YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy
Abstract
1. Introduction
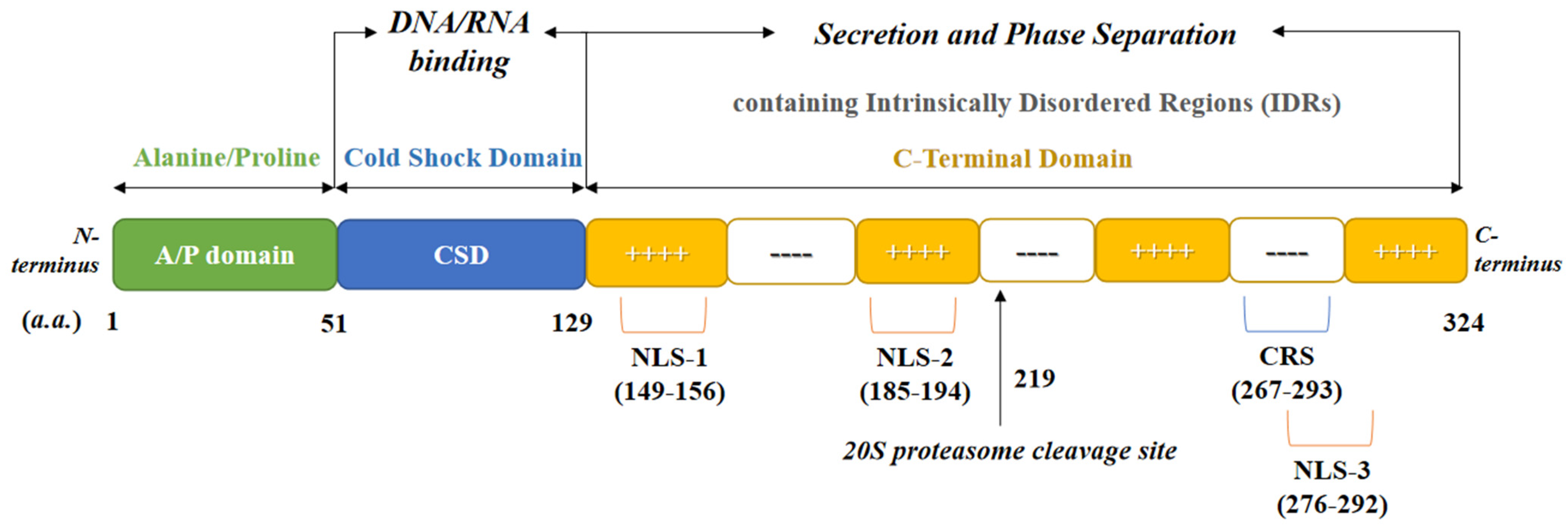
2. Protein Structure of YB-1
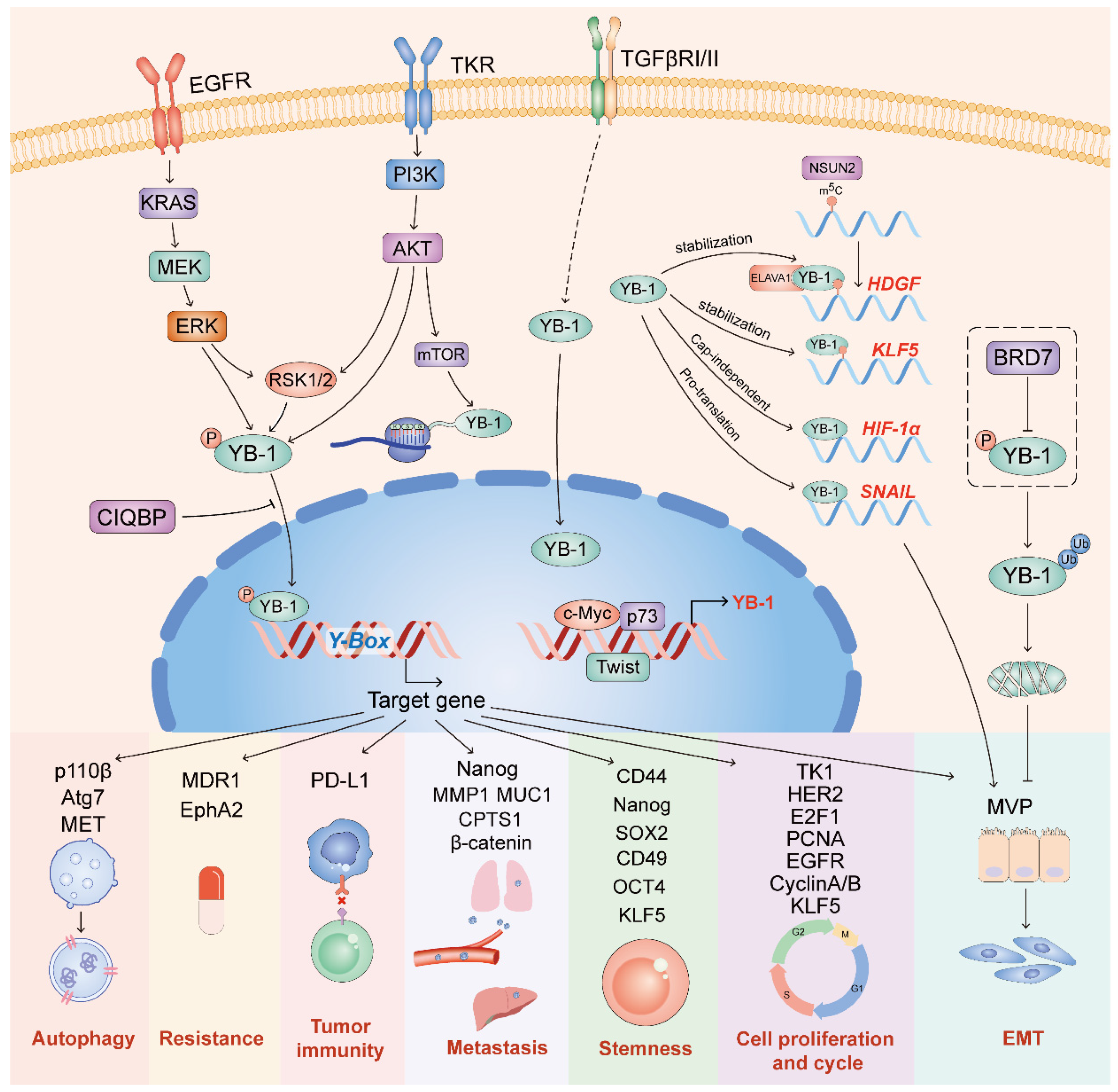
3. The Functions and Mechanisms of YB-1
3.1. The Function of YB-1 in Cancers
3.2. Cell Proliferation and Cell Cycle Progression
3.3. Cancer Stem-like Properties
3.4. Invasion and Metastasis
3.5. DNA Damage Repair (DDR)
3.6. Autophagy
3.7. Tumor Immunity
3.8. Multidrug Resistance
3.9. YB-1 as an RNA 5-Methylcytosine (m5C)-Binding Protein
3.10. Phase Separation
3.11. YB-1 as a Secreted Protein
4. Upstream Regulation of YB-1
4.1. Upstream Regulators of YB-1
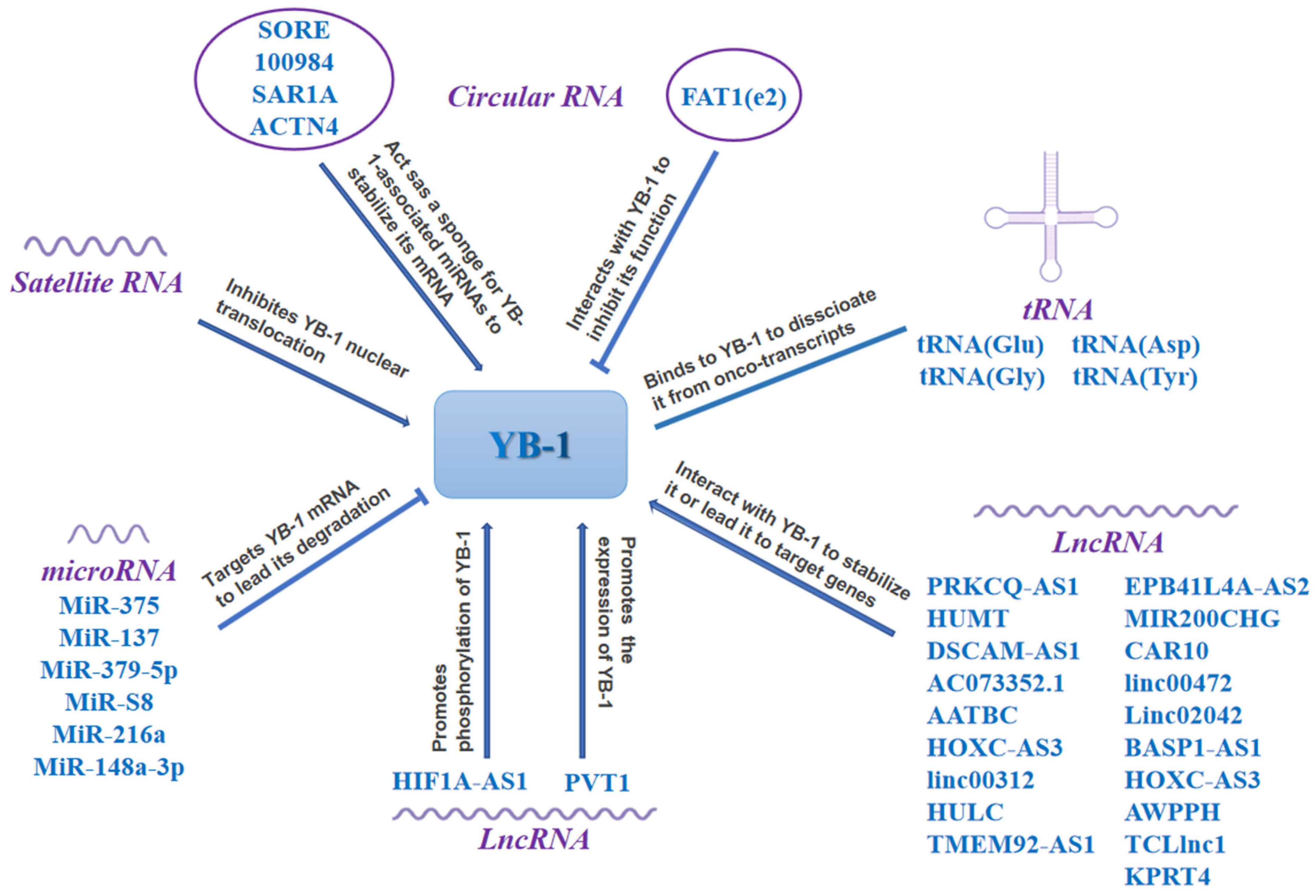
4.2. Multiple Non-Coding RNAs Regulate YB-1 in Cancer
4.2.1. LncRNAs Regulate YB-1 in Cancer
4.2.2. MicroRNAs (miRNAs) Target YB-1 in Cancer
4.2.3. CircRNAs Regulate YB-1 in Cancer
4.2.4. Other RNAs Regulate YB-1 in Cancer
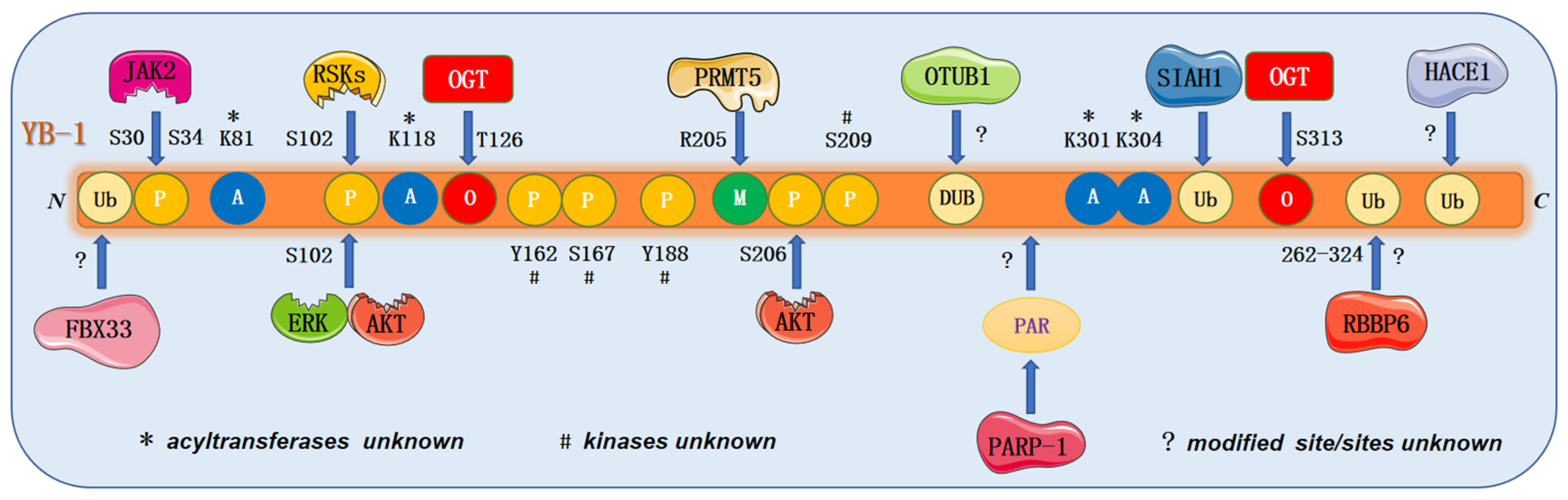
4.3. Post-Translational Modification of YB-1
4.3.1. Phosphorylation
4.3.2. Acetylation
4.3.3. Methylation
4.3.4. Ubiquitination and de-Ubiquitination
4.3.5. O-glycosylation
4.3.6. PARylation
4.4. Nuclear-Cytoplasmic Transport of YB-1
5. Targeting YB-1 for Cancer Therapy
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Spitkovsky, D.D.; Royer-Pokora, B.; Delius, H.; Kisseljov, F.; Jenkins, N.A.; Gilbert, D.J.; Copeland, N.G.; Royer, H.D. Tissue restricted expression and chromosomal localization of the YB-1 gene encoding a 42 kD nuclear CCAAT binding protein. Nucleic Acids Res. 1992, 20, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.A.; Kleene, K.C. Developmental expression of Y-box protein 1 mRNA and alternatively spliced Y-box protein 3 mRNAs in spermatogenic cells in mice. Mol. Hum. Reprod. 2000, 6, 779–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sapru, M.K.; Gao, J.P.; Walke, W.; Burmeister, M.; Goldman, D. Cloning and characterization of a novel transcriptional repressor of the nicotinic acetylcholine receptor delta-subunit gene. J. Biol. Chem. 1996, 271, 7203–7211. [Google Scholar] [CrossRef] [PubMed]
- Mordovkina, D.; Lyabin, D.N.; Smolin, E.A.; Sogorina, E.M.; Ovchinnikov, L.P.; Eliseeva, I. Y-Box Binding Proteins in mRNP Assembly, Translation, and Stability Control. Biomolecules 2020, 10, 591. [Google Scholar] [CrossRef]
- Gu, W.; Tekur, S.; Reinbold, R.; Eppig, J.J.; Choi, Y.C.; Zheng, J.Z.; Murray, M.T.; Hecht, N.B. Mammalian male and female germ cells express a germ cell-specific Y-Box protein, MSY2. Biol. Reprod. 1998, 59, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Books, J.T.; Ley, T.J. Cold shock domain family members YB-1 and MSY4 share essential functions during murine embryogenesis. Mol. Cell. Biol. 2006, 26, 8410–8417. [Google Scholar] [CrossRef]
- Yu, J.; Hecht, N.B.; Schultz, R.M. Expression of MSY2 in mouse oocytes and preimplantation embryos. Biol. Reprod. 2001, 65, 1260–1270. [Google Scholar] [CrossRef]
- Davies, H.G.; Giorgini, F.; Fajardo, M.A.; Braun, R.E. A sequence-specific RNA binding complex expressed in murine germ cells contains MSY2 and MSY4. Dev. Biol. 2000, 221, 87–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giorgini, F.; Davies, H.G.; Braun, R.E. Translational repression by MSY4 inhibits spermatid differentiation in mice. Development 2002, 129, 3669–3679. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Bettencourt, M.; Alho, I.; Costa, A.L.; Sousa, A.R.; Mansinho, A.; Abreu, C.; Pulido, C.; Macedo, D.; Vendrell, I.; et al. Serum YB-1 (Y-box binding protein 1) as a biomarker of bone disease progression in patients with breast cancer and bone metastases. J. Bone Oncol. 2017, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Fotovati, A.; Reipas, K.M.; Law, J.H.; Hu, K.; Wang, J.; Astanehe, A.; Davies, A.H.; Lee, L.; Stratford, A.L.; et al. Y-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res. 2010, 70, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, M.; Oda, Y.; Izumi, H.; Yang, S.J.; Uchiumi, T.; Iwamoto, Y.; Toi, M.; Fujii, T.; Yamana, H.; Kinoshita, H.; et al. The role of nuclear Y-box binding protein 1 as a global marker in drug resistance. Mol. Cancer Ther. 2004, 3, 1485–1492. [Google Scholar]
- Harada, M.; Kotake, Y.; Ohhata, T.; Kitagawa, K.; Niida, H.; Matsuura, S.; Funai, K.; Sugimura, H.; Suda, T.; Kitagawa, M. YB-1 promotes transcription of cyclin D1 in human non-small-cell lung cancers. Genes Cells 2014, 19, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Kloks, C.P.; Spronk, C.A.; Lasonder, E.; Hoffmann, A.; Vuister, G.W.; Grzesiek, S.; Hilbers, C.W. The solution structure and DNA-binding properties of the cold-shock domain of the human Y-box protein YB-1. J. Mol. Biol. 2002, 316, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kleene, K.C. Y-box proteins combine versatile cold shock domains and arginine-rich motifs (ARMs) for pleiotropic functions in RNA biology. Biochem. J. 2018, 475, 2769–2784. [Google Scholar] [CrossRef] [PubMed]
- Alkrekshi, A.; Wang, W.; Rana, P.S.; Markovic, V.; Sossey-Alaoui, K. A comprehensive review of the functions of YB-1 in cancer stemness, metastasis and drug resistance. Cell Signal. 2021, 85, 110073. [Google Scholar] [CrossRef]
- Sangermano, F.; Delicato, A.; Calabrò, V. Y box binding protein 1 (YB-1) oncoprotein at the hub of DNA proliferation, damage and cancer progression. Biochimie 2020, 179, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P.; Tafuri, S.; Ranjan, M.; Familari, M. The Y-box factors: A family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992, 4, 290–298. [Google Scholar] [PubMed]
- Guryanov, S.G.; Filimonov, V.V.; Timchenko, A.A.; Melnik, B.S.; Kihara, H.; Kutyshenko, V.P.; Ovchinnikov, L.P.; Semisotnov, G.V. The major mRNP protein YB-1: Structural and association properties in solution. Biochim. Biophys. Acta 2013, 1834, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Zhu, H.; Mu, S.R.; Wei, W.J.; Yuan, X.; Wang, M.; Liu, Y.; Hui, J.; Huang, Y. Crystal structure of a Y-box binding protein 1 (YB-1)-RNA complex reveals key features and residues interacting with RNA. J. Biol. Chem. 2019, 294, 10998–11010. [Google Scholar] [CrossRef] [PubMed]
- Bargou, R.C.; Jürchott, K.; Wagener, C.; Bergmann, S.; Metzner, S.; Bommert, K.; Mapara, M.Y.; Winzer, K.J.; Dietel, M.; Dörken, B.; et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997, 3, 447–450. [Google Scholar] [CrossRef]
- Kamura, T.; Yahata, H.; Amada, S.; Ogawa, S.; Sonoda, T.; Kobayashi, H.; Mitsumoto, M.; Kohno, K.; Kuwano, M.; Nakano, H. Is nuclear expression of Y box-binding protein-1 a new prognostic factor in ovarian serous adenocarcinoma? Cancer 1999, 85, 2450–2454. [Google Scholar] [CrossRef]
- Yasen, M.; Kajino, K.; Kano, S.; Tobita, H.; Yamamoto, J.; Uchiumi, T.; Kon, S.; Maeda, M.; Obulhasim, G.; Arii, S.; et al. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, K.; Sugio, K.; Osaki, T.; Uchiumi, T.; Maehara, Y.; Kohno, K.; Yasumoto, K.; Sugimachi, K.; Kuwano, M. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 3151–3155. [Google Scholar]
- Shibao, K.; Takano, H.; Nakayama, Y.; Okazaki, K.; Nagata, N.; Izumi, H.; Uchiumi, T.; Kuwano, M.; Kohno, K.; Itoh, H. Enhanced coexpression of YB-1 and DNA topoisomerase II alpha genes in human colorectal carcinomas. Int. J. Cancer 1999, 83, 732–737. [Google Scholar] [CrossRef]
- Giménez-Bonafé, P.; Fedoruk, M.N.; Whitmore, T.G.; Akbari, M.; Ralph, J.L.; Ettinger, S.; Gleave, M.E.; Nelson, C.C. YB-1 is upregulated during prostate cancer tumor progression and increases P-glycoprotein activity. Prostate 2004, 59, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Rancso, C.; Stuhmer, T.; Eckstein, N.; Andrulis, M.; Gerecke, C.; Lorentz, H.; Royer, H.D.; Bargou, R.C. The Y-box binding protein YB-1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood 2008, 111, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Psenner, K.; Sauer, B.; Meier, F.; Iftner, T.; Garbe, C. The increased expression of Y box-binding protein 1 in melanoma stimulates proliferation and tumor invasion, antagonizes apoptosis and enhances chemoresistance. Int. J. Cancer 2007, 120, 2110–2118. [Google Scholar] [CrossRef]
- Oda, Y.; Sakamoto, A.; Shinohara, N.; Ohga, T.; Uchiumi, T.; Kohno, K.; Tsuneyoshi, M.; Kuwano, M.; Iwamoto, Y. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human osteosarcoma. Clin. Cancer Res. 1998, 4, 2273–2277. [Google Scholar] [PubMed]
- Faury, D.; Nantel, A.; Dunn, S.E.; Guiot, M.C.; Haque, T.; Hauser, P.; Garami, M.; Bognar, L.; Hanzely, Z.; Liberski, P.P.; et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J. Clin. Oncol. 2007, 25, 1196–1208. [Google Scholar] [CrossRef]
- Goswami, C.P.; Nakshatri, H. PROGgeneV2: Enhancements on the existing database. BMC Cancer 2014, 14, 970. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, A.; Sun, B.F.; Yang, Y.; Han, Y.N.; Yuan, X.; Chen, R.X.; Wei, W.S.; Liu, Y.; Gao, C.C.; et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 2019, 21, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Lasham, A.; Print, C.G.; Woolley, A.G.; Dunn, S.E.; Braithwaite, A.W. YB-1: Oncoprotein, prognostic marker and therapeutic target? Biochem. J. 2013, 449, 11–23. [Google Scholar] [CrossRef]
- Lasham, A.; Samuel, W.; Cao, H.; Patel, R.; Mehta, R.; Stern, J.L.; Reid, G.; Woolley, A.G.; Miller, L.D.; Black, M.A.; et al. YB-1, the E2F pathway, and regulation of tumor cell growth. J. Natl. Cancer Inst. 2012, 104, 133–146. [Google Scholar] [CrossRef]
- Jurchott, K.; Bergmann, S.; Stein, U.; Walther, W.; Janz, M.; Manni, I.; Piaggio, G.; Fietze, E.; Dietel, M.; Royer, H.D. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J. Biol. Chem. 2003, 278, 27988–27996. [Google Scholar] [CrossRef]
- Ise, T.; Nagatani, G.; Imamura, T.; Kato, K.; Takano, H.; Nomoto, M.; Izumi, H.; Ohmori, H.; Okamoto, T.; Ohga, T.; et al. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999, 59, 342–346. [Google Scholar]
- Ladomery, M.; Sommerville, J. A role for Y-box proteins in cell proliferation. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 9–11. [Google Scholar] [CrossRef]
- Swamynathan, S.K.; Nambiar, A.; Guntaka, R.V. Role of single-stranded DNA regions and Y-box proteins in transcriptional regulation of viral and cellular genes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Qiu, T.; Peng, J.; Li, S.; Tala; Ren, W.; Yang, C.; Wen, Y.; Chen, C.H.; Sun, J.; et al. YB-1 is a positive regulator of KLF5 transcription factor in basal-like breast cancer. Cell Death Differ. 2022. [Google Scholar] [CrossRef]
- Bergmann, S.; Royer-Pokora, B.; Fietze, E.; Jürchott, K.; Hildebrandt, B.; Trost, D.; Leenders, F.; Claude, J.C.; Theuring, F.; Bargou, R.; et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005, 65, 4078–4087. [Google Scholar] [CrossRef] [PubMed]
- Swamynathan, S.K.; Varma, B.R.; Weber, K.T.; Guntaka, R.V. Targeted disruption of one allele of the Y-box protein gene, Chk-YB-1b, in DT40 cells results in major defects in cell cycle. Biochem. Biophys. Res. Commun. 2002, 296, 451–457. [Google Scholar] [CrossRef]
- Basaki, Y.; Taguchi, K.; Izumi, H.; Murakami, Y.; Kubo, T.; Hosoi, F.; Watari, K.; Nakano, K.; Kawaguchi, H.; Ohno, S.; et al. Y-box binding protein-1 (YB-1) promotes cell cycle progression through CDC6-dependent pathway in human cancer cells. Eur. J. Cancer 2010, 46, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Izumi, H.; Onitsuka, T.; Miyamoto, N.; Kashiwagi, E.; Kidani, A.; Yokomizo, A.; Naito, S.; Kohno, K. Twist promotes tumor cell growth through YB-1 expression. Cancer Res. 2008, 68, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, L.C.; Wilson, L.A.; Sawicka, K.; King, H.A.; Kondrashov, A.V.; Spriggs, K.A.; Bushell, M.; Willis, A.E. Upregulated c-myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB-1 and YB-1. Oncogene 2010, 29, 2884–2891. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, W.; Zhou, Y.; Hong, Z.; Ni, J.; Zhang, X.; Li, Z.; Li, M.; He, W.; Zhang, D.; et al. Therapeutic inhibition of GAS6-AS1/YBX1/MYC axis suppresses cell propagation and disease progression of acute myeloid leukemia. J. Exp. Clin. Cancer Res. 2021, 40, 353. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ma, Y.; Yong, L.; Yang, C.; Wang, P.; Liu, X.; Zhu, B.; Zhou, H.; Liu, X.; Liu, Z. Y-box binding protein-1 promotes tumorigenesis and progression via the epidermal growth factor receptor/AKT pathway in spinal chordoma. Cancer Sci. 2019, 110, 166–179. [Google Scholar] [CrossRef]
- Koren, S.; Bentires-Alj, M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol. Cell 2015, 60, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mylona, E.; Melissaris, S.; Giannopoulou, I.; Theohari, I.; Papadimitriou, C.; Keramopoulos, A.; Nakopoulou, L. Y-box-binding protein 1 (YB1) in breast carcinomas: Relation to aggressive tumor phenotype and identification of patients at high risk for relapse. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014, 40, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.H.; Reipas, K.; Hu, K.; Berns, R.; Firmino, N.; Stratford, A.L.; Dunn, S.E. Inhibition of RSK with the novel small-molecule inhibitor LJI308 overcomes chemoresistance by eliminating cancer stem cells. Oncotarget 2015, 6, 20570–20577. [Google Scholar] [CrossRef]
- Bledzka, K.; Schiemann, B.; Schiemann, W.P.; Fox, P.; Plow, E.F.; Sossey-Alaoui, K. The WAVE3-YB1 interaction regulates cancer stem cells activity in breast cancer. Oncotarget 2017, 8, 104072–104089. [Google Scholar] [CrossRef]
- Guo, T.; Kong, J.; Liu, Y.; Li, Z.; Xia, J.; Zhang, Y.; Zhao, S.; Li, F.; Li, J.; Gu, C. Transcriptional activation of NANOG by YBX1 promotes lung cancer stem-like properties and metastasis. Biochem. Biophys. Res. Commun. 2017, 487, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.M.; Huang, H.X.; Chang, P.H.; Tseng, K.C.; Miyajima, A.; Chern, E. Y-box binding protein-1 promotes hepatocellular carcinoma-initiating cell progression and tumorigenesis via Wnt/β-catenin pathway. Oncotarget 2017, 8, 2604–2616. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Shyamasundar, S.; Gunaratne, J.; Scully, O.J.; Matsumoto, K.; Bay, B.H. YBX1 gene silencing inhibits migratory and invasive potential via CORO1C in breast cancer in vitro. BMC Cancer 2017, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Stratford, A.L.; Habibi, G.; Astanehe, A.; Jiang, H.; Hu, K.; Park, E.; Shadeo, A.; Buys, T.P.; Lam, W.; Pugh, T.; et al. Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y-box binding protein-1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res. BCR 2007, 9, R61. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Nair, S.; Shyamasundar, S.; Chua, P.J.; Muniasamy, U.; Matsumoto, K.; Gunaratne, J.; Bay, B.H. Silencing Y-box binding protein-1 inhibits triple-negative breast cancer cell invasiveness via regulation of MMP1 and beta-catenin expression. Cancer Lett. 2019, 452, 119–131. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Li, Y.; Guo, W.; Chen, L.; Chen, M.; Chen, X.; Zhang, W.; Jin, X.; Jiang, M.; et al. CTPS1 promotes malignant progression of triple-negative breast cancer with transcriptional activation by YBX1. J. Transl. Med. 2022, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.G.; Schelch, K.; Cheng, Y.Y.; Williams, M.; Sarun, K.H.; Kirschner, M.B.; Kao, S.; Linton, A.; Klebe, S.; McCaughan, B.C.; et al. Dysregulated Expression of the MicroRNA miR-137 and Its Target YBX1 Contribute to the Invasive Characteristics of Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.T.; Yu, Y.N.; Yip, G.W.; Matsumoto, K.; Bay, B.H. Silencing the YB-1 gene inhibits cell migration in gastric cancer in vitro. Anat. Rec. 2013, 296, 891–898. [Google Scholar] [CrossRef]
- Zhou, L.L.; Ni, J.; Feng, W.T.; Yao, R.; Yue, S.; Zhu, Y.N.; Tang, H.Y.; Lv, L.Y.; Feng, J.F.; Zhu, W.G. High YBX1 expression indicates poor prognosis and promotes cell migration and invasion in nasopharyngeal carcinoma. Exp. Cell Res. 2017, 361, 126–134. [Google Scholar] [CrossRef]
- Kosnopfel, C.; Sinnberg, T.; Sauer, B.; Busch, C.; Niessner, H.; Schmitt, A.; Forchhammer, S.; Grimmel, C.; Mertens, P.R.; Hailfinger, S.; et al. YB-1 Expression and Phosphorylation Regulate Tumorigenicity and Invasiveness in Melanoma by Influencing EMT. Mol. Cancer Res. 2018, 16, 1149–1160. [Google Scholar] [CrossRef]
- Wu, Y.; Yamada, S.; Izumi, H.; Li, Z.; Shimajiri, S.; Wang, K.Y.; Liu, Y.P.; Kohno, K.; Sasaguri, Y. Strong YB-1 expression is associated with liver metastasis progression and predicts shorter disease-free survival in advanced gastric cancer. J. Surg. Oncol. 2012, 105, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, Y.; Jia, J.; Li, C.; Duan, Q.; Li, R.; Wang, X.; Shao, Y.; Chen, C.; Yan, H. Antimicrobial peptide LL-37 promotes the viability and invasion of skin squamous cell carcinoma by upregulating YB-1. Exp. Ther. Med. 2017, 14, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Tailor, D.; Resendez, A.; Garcia-Marques, F.J.; Pandrala, M.; Going, C.C.; Bermudez, A.; Kumar, V.; Rafat, M.; Nambiar, D.K.; Honkala, A.; et al. Y box binding protein 1 inhibition as a targeted therapy for ovarian cancer. Cell Chem. Biol. 2021, 28, 1206–1220.e6. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Narita, S.; Habuchi, T.; Eto, M. Validated prognostic significance of YB-1 genetic variation in metastatic prostate cancer. Pharm. J. 2021, 21, 102–105. [Google Scholar] [CrossRef]
- Xie, Q.; Zhao, S.; Liu, W.; Cui, Y.; Li, F.; Li, Z.; Guo, T.; Yu, W.; Guo, W.; Deng, W.; et al. YBX1 Enhances Metastasis and Stemness by Transcriptionally Regulating MUC1 in Lung Adenocarcinoma. Front. Oncol. 2021, 11, 702491. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Brandt, S.; Bernhardt, A.; Gorny, X.; Schindele, D.; Jandrig, B.; Schostak, M.; Isermann, B.; Lindquist, J.A.; Mertens, P.R. Crosstalk between Akt signaling and cold shock proteins in mediating invasive cell phenotypes. Oncotarget 2018, 9, 19039–19049. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Ünsal-Kaçmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Lasham, A.; Moloney, S.; Hale, T.; Homer, C.; Zhang, Y.F.; Murison, J.G.; Braithwaite, A.W.; Watson, J. The Y-box-binding protein, YB1, is a potential negative regulator of the p53 tumor suppressor. J. Biol. Chem. 2003, 278, 35516–35523. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, I.; Guay, D.; Lebel, M. YB-1 promotes strand separation in vitro of duplex DNA containing either mispaired bases or cisplatin modifications, exhibits endonucleolytic activities and binds several DNA repair proteins. Nucleic Acids Res. 2004, 32, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Senarisoy, M.; Barette, C.; Lacroix, F.; De Bonis, S.; Stelter, M.; Hans, F.; Kleman, J.P.; Fauvarque, M.O.; Timmins, J. Förster Resonance Energy Transfer Based Biosensor for Targeting the hNTH1-YB1 Interface as a Potential Anticancer Drug Target. ACS Chem. Biol. 2020, 15, 990–1003. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Pestryakov, P.E.; Sukhanova, M.V.; Kretov, D.A.; Moor, N.A.; Curmi, P.A.; Ovchinnikov, L.P.; Lavrik, O.I. Poly(ADP-ribosyl)ation as a new posttranslational modification of YB-1. Biochimie 2015, 119, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; Ryan, K.M. The role of autophagy in tumour development and cancer therapy. Expert Rev. Mol. Med. 2009, 11, e36. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, F.; Xie, Q.; Zhao, S.; Guo, T.; Guo, P.; Hu, S.; Hao, J.; Tian, C.; Yu, W.; et al. YBX1 mediates autophagy by targeting p110β and decreasing the sensitivity to cisplatin in NSCLC. Cell Death Dis. 2020, 11, 476. [Google Scholar] [CrossRef]
- Su, W.; Wang, L.; Zhao, H.; Hu, S.; Zhou, Y.; Guo, C.; Wu, B.; Li, L.; Yang, Z.; Beer, D.G.; et al. LINC00857 Interacting with YBX1 to Regulate Apoptosis and Autophagy via MET and Phosphor-AMPKa Signaling. Mol. Ther. Nucleic Acids 2020, 22, 1164–1175. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, J.; Xu, X.; Shen, B.; Shen, Z.; Li, B.; Li, F.; Gu, T.; Cai, X.; Dong, H.; et al. TGF-β/YB-1/Atg7 axis promotes the proliferation of hepatic progenitor cells and liver fibrogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166290. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Ruan, H.; Sun, L.; Kuang, D.; Song, Y.; Wang, Q.; Wang, T.; Hao, Y.; Chen, K. Targeting the YB-1/PD-L1 Axis to Enhance Chemotherapy and Antitumor Immunity. Cancer Immunol. Res. 2019, 7, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Asakuno, K.; Kohno, K.; Uchiumi, T.; Kubo, T.; Sato, S.; Isono, M.; Kuwano, M. Involvement of a DNA binding protein, MDR-NF1/YB-1, in human MDR1 gene expression by actinomycin D. Biochem. Biophys. Res. Commun. 1994, 199, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Uchiumi, T.; Ohga, T.; Toh, S.; Wada, M.; Kohno, K.; Kuwano, M. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 1997, 417, 390–394. [Google Scholar] [CrossRef]
- Tang, L.; Wei, D.; Xu, X.; Mao, X.; Mo, D.; Yan, L.; Xu, W.; Yan, F. Long non-coding RNA MIR200CHG promotes breast cancer proliferation, invasion, and drug resistance by interacting with and stabilizing YB-1. NPJ Breast Cancer 2021, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Guay, D.; Evoy, A.A.; Paquet, E.; Garand, C.; Bachvarova, M.; Bachvarov, D.; Lebel, M. The strand separation and nuclease activities associated with YB-1 are dispensable for cisplatin resistance but overexpression of YB-1 in MCF7 and MDA-MB-231 breast tumor cells generates several chemoresistance signatures. Int. J. Biochem. Cell Biol. 2008, 40, 2492–2507. [Google Scholar] [CrossRef] [PubMed]
- Gluz, O.; Mengele, K.; Schmitt, M.; Kates, R.; Diallo-Danebrock, R.; Neff, F.; Royer, H.D.; Eckstein, N.; Mohrmann, S.; Ting, E.; et al. Y-box-binding protein YB-1 identifies high-risk patients with primary breast cancer benefiting from rapidly cycled tandem high-dose adjuvant chemotherapy. J. Clin. Oncol. 2009, 27, 6144–6151. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ito, K.; Izumi, H.; Kimura, M.; Sano, M.; Nakagomi, H.; Maeno, K.; Hama, Y.; Shingu, K.; Tsuchiya, S.; et al. Increased nuclear localization of transcription factor Y-box binding protein 1 accompanied by up-regulation of P-glycoprotein in breast cancer pretreated with paclitaxel. Clin. Cancer Res. 2005, 11, 8837–8844. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Li, S.; Bao, L.; Zhang, X. Enhanced YB1/EphA2 axis signaling promotes acquired resistance to sunitinib and metastatic potential in renal cell carcinoma. Oncogene 2020, 39, 6113–6128. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Ohishi, Y.; Saito, T.; Hinoshita, E.; Uchiumi, T.; Kinukawa, N.; Iwamoto, Y.; Kohno, K.; Kuwano, M.; Tsuneyoshi, M. Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. J. Pathol. 2003, 199, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Han, X.; Yang, W.L.; Zhang, M.; Ma, H.L.; Sun, B.F.; Li, A.; Xia, J.; Chen, J.; et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol. Cell 2019, 75, 1188–1202.e11. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Tu, R.; Duan, B.; Yang, Z.; Ping, Z.; Song, X.; Chen, S.; Price, A.; Li, H.; Scott, A.; et al. Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc. Natl. Acad Sci. USA 2020, 117, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Evdokimova, V.; Tognon, C.; Ng, T.; Ruzanov, P.; Melnyk, N.; Fink, D.; Sorokin, A.; Ovchinnikov, L.P.; Davicioni, E.; Triche, T.J.; et al. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell 2009, 15, 402–415. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.M.; Veinotte, C.J.; Cheng, H.; Grunewald, T.G.; Negri, G.L.; Somasekharan, S.P.; Corkery, D.P.; Tirode, F.; Mathers, J.; Khan, D.; et al. Translational Activation of HIF1α by YB-1 Promotes Sarcoma Metastasis. Cancer Cell 2015, 27, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Tan, Y.; Li, X.; Li, X.; Zeng, Z.; Xiong, W.; Li, G.; Xiang, B.; Yi, M. RNA-binding protein YBX1 promotes cell proliferation and invasiveness of nasopharyngeal carcinoma cells via binding to AURKA mRNA. J. Cancer 2021, 12, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.N.; Wang, Z.; Zhang, H. Liquid-liquid phase separation in autophagy. J. Cell Biol. 2020, 219, e202004062. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.H.; Hsu, K.W.; Wu, K.J. Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology. Am. J. Cancer Res. 2021, 11, 3766–3776. [Google Scholar] [PubMed]
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China. Life Sci. 2020, 63, 953–985. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Ma, L.; Schekman, R. Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. eLife 2021, 10, e71982. [Google Scholar] [CrossRef] [PubMed]
- Paltridge, J.L.; Belle, L.; Khew-Goodall, Y. The secretome in cancer progression. Biochim. Biophys. Acta 2013, 1834, 2233–2241. [Google Scholar] [CrossRef]
- Frye, B.C.; Halfter, S.; Djudjaj, S.; Muehlenberg, P.; Weber, S.; Raffetseder, U.; En-Nia, A.; Knott, H.; Baron, J.M.; Dooley, S.; et al. Y-box protein-1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep. 2009, 10, 783–789. [Google Scholar] [CrossRef]
- Kang, S.; Lee, T.A.; Ra, E.A.; Lee, E.; Choi, H.; Lee, S.; Park, B. Differential control of interleukin-6 mRNA levels by cellular distribution of YB-1. PLoS ONE 2014, 9, e112754. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.M.; Troiano, A.; Pizzo, E.; Bosso, A.; Vivo, M.; Pinto, G.; Amoresano, A.; Pollice, A.; La Mantia, G.; Calabrò, V. Oxidative Stress Causes Enhanced Secretion of YB-1 Protein that Restrains Proliferation of Receiving Cells. Genes 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Kosnopfel, C.; Sinnberg, T.; Sauer, B.; Niessner, H.; Muenchow, A.; Fehrenbacher, B.; Schaller, M.; Mertens, P.R.; Garbe, C.; Thakur, B.K.; et al. Tumour Progression Stage-Dependent Secretion of YB-1 Stimulates Melanoma Cell Migration and Invasion. Cancers 2020, 12, 2328. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Huang, J.; Yu, K.; Chen, X.; He, Y.; Qi, D.; Wu, Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer 2020, 20, 996. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.W.; Kucab, J.; Wu, J.; Lee, C.; Cheang, M.C.; Yorida, E.; Turbin, D.; Dedhar, S.; Nelson, C.; Pollak, M.; et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene 2005, 24, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xu, W.; Luo, W.; Zhou, L.; Yong, W.; Chen, F.; Wu, C.; Chen, Q.; Han, X. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp. Hematol. 2011, 39, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Stratford, A.L.; Fry, C.J.; Desilets, C.; Davies, A.H.; Cho, Y.Y.; Li, Y.; Dong, Z.; Berquin, I.M.; Roux, P.P.; Dunn, S.E. Y-box binding protein-1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal-like breast cancer cells. Breast Cancer Res. 2008, 10, R99. [Google Scholar] [CrossRef] [PubMed]
- Lyabin, D.N.; Eliseeva, I.A.; Ovchinnikov, L.P. YB-1 synthesis is regulated by mTOR signaling pathway. PLoS ONE 2012, 7, e52527. [Google Scholar] [CrossRef] [PubMed]
- Astanehe, A.; Finkbeiner, M.R.; Hojabrpour, P.; To, K.; Fotovati, A.; Shadeo, A.; Stratford, A.L.; Lam, W.L.; Berquin, I.M.; Duronio, V.; et al. The transcriptional induction of PIK3CA in tumor cells is dependent on the oncoprotein Y-box binding protein-1. Oncogene 2009, 28, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Lee, E.B.; Cui, J.; Kim, Y.; Jang, H.H. YB-1 overexpression promotes a TGF-β1-induced epithelial-mesenchymal transition via Akt activation. Biochem. Biophys. Res. Commun. 2015, 458, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Izumi, H.; Ise, T.; Tada, M.; Uchiumi, T.; Kuwano, M.; Yasumoto, K.; Funa, K.; Kohno, K. p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression. J. Biol. Chem. 2002, 277, 31694–31702. [Google Scholar] [CrossRef] [PubMed]
- di Martino, O.; Troiano, A.; Guarino, A.M.; Pollice, A.; Vivo, M.; La Mantia, G.; Calabrò, V. ΔNp63α controls YB-1 protein stability: Evidence on YB-1 as a new player in keratinocyte differentiation. Genes Cells 2016, 21, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zheng, Y.; Wang, W.; Shao, Y.; Li, Z.; Wang, Q.; Wang, Y.; Yan, H. Antimicrobial peptide LL-37 promotes YB-1 expression, and the viability, migration and invasion of malignant melanoma cells. Mol. Med. Rep. 2017, 15, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Wang, Y.; Sun, Y.; Niu, Y.; Chang, C. C1QBP Regulates YBX1 to Suppress the Androgen Receptor (AR)-Enhanced RCC Cell Invasion. Neoplasia 2017, 19, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Luo, Y.; Zhou, Y.; Li, M.; Wu, C.; Duan, Y.; Wang, H.; Fan, S.; Li, Z.; Xiong, W.; et al. BRD7 suppresses invasion and metastasis in breast cancer by negatively regulating YB1-induced epithelial-mesenchymal transition. J. Exp. Clin. Cancer Res. 2020, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Long, X.; Li, J.; Feng, M. Homeodomain protein DLX4 facilitates nasopharyngeal carcinoma progression via up-regulation of YB-1. Genes Cells 2020, 25, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.M.; Zhou, Y.C.; Wen, Z.S.; Zhou, B.; Huang, Y.C.; Wang, G.Z.; Zhao, X.C.; Pan, H.L.; Qu, L.W.; Zhang, J.; et al. Long non-coding RNA stabilizes the Y-box-binding protein 1 and regulates the epidermal growth factor receptor to promote lung carcinogenesis. Oncotarget 2016, 7, 59556–59571. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiong, W.; Jiang, X.; Zhang, S.; Li, Z.; Zhou, Y.; Xiang, B.; Zhou, M.; Li, X.; Li, G.; et al. LncRNA LINC00472 regulates cell stiffness and inhibits the migration and invasion of lung adenocarcinoma by binding to YBX1. Cell Death Dis. 2020, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Diao, Y.; Li, X.; Jiang, Z.; Xu, Y.; Zhou, H.; Qiang, Y.; Wu, H.; Shen, Y. Long non-coding RNA linc00665 interacts with YB-1 and promotes angiogenesis in lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2020, 527, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, J.; Shan, B.; Li, B.; Peng, W.; Dong, Y.; Shi, W.; Zhao, W.; He, D.; Duan, M.; et al. The long noncoding RNA LINC00312 induces lung adenocarcinoma migration and vasculogenic mimicry through directly binding YBX1. Mol. Cancer 2018, 17, 167. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, L.; Zou, Y.; Liang, J.Y.; Liu, P.; Gao, G.; Yang, A.; Tang, H.; Xie, X. Long non-coding RNA HUMT hypomethylation promotes lymphangiogenesis and metastasis via activating FOXK1 transcription in triple-negative breast cancer. J. Hematol. Oncol. 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.X.; Wang, D.L.; Yang, B.; Yan, H.Y.; Lin, L.H.; Li, Y.; Chen, J.; Xie, L.M.; Huang, Y.S.; et al. LncRNA DSCAM-AS1 interacts with YBX1 to promote cancer progression by forming a positive feedback loop that activates FOXA1 transcription network. Theranostics 2020, 10, 10823–10837. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, J.; Li, Y.; Duan, W.; Qi, Q.; Wang, T.; Yang, Q.; Du, L.; Mao, H.; Wang, C. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dai, M.; Wang, D.; Tang, T.; Xiong, F.; Xiang, B.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; et al. The long noncoding RNA AATBC promotes breast cancer migration and invasion by interacting with YBX1 and activating the YAP1/Hippo signaling pathway. Cancer Lett. 2021, 512, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yu, B.; Zhang, C.; Yi, P.; Li, H.; Xu, C.; Cao, L.; Chen, P.; Li, M.; Shen, K.; et al. Long noncoding RNA HOXC-AS3 indicates a poor prognosis and regulates tumorigenesis by binding to YBX1 in breast cancer. Am. J. Transl. Res. 2020, 12, 6335–6350. [Google Scholar]
- Li, D.; Liu, X.; Zhou, J.; Hu, J.; Zhang, D.; Liu, J.; Qiao, Y.; Zhan, Q. Long noncoding RNA HULC modulates the phosphorylation of YB-1 through serving as a scaffold of extracellular signal-regulated kinase and YB-1 to enhance hepatocarcinogenesis. Hepatology 2017, 65, 1612–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; He, X.; Zhang, C.; Su, J.; Lu, X.; Si, X.; Chen, J.; Yin, D.; Han, L.; De, W. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Huang, M.; Chen, Q.; Niu, Y.; Hu, Y.; Hu, P.; Chen, D.; He, C.; Huang, K.; Zeng, Z.; et al. LncRNA HIF1A-AS1 Promotes Gemcitabine Resistance of Pancreatic Cancer by Enhancing Glycolysis through Modulating the AKT/YB1/HIF1α Pathway. Cancer Res. 2021, 81, 5678–5691. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Yu, S. Long noncoding RNA AWPPH promotes hepatocellular carcinoma progression through YBX1 and serves as a prognostic biomarker. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1805–1816. [Google Scholar] [CrossRef]
- Song, S.; He, X.; Wang, J.; Song, H.; Wang, Y.; Liu, Y.; Zhou, Z.; Yu, Z.; Miao, D.; Xue, Y. A novel long noncoding RNA, TMEM92-AS1, promotes gastric cancer progression by binding to YBX1 to mediate CCL5. Mol. Oncol. 2021, 15, 1256–1273. [Google Scholar] [CrossRef]
- Zhao, P.; Ji, M.M.; Fang, Y.; Li, X.; Yi, H.M.; Yan, Z.X.; Cheng, S.; Xu, P.P.; Janin, A.; Wang, C.F.; et al. A novel lncRNA TCLlnc1 promotes peripheral T cell lymphoma progression through acting as a modular scaffold of HNRNPD and YBX1 complexes. Cell Death Dis. 2021, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Hu, X.; Jiang, X.; Yin, L.; Chen, J.; Wen, J.; Fan, Y.; Peng, F.; Qian, L.; Wu, J.; et al. LncRNA EPB41L4A-AS2 represses Nasopharyngeal Carcinoma Metastasis by binding to YBX1 in the Nucleus and Sponging MiR-107 in the Cytoplasm. Int. J. Biol. Sci. 2021, 17, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, G.; Qiu, H.; Yu, H.; Yuan, W. A novel positive feedback loop of linc02042 and c-Myc mediated by YBX1 promotes tumorigenesis and metastasis in esophageal squamous cell carcinoma. Cancer Cell Int. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Niu, X.; Tang, M.; Li, J.; Song, B.; Guan, X. LncRNA BASP1-AS1 interacts with YBX1 to regulate Notch transcription and drives the malignancy of melanoma. Cancer Sci. 2021, 112, 4526–4542. [Google Scholar] [CrossRef]
- Zhao, P.; Deng, Y.; Wu, Y.; Guo, Q.; Zhou, L.; Yang, X.; Wang, C. Long noncoding RNA SNHG6 promotes carcinogenesis by enhancing YBX1-mediated translation of HIF1α in clear cell renal cell carcinoma. FASEB J. 2021, 35, e21160. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, Y.; Zhu, H.; Chen, D.; Hu, W. Downregulation of miR-216a-5p by long noncoding RNA PVT1 suppresses colorectal cancer progression via modulation of YBX1 expression. Cancer Manag. Res. 2019, 11, 6981–6993. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, H.; Li, L. Long noncoding RNA PRKCQ-AS1 promotes CRC cell proliferation and migration via modulating miR-1287-5p/YBX1 axis. J. Cell. Biochem. 2020, 121, 4166–4175. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, J.; Li, Z.; Zhang, Q.; Liu, W.; Yang, C.; Zhao, P.; Wang, X.; Yin, Q.; Luo, Y.; et al. A feedforward circuit between KLF5 and lncRNA KPRT4 contributes to basal-like breast cancer. Cancer Lett. 2022, 534, 215618. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar]
- Liu, S.L.; Sui, Y.F.; Lin, M.Z. MiR-375 is epigenetically downregulated due to promoter methylation and modulates multi-drug resistance in breast cancer cells via targeting YBX1. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3223–3229. [Google Scholar]
- Yang, F.; Wei, J.; Zhang, S.; Zhang, X. Shrimp miR-S8 Suppresses the Stemness of Human Melanoma Stem-like Cells by Targeting the Transcription Factor YB-1. Cancer Res. 2017, 77, 5543–5553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Duan, C.; Yin, S.; Tian, Y. MicroRNA-379-5p/YBX1 Axis Regulates Cellular EMT to Suppress Migration and Invasion of Nasopharyngeal Carcinoma Cells. Cancer Manag. Res. 2020, 12, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fan, G.; Huang, J.; Qiu, X. YBX1 regulated by Runx3-miR-148a-3p axis facilitates non-small-cell lung cancer progression. Cell Signal. 2021, 85, 110049. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ji, L.; Liang, Y.; Wan, Z.; Zheng, W.; Song, X.; Gorshkov, K.; Sun, Q.; Lin, H.; Zheng, X.; et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal. Transduct. Target. Ther. 2020, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yang, H.; Xiong, W.; Tang, G.; Zu, X.; Qi, L. circ_100984-miR-432-3p axis regulated c-Jun/YBX-1/β-catenin feedback loop promotes bladder cancer progression. Cancer Sci. 2021, 112, 1429–1442. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Z.; Xu, W.; Liu, H.; Chang, J.; Xu, W.; Li, S.; Cao, S.; Hou, J. Circ-SAR1A Promotes Renal Cell Carcinoma Progression Through miR-382/YBX1 Axis. Cancer Manag. Res. 2020, 12, 7353–7361. [Google Scholar] [CrossRef]
- Fang, J.; Hong, H.; Xue, X.; Zhu, X.; Jiang, L.; Qin, M.; Liang, H.; Gao, L. A novel circular RNA, circFAT1(e2), inhibits gastric cancer progression by targeting miR-548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett. 2019, 442, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Li, Z.; Li, F.; Liang, L.; Zou, Y.; Shen, H.; Li, J.; Xia, Y.; Cheng, Z.; et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J. Hepatol. 2022, 76, 135–147. [Google Scholar] [CrossRef]
- Plohl, M.; Meštrović, N.; Mravinac, B. Centromere identity from the DNA point of view. Chromosoma 2014, 123, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.A.; Canapa, A.; Forconi, M.; Olmo, E.; Barucca, M. Transcription of tandemly repetitive DNA: Functional roles. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2015, 23, 463–477. [Google Scholar] [CrossRef]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 2015, 161, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, T.; Otsuka, M.; Yoshikawa, T.; Ohno, M.; Ijichi, H.; Koike, K. Satellite RNAs promote pancreatic oncogenic processes via the dysfunction of YBX1. Nat. Commun. 2016, 7, 13006. [Google Scholar] [CrossRef]
- Jayavelu, A.K.; Schnöder, T.M.; Perner, F.; Herzog, C.; Meiler, A.; Krishnamoorthy, G.; Huber, N.; Mohr, J.; Edelmann-Stephan, B.; Austin, R.; et al. Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms. Nature 2020, 588, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Hua, L.; Wang, B.; Wei, H.; Prabhu, L.; Hartley, A.V.; Jiang, G.; Liu, Y.; Lu, T. Novel Serine 176 Phosphorylation of YBX1 Activates NF-κB in Colon Cancer. J. Biol. Chem. 2017, 292, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; D’Souza, R.C.; Tyanova, S.; Schaab, C.; Wiśniewski, J.R.; Cox, J.; Mann, M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 2014, 8, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, L.; Mundade, R.; Wang, B.; Wei, H.; Hartley, A.V.; Martin, M.; McElyea, K.; Temm, C.J.; Sandusky, G.; Liu, Y.; et al. Critical role of phosphorylation of serine 165 of YBX1 on the activation of NF-κB in colon cancer. Oncotarget 2015, 6, 29396–29412. [Google Scholar] [CrossRef] [PubMed]
- Kasyapa, C.; Gu, T.L.; Nagarajan, L.; Polakiewicz, R.; Cowell, J.K. Phosphorylation of the SSBP2 and ABL proteins by the ZNF198-FGFR1 fusion kinase seen in atypical myeloproliferative disorders as revealed by phosphopeptide-specific MS. Proteomics 2009, 9, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, M.V.; Larsen, D.H.; Bunkenborg, J.; Bartek, J.; Lukas, J.; Andersen, J.S. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol. Cell. Proteom. MCP 2010, 9, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, A.N.; Schweppe, D.K.; Faherty, B.K.; Pechenick, D.; Pletnev, A.A.; Gerber, S.A. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 2011, 4, rs5. [Google Scholar] [CrossRef]
- Sogorina, E.M.; Kim, E.R.; Sorokin, A.V.; Lyabin, D.N.; Ovchinnikov, L.P.; Mordovkina, D.A.; Eliseeva, I.A. YB-1 Phosphorylation at Serine 209 Inhibits Its Nuclear Translocation. Int. J. Mol. Sci. 2021, 23, 428. [Google Scholar] [CrossRef]
- van Roeyen, C.R.; Scurt, F.G.; Brandt, S.; Kuhl, V.A.; Martinkus, S.; Djudjaj, S.; Raffetseder, U.; Royer, H.D.; Stefanidis, I.; Dunn, S.E.; et al. Cold shock Y-box protein-1 proteolysis autoregulates its transcriptional activities. Cell Commun. Signal. CCS 2013, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, E.M.; Hunzicker-Dunn, M.E. Extracellular Signal-regulated Kinase (ERK)-dependent Phosphorylation of Y-Box-binding Protein 1 (YB-1) Enhances Gene Expression in Granulosa Cells in Response to Follicle-stimulating Hormone (FSH). J. Biol. Chem. 2016, 291, 12145–12160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, L.; Zhao, H.; Li, Q.; Hu, R.; Wang, H. Integrin β8 facilitates tumor growth and drug resistance through a Y-box binding protein 1-dependent signaling pathway in bladder cancer. Cancer Sci. 2020, 111, 2423–2430. [Google Scholar] [CrossRef]
- Liu, D.; Ke, J.; Liu, Y.; Rao, H.; Tang, Z.; Liu, Y.; Zhang, Z.; You, L.; Luo, X.; Sun, Z.; et al. The interaction between PDCD4 and YB1 is critical for cervical cancer stemness and cisplatin resistance. Mol. Carcinog. 2021, 60, 813–825. [Google Scholar] [CrossRef]
- Ewert, L.; Fischer, A.; Brandt, S.; Scurt, F.G.; Philipsen, L.; Müller, A.J.; Girndt, M.; Zenclussen, A.C.; Lindquist, J.A.; Gorny, X.; et al. Cold shock Y-box binding protein-1 acetylation status in monocytes is associated with systemic inflammation and vascular damage. Atherosclerosis 2018, 278, 156–165. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.M.; Somasekharan, S.P.; Wang, Y.; Cheng, H.; Negri, G.L.; Pan, M.; Wang, X.Q.; Delaidelli, A.; Rafn, B.; Cran, J.; et al. Class I HDAC inhibitors enhance YB-1 acetylation and oxidative stress to block sarcoma metastasis. EMBO Rep. 2019, 20, e48375. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, X.; Liu, L.; Shu, Y.; Zhang, M.; Zhong, Y. Role of protein arginine methyltransferase 5 in human cancers. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 114, 108790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S.; Zhu, L.; Chen, X.; Zhao, Y.; Chao, L.; Zhou, J.; Wang, X.; Zhang, X.; Ma, N. Arginine methyltransferase inhibitor 1 inhibits gastric cancer by downregulating eIF4E and targeting PRMT5. Toxicol. Appl. Pharmacol. 2017, 336, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.V.; Wang, B.; Mundade, R.; Jiang, G.; Sun, M.; Wei, H.; Sun, S.; Liu, Y.; Lu, T. PRMT5-mediated methylation of YBX1 regulates NF-κB activity in colorectal cancer. Sci. Rep. 2020, 10, 15934. [Google Scholar] [CrossRef]
- Yan, S.; Hu, J.; Li, J.; Wang, P.; Wang, Y.; Wang, Z. PRMT4 drives post-ischemic angiogenesis via YB1/VEGF signaling. J. Mol. Med. 2021, 99, 993–1008. [Google Scholar] [CrossRef]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Wempe, F.; Bahr, I.; Zopf, D.; von Melchner, H. Proteasomal degradation of the multifunctional regulator YB-1 is mediated by an F-Box protein induced during programmed cell death. FEBS Lett. 2006, 580, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Chibi, M.; Meyer, M.; Skepu, A.; DJ, G.R.; Moolman-Smook, J.C.; Pugh, D.J. RBBP6 interacts with multifunctional protein YB-1 through its RING finger domain, leading to ubiquitination and proteosomal degradation of YB-1. J. Mol. Biol. 2008, 384, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, L.; Lin, L.; Yang, M.; Li, T.; Wei, H.; Sha, C.; Xing, J.; Zhang, M.; Zhao, S.; et al. SIAH1 reverses chemoresistance in epithelial ovarian cancer via ubiquitination of YBX-1. Oncogenesis 2022, 11, 13. [Google Scholar] [CrossRef]
- Palicharla, V.R.; Maddika, S. HACE1 mediated K27 ubiquitin linkage leads to YB-1 protein secretion. Cell Signal. 2015, 27, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, H.; Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Kohli, S.; Hoffmann, J.; Dhople, V.M.; et al. Activated Protein C Ameliorates Renal Ischemia-Reperfusion Injury by Restricting Y-Box Binding Protein-1 Ubiquitination. J. Am. Soc. Nephrol. JASN 2015, 26, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Comer, F.I.; Hart, G.W. O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J. Biol. Chem. 2000, 275, 29179–29182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tao, T.; Liu, F.; Ni, R.; Lu, C.; Shen, A. Hyper-O-GlcNAcylation of YB-1 affects Ser102 phosphorylation and promotes cell proliferation in hepatocellular carcinoma. Exp. Cell Res. 2016, 349, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Alemasova, E.E.; Naumenko, K.N.; Kurgina, T.A.; Anarbaev, R.O.; Lavrik, O.I. The multifunctional protein YB-1 potentiates PARP1 activity and decreases the efficiency of PARP1 inhibitors. Oncotarget 2018, 9, 23349–23365. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, K.N.; Sukhanova, M.V.; Hamon, L.; Kurgina, T.A.; Alemasova, E.E.; Kutuzov, M.M.; Pastré, D.; Lavrik, O.I. Regulation of Poly(ADP-Ribose) Polymerase 1 Activity by Y-Box-Binding Protein 1. Biomolecules 2020, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.G.; Algie, M.; Samuel, W.; Harfoot, R.; Wiles, A.; Hung, N.A.; Tan, P.H.; Hains, P.; Valova, V.A.; Huschtscha, L.; et al. Prognostic association of YB-1 expression in breast cancers: A matter of antibody. PLoS ONE 2011, 6, e20603. [Google Scholar] [CrossRef] [PubMed]
- Mordovkina, D.A.; Kim, E.R.; Buldakov, I.A.; Sorokin, A.V.; Eliseeva, I.A.; Lyabin, D.N.; Ovchinnikov, L.P. Transportin-1-dependent YB-1 nuclear import. Biochem. Biophys. Res. Commun. 2016, 480, 629–634. [Google Scholar] [CrossRef]
- Stein, U.; Jürchott, K.; Walther, W.; Bergmann, S.; Schlag, P.M.; Royer, H.D. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J. Biol. Chem. 2001, 276, 28562–28569. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Frye, B.C.; Wang, J.; Raffetseder, U.; Alidousty, C.; En-Nia, A.; Floege, J.; Mertens, P.R. Cold shock protein YB-1 is involved in hypoxia-dependent gene transcription. Biochem. Biophys. Res. Commun. 2016, 478, 982–987. [Google Scholar] [CrossRef]
- Ohga, T.; Koike, K.; Ono, M.; Makino, Y.; Itagaki, Y.; Tanimoto, M.; Kuwano, M.; Kohno, K. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996, 56, 4224–4228. [Google Scholar] [PubMed]
- Kim, E.R.; Selyutina, A.A.; Buldakov, I.A.; Evdokimova, V.; Ovchinnikov, L.P.; Sorokin, A.V. The proteolytic YB-1 fragment interacts with DNA repair machinery and enhances survival during DNA damaging stress. Cell Cycle 2013, 12, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Basaki, Y.; Hosoi, F.; Oda, Y.; Fotovati, A.; Maruyama, Y.; Oie, S.; Ono, M.; Izumi, H.; Kohno, K.; Sakai, K.; et al. Akt-dependent nuclear localization of Y-box-binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene 2007, 26, 2736–2746. [Google Scholar] [CrossRef]
- Higashi, K.; Inagaki, Y.; Suzuki, N.; Mitsui, S.; Mauviel, A.; Kaneko, H.; Nakatsuka, I. Y-box-binding protein YB-1 mediates transcriptional repression of human alpha 2(I) collagen gene expression by interferon-gamma. J. Biol. Chem. 2003, 278, 5156–5162. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Selyutina, A.A.; Skabkin, M.A.; Guryanov, S.G.; Nazimov, I.V.; Richard, C.; Th’ng, J.; Yau, J.; Sorensen, P.H.; Ovchinnikov, L.P.; et al. Proteasome-mediated cleavage of the Y-box-binding protein 1 is linked to DNA-damage stress response. EMBO J. 2005, 24, 3602–3612. [Google Scholar] [CrossRef]
- Kretov, D.A.; Mordovkina, D.A.; Eliseeva, I.A.; Lyabin, D.N.; Polyakov, D.N.; Joshi, V.; Desforges, B.; Hamon, L.; Lavrik, O.I.; Pastré, D.; et al. Inhibition of Transcription Induces Phosphorylation of YB-1 at Ser102 and Its Accumulation in the Nucleus. Cells 2019, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; McKinney, C.; Algie, M.; Verma, C.S.; Kannan, S.; Harfoot, R.; Bartolec, T.K.; Bhatia, P.; Fisher, A.J.; Gould, M.L.; et al. Dephosphorylation of YB-1 is Required for Nuclear Localisation During G(2) Phase of the Cell Cycle. Cancers 2020, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Rebholz, S.; Maier, E.; Dehghan Harati, M.; Zips, D.; Sers, C.; Rodemann, H.P.; Toulany, M. Stress-Induced Phosphorylation of Nuclear YB-1 Depends on Nuclear Trafficking of p90 Ribosomal S6 Kinase. Int. J. Mol. Sci. 2018, 19, 2441. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kasai, M.; Kobayashi, S. Mechanism responsible for inhibitory effect of indirubin 3’-oxime on anticancer agent-induced YB-1 nuclear translocation in HepG2 human hepatocellular carcinoma cells. Exp. Cell Res. 2018, 370, 454–460. [Google Scholar] [CrossRef]
- Reipas, K.M.; Law, J.H.; Couto, N.; Islam, S.; Li, Y.; Li, H.; Cherkasov, A.; Jung, K.; Cheema, A.S.; Jones, S.J.; et al. Luteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y-box binding protein-1 (YB-1). Oncotarget 2013, 4, 329–345. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Yang, Y.; Liu, J.; Chen, Z. Sesquiterpene lactone 6-O-angeloylplenolin reverses vincristine resistance by inhibiting YB-1 nuclear translocation in colon carcinoma cells. Oncol. Lett. 2018, 15, 9673–9680. [Google Scholar] [CrossRef]
- Gunasekaran, V.P.; Nishi, K.; Sivakumar, D.; Sivaraman, T.; Mathan, G. Identification of 2,4-dihydroxy-5-pyrimidinyl imidothiocarbomate as a novel inhibitor to Y box binding protein-1 (YB-1) and its therapeutic actions against breast cancer. Eur. J. Pharm. Sci. 2018, 116, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Hung, C.M.; Lin, Y.C.; Ho, C.T.; Kao, J.Y.; Way, T.D. Aloe-emodin inhibits HER-2 expression through the downregulation of Y-box binding protein-1 in HER-2-overexpressing human breast cancer cells. Oncotarget 2016, 7, 58915–58930. [Google Scholar] [CrossRef][Green Version]
- Tanaka, T.; Saito, H.; Miyairi, S.; Kobayashi, S. 7-Hydorxyindirubin is capable of specifically inhibiting anticancer drug-induced YB-1 nuclear translocation without showing cytotoxicity in HepG2 hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2021, 544, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gibbert, L.; Djudjaj, S.; Alidousty, C.; Rauen, T.; Kunter, U.; Rembiak, A.; Enders, D.; Jankowski, V.; Braun, G.S.; et al. Therapeutic nuclear shuttling of YB-1 reduces renal damage and fibrosis. Kidney Int. 2016, 90, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Tomigahara, Y.; Shiraki, H.; Miyata, K.; Mikami, T.; Kimura, T.; Moro, T.; Inagaki, Y.; Kaneko, H. A novel small compound that promotes nuclear translocation of YB-1 ameliorates experimental hepatic fibrosis in mice. J. Biol. Chem. 2011, 286, 4485–4492. [Google Scholar] [CrossRef] [PubMed]
- Houles, T.; Roux, P.P. Defining the role of the RSK isoforms in cancer. Semin. Cancer Biol. 2018, 48, 53–61. [Google Scholar] [CrossRef] [PubMed]
| Inhibitors | Structure | Function and Mechanism | Disease | References |
|---|---|---|---|---|
| luteolin |  | suppresses Notch4 signaling by blocking the activation of YB-1 | TNBC | [195] |
| Sesquiterpene lactone 6-O-angeloylplenolin | 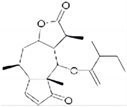 | reverses vincristine resistance by inhibiting YB-1 nuclear translocation | colon carcinoma | [196] |
| Aloe-emodin | 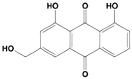 | inhibits HER-2 expression through the downregulation of YB-1 | HER-2 positive breast cancer | [198] |
| DPI | 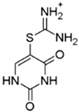 | inhibits YB-1 nuclear translocation and increases the therapeutic potential of doxorubicin | breast cancer | [197] |
| MS-275 | 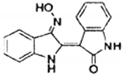 | inhibits YB-1 deacetylation and reduces sarcoma metastasis | sarcoma | [167] |
| 7-hydroxyindirubin | 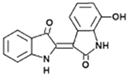 | inhibits the actinomycin D-induced nuclear translocation of YB-1 | HCC | [199] |
| SU056 | 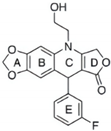 | binds to YB-1 and inhibits its expression | ovarian cancer | [63] |
| HSc025 | 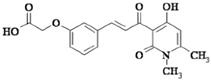 | promotes YB-1 entry into the nucleus and reduces fibrosis in the liver and kidney | hepatic fibrosis and renal fibrosis | [200,201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Q.; Zheng, M.; Luo, Q.; Jiang, D.; Zhang, H.; Chen, C. YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy. Cells 2022, 11, 1217. https://doi.org/10.3390/cells11071217
Yin Q, Zheng M, Luo Q, Jiang D, Zhang H, Chen C. YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy. Cells. 2022; 11(7):1217. https://doi.org/10.3390/cells11071217
Chicago/Turabian StyleYin, Qiyan, Min Zheng, Qianmei Luo, Dewei Jiang, Huifeng Zhang, and Ceshi Chen. 2022. "YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy" Cells 11, no. 7: 1217. https://doi.org/10.3390/cells11071217
APA StyleYin, Q., Zheng, M., Luo, Q., Jiang, D., Zhang, H., & Chen, C. (2022). YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy. Cells, 11(7), 1217. https://doi.org/10.3390/cells11071217








