Heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef]
- Wu, Y.L.; Xie, J.; An, S.W.; Oliver, N.; Barrezueta, N.X.; Lin, M.H.; Birnbaumer, L.; Huang, C.L. Inhibition of TRPC6 Channels Ameliorates Renal Fibrosis and Contributes to Renal Protection by Soluble Klotho. Kidney Int. 2017, 91, 830–841. [Google Scholar] [CrossRef]
- Xie, J.; An, S.W.; Jin, X.; Gui, Y.; Huang, C.L. Munc13 Mediates Klotho-Inhibitable Diacylglycerol-Stimulated Exocytotic Insertion of Pre-Docked TRPC6 Vesicles. PLoS ONE 2020, 15, e0229799. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of Size and Temperature on Metabolic Rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Keil, G.; Cummings, E.; de Magalhães, J.P. Being Cool: How Body Temperature Influences Ageing and Longevity. Biogerontology 2015, 16, 383. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.K.; Walford, R.L. The Effect of Lowered Body Temperature on Lifespan and Immune and Non-Immune Processes. Gerontologia 1972, 18, 363–388. [Google Scholar] [CrossRef]
- Loeb, J.; Northrop, J.H. Is There a Temperature Coefficient for the Duration of Life? Proc. Natl. Acad. Sci. USA 1916, 2, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.H.; Feuers, R.J.; Leakey, J.A.; Nakamura, K.D.; Turturro, A.; Hart, R.W. Effect of Chronic Caloric Restriction on Physiological Variables Related to Energy Metabolism in the Male Fischer 344 Rat. Mech. Ageing Dev. 1989, 48, 117–133. [Google Scholar] [CrossRef]
- Kim, K.; Lin, Y.R.; Park, Y. Enhancement of Stress Resistances and Downregulation of Imd Pathway by Lower Developmental Temperature in Drosophila Melanogaster. Exp. Gerontol. 2010, 45, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rikke, B.A.; Johnson, T.E. Lower Body Temperature as a Potential Mechanism of Life Extension in Homeotherms. Exp. Gerontol. 2004, 39, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, B.; Dong, Y.; Gong, J.; Xu, T.; Liu, J.; Xu, X.Z.S. A Genetic Program Promotes C. elegans Longevity at Cold Temperatures via a Thermosensitive TRP Channel. Cell 2013, 152, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, R.; Ronan, E.A.; He, Y.; Hsu, A.L.; Liu, J.; Xu, X.Z.S. Environmental Temperature Differentially Modulates C. elegans Longevity through a Thermosensitive TRP Channel. Cell Rep. 2015, 11, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Riera, C.E.; Huising, M.O.; Follett, P.; Leblanc, M.; Halloran, J.; van Andel, R.; de Magalhaes Filho, C.D.; Merkwirth, C.; Dillin, A. TRPV1 Pain Receptors Regulate Longevity and Metabolism by Neuropeptide Signaling. Cell 2014, 157, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Ashrafi, K. A TRPV Channel Modulates C. elegans Neurosecretion, Larval Starvation Survival, and Adult Lifespan. PLoS Genet. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Murshid, A.; Prince, T. The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging—A Mini-Review. Gerontology 2009, 55, 550–558. [Google Scholar] [CrossRef]
- Volovik, Y.; Maman, M.; Dubnikov, T.; Bejerano-Sagie, M.; Joyce, D.; Kapernick, E.A.; Cohen, E.; Dillin, A. Temporal Requirements of Heat Shock Factor-1 for Longevity Assurance. Aging Cell 2012, 11, 491–499. [Google Scholar] [CrossRef]
- Seo, K.; Choi, E.; Lee, D.; Jeong, D.E.; Jang, S.K.; Lee, S.J. Heat Shock Factor 1 Mediates the Longevity Conferred by Inhibition of TOR and Insulin/IGF-1 Signaling Pathways in C. elegans. Aging Cell 2013, 12, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the Cellular Heat Shock Response in Caenorhabditis Elegans by Thermosensory Neurons. Science 2008, 320, 811. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, H.J.; Tseng, W.C.; Hsu, J.M.; Huang, T.T.; Chen, C.H.; Pan, C.L. A C. elegans Thermosensory Circuit Regulates Longevity through Crh-1/CREB-Dependent Flp-6 Neuropeptide Signaling. Dev. Cell 2016, 39, 209–223. [Google Scholar] [CrossRef]
- Silva, M.C.; Amaral, M.D.; Morimoto, R.I. Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response. PLoS Genet. 2013, 9, e1003711. [Google Scholar] [CrossRef] [PubMed]
- Waterson, M.J.; Chung, B.Y.; Harvanek, Z.M.; Ostojic, I.; Alcedo, J.; Pletcher, S.D. Water Sensor Ppk28 Modulates Drosophila Lifespan and Physiology through AKH Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 8137–8142. [Google Scholar] [CrossRef]
- Zelle, K.M.; Lu, B.; Pyfrom, S.C.; Ben-Shahar, Y. The Genetic Architecture of Degenerin/Epithelial Sodium Channels in Drosophila. G3 Genes Genomes Genet. 2013, 3, 441–450. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Wang, Z. The Amiloride-Sensitive Epithelial Na+ Channel PPK28 Is Essential for Drosophila Gustatory Water Reception. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.C.; Hsu, J.M.; Yen, C.P.; Chao, C.C.; Chen, R.H.; Pan, C.L. Neural Activity and CaMKII Protect Mitochondria from Fragmentation in Aging Caenorhabditis Elegans Neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 8768–8773. [Google Scholar] [CrossRef]
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. OSM-9, a Novel Protein with Structural Similarity to Channels, Is Required for Olfaction, Mechanosensation, and Olfactory Adaptation in Caenorhabditis Elegans. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 8259–8269. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, H.; Ditano, J.; Manion, D.; King, B.L.; Strange, K. Abnormal Osmotic Avoidance Behavior in C. elegans Is Associated with Increased Hypertonic Stress Resistance and Improved Proteostasis. PLoS ONE 2016, 11, e0154156. [Google Scholar] [CrossRef] [PubMed]
- Odell, K.M.C. The Effect of the Inactive Mutation on Longevity, Sex, Rhythm and Resistance to p-Cresol in Drosophila Melanogaster. Heredity 1993, 70, 393–399. [Google Scholar] [CrossRef][Green Version]
- Gong, Z. Two Interdependent TRPV Channel Subunits, Inactive and Nanchung, Mediate Hearing in Drosophila. J. Neurosci. 2004, 24, 9059–9066. [Google Scholar] [CrossRef]
- Gendron, C.M.; Kuo, T.H.; Harvanek, Z.M.; Chung, B.Y.; Yew, J.Y.; Dierick, H.A.; Pletcher, S.D. Drosophila Life Span and Physiology Are Modulated by Sexual Perception and Reward. Science 2014, 343, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; LaMora, A.; Sun, Y.; Welsh, M.J.; Ben-Shahar, Y. Ppk23-Dependent Chemosensory Functions Contribute to Courtship Behavior in Drosophila Melanogaster. PLoS Genet. 2012, 8, e1002587. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Thistle, R.; Liu, T.; Starostina, E.; Pikielny, C.W. Drosophila Pheromone-Sensing Neurons Expressing the Ppk25 Ion Channel Subunit Stimulate Male Courtship and Female Receptivity. PLoS Genet. 2014, 10, e1004238. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Zwiener, J.; Chu, X.; VanVoorhies, W.; Roman, G.; Pletcher, S.D. Regulation of Drosophila Life Span by Olfaction and Food-Derived Odors. Science 2007, 315, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila Odorant Receptors Are Both Ligand-Gated and Cyclic-Nucleotide-Activated Cation Channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect Olfactory Receptors Are Heteromeric Ligand-Gated Ion Channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef] [PubMed]


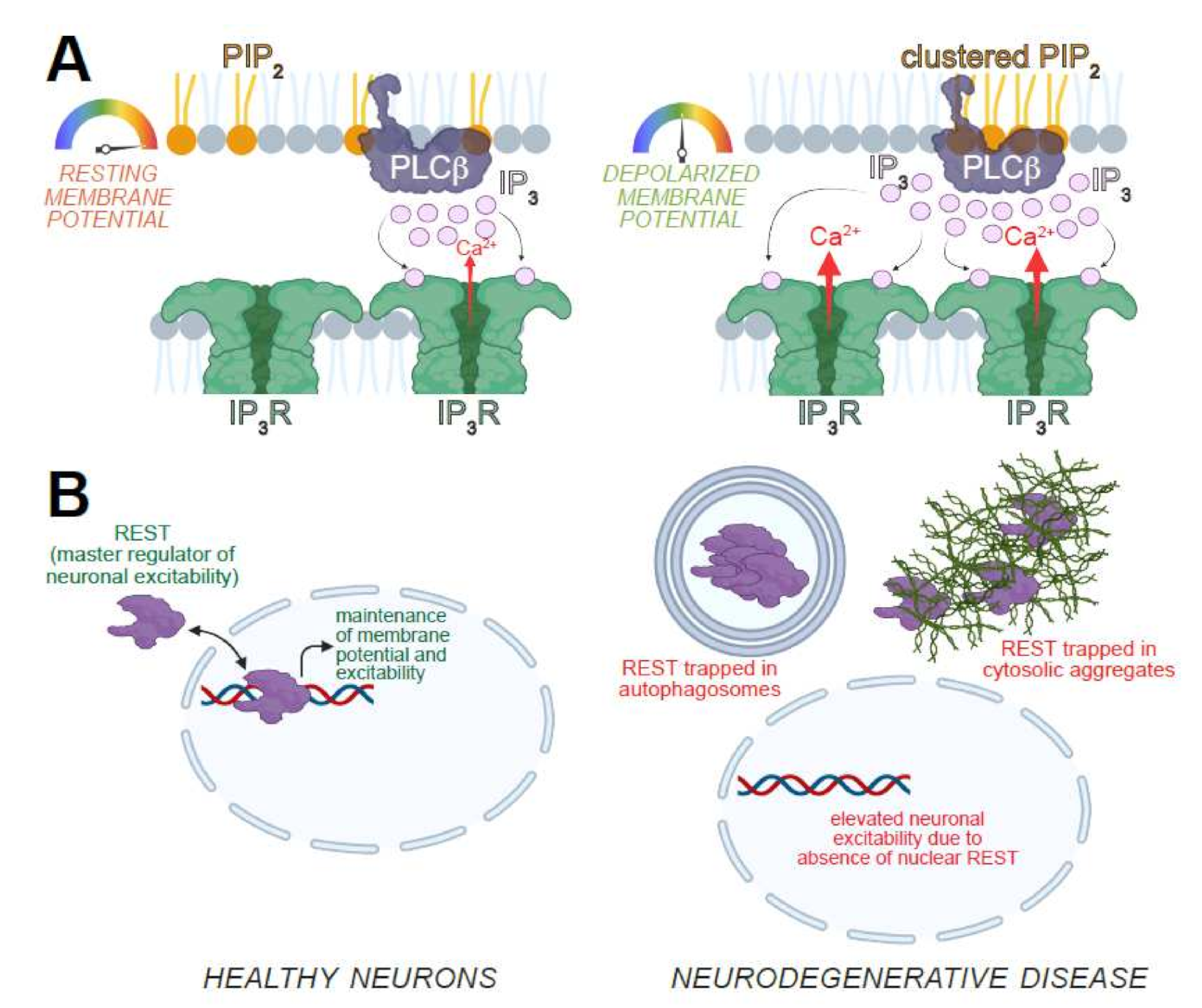
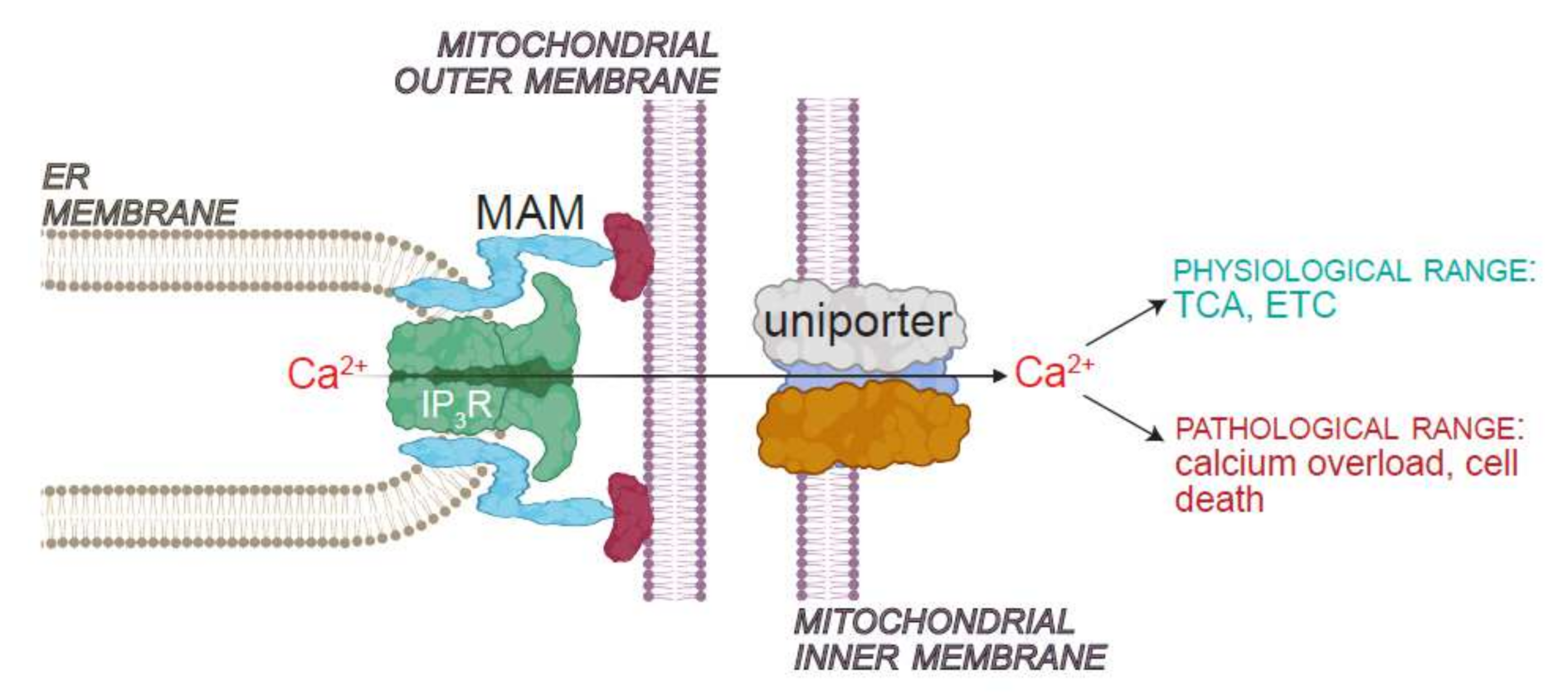



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K. Regulation of Aging and Longevity by Ion Channels and Transporters. Cells 2022, 11, 1180. https://doi.org/10.3390/cells11071180
Venkatachalam K. Regulation of Aging and Longevity by Ion Channels and Transporters. Cells. 2022; 11(7):1180. https://doi.org/10.3390/cells11071180
Chicago/Turabian StyleVenkatachalam, Kartik. 2022. "Regulation of Aging and Longevity by Ion Channels and Transporters" Cells 11, no. 7: 1180. https://doi.org/10.3390/cells11071180
APA StyleVenkatachalam, K. (2022). Regulation of Aging and Longevity by Ion Channels and Transporters. Cells, 11(7), 1180. https://doi.org/10.3390/cells11071180





