Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Samples
2.2. Chemicals and Reagents
2.3. NAbs Measurements
2.4. Plasma Sample Preparation
2.5. Data Acquisition
2.6. Data Processing
2.7. Study Approval
3. Results
3.1. Cohort Characteristics
3.2. NMR Results
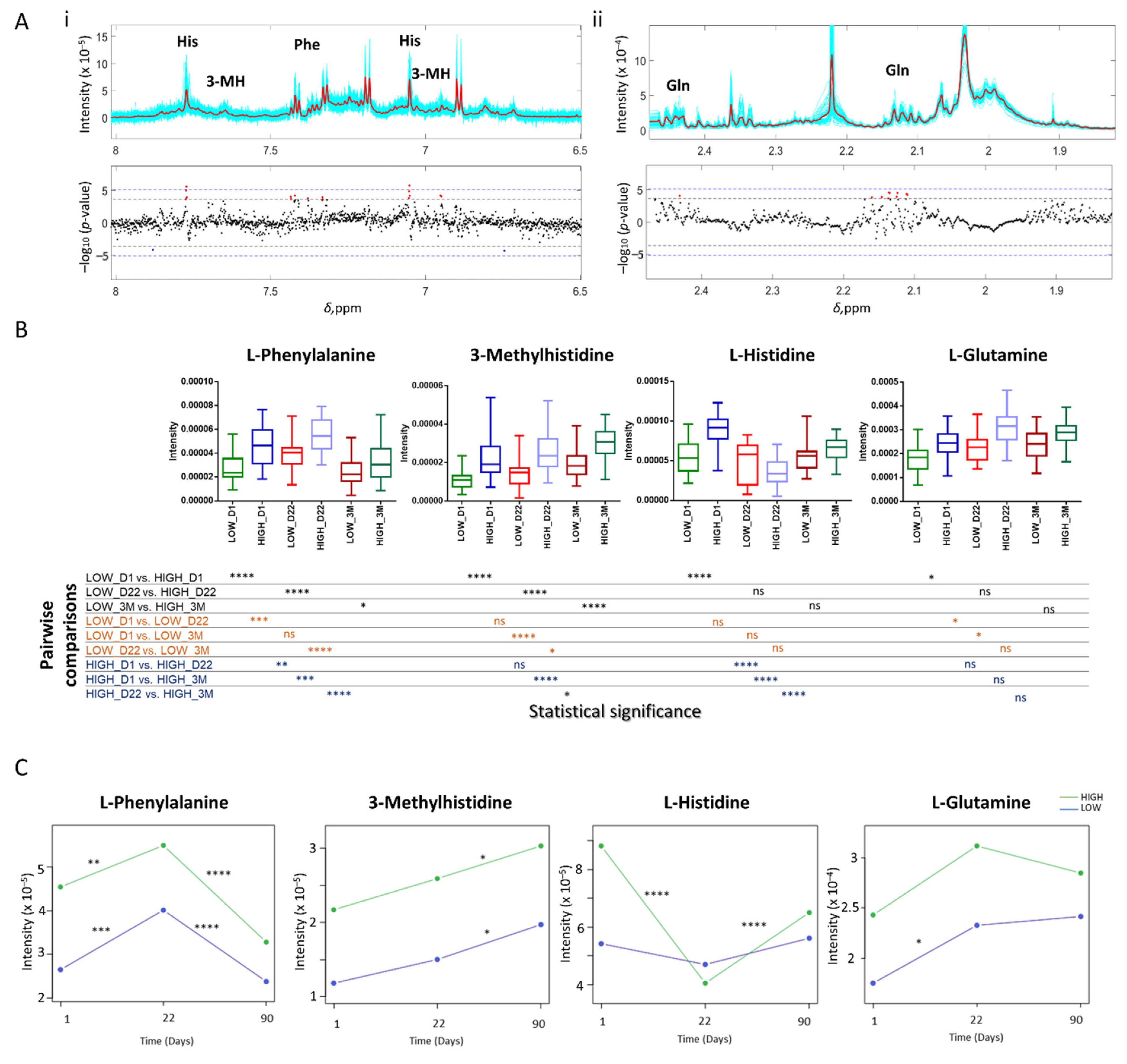
3.2.1. Metabolite Identification
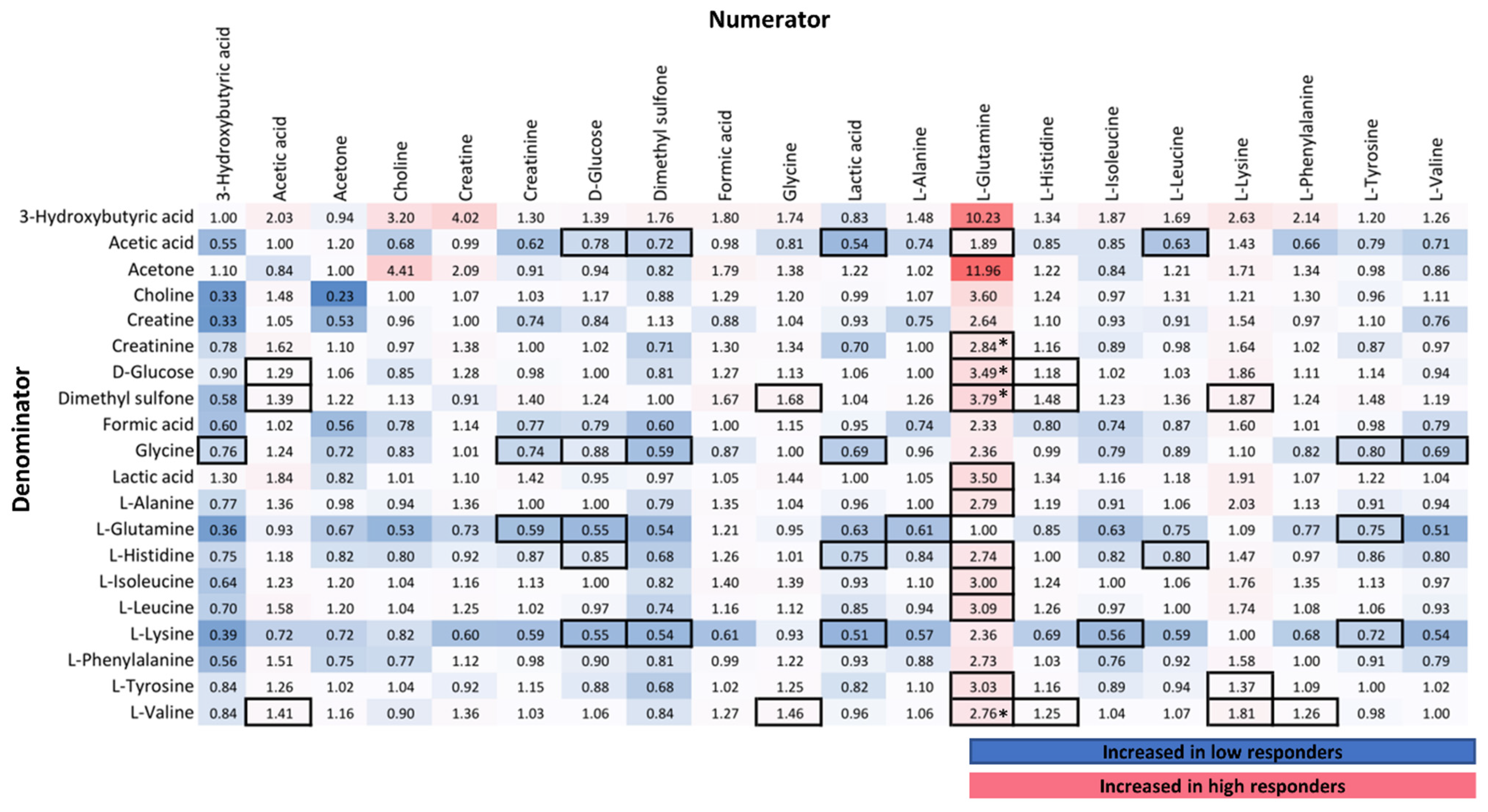
3.2.2. Correlation of NMR Metabolic Features with Immune Response
3.2.3. NMR Targeted Metabolite Quantification
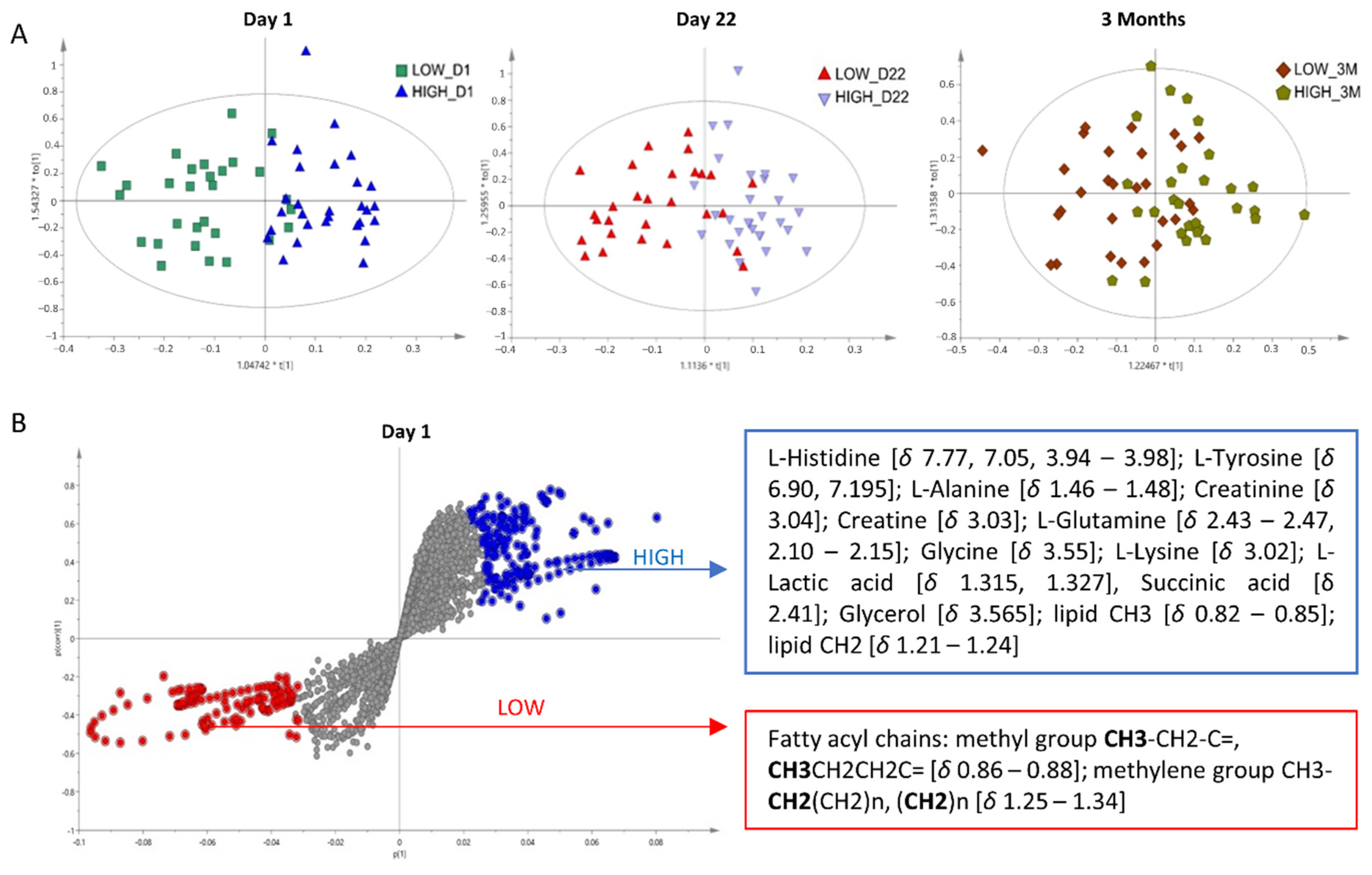
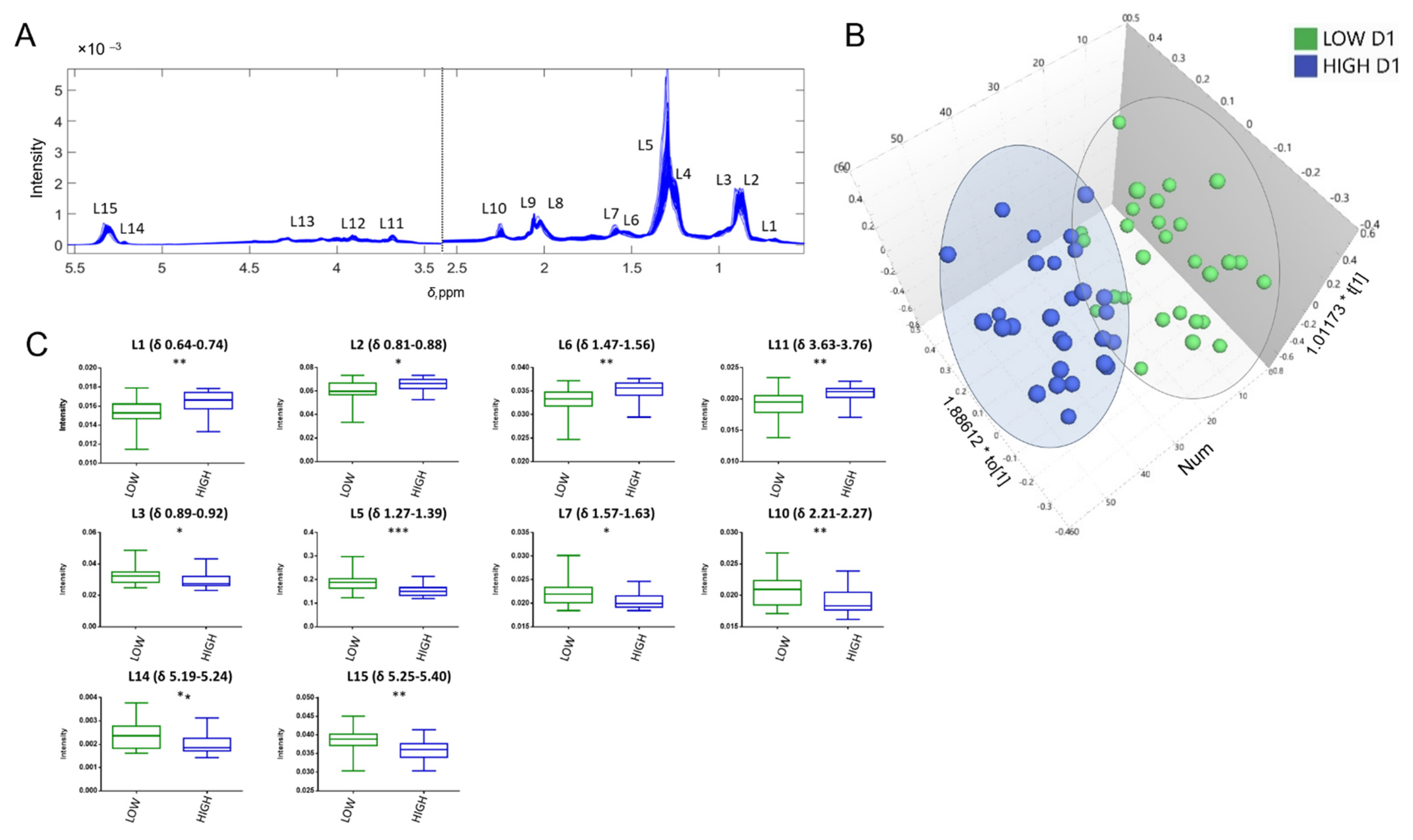
3.2.4. Multivariate Analysis
3.3. LC–MS Results
3.3.1. Multivariate Analysis
3.3.2. Annotation of the Selected Variables
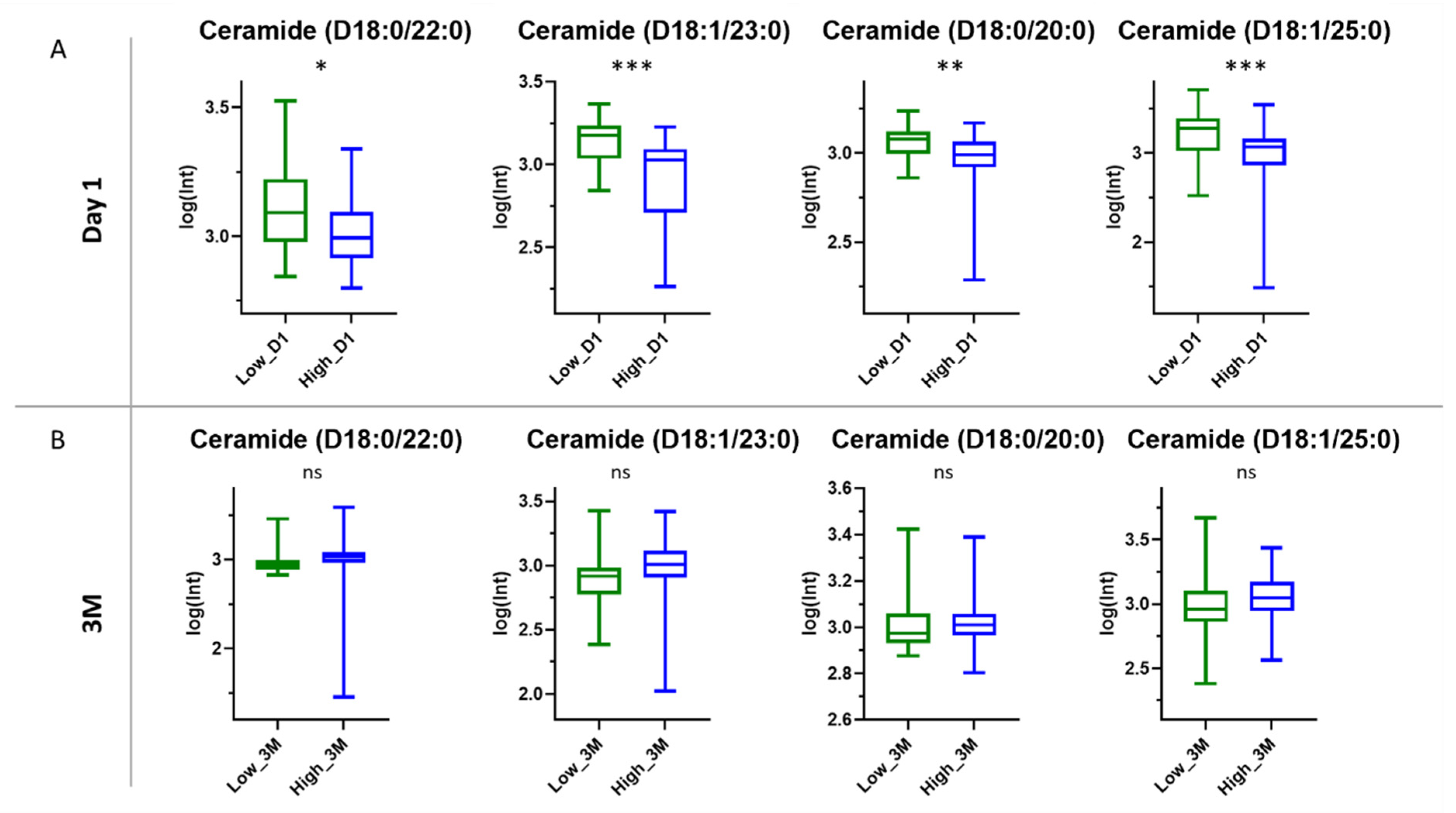
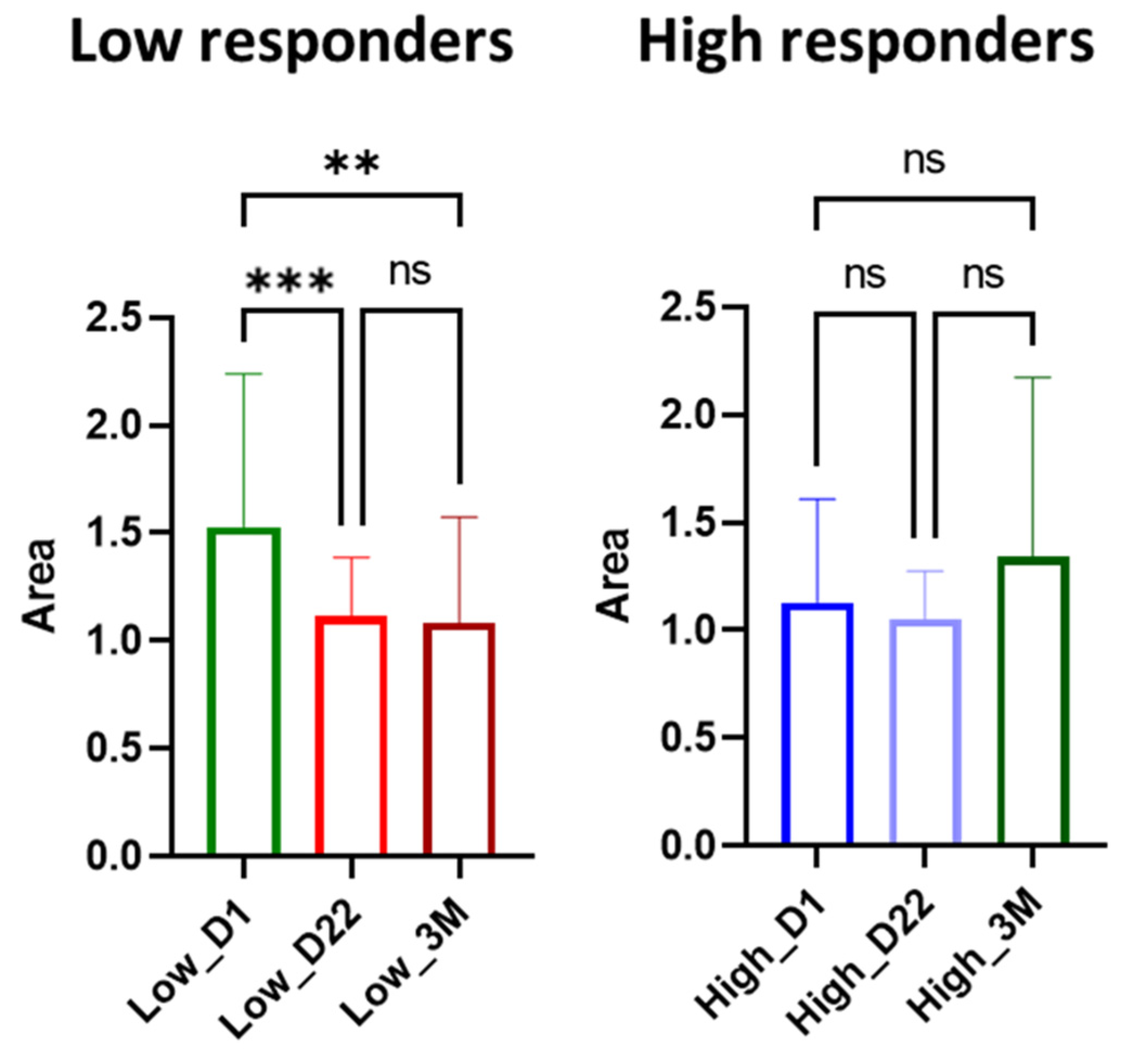
3.3.3. Ceramides
4. Discussion
4.1. Amino Acid Metabolism in Immune Response
4.2. Lipoproteins and Lipids
4.3. Role of Ceramides in SARS-CoV-2 Antibody Production
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological mechanism and beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Karalis, V.; Ntanasis-Stathopoulos, I.; Gumeni, S.; Apostolakou, F.; Sklirou, A.D.; Gavriatopoulou, M.; Skourti, S.; Kastritis, E.; et al. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2; A Prospective Study in 283 Health Workers. Cells 2021, 10, 1942. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.D.; Liacos, C.I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.H.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddington, K.E.; Papadaki, A.; Coelewij, L.; Adriani, M.; Nytrova, P.; Kubala Havrdova, E.; Fogdell-Hahn, A.; Farrell, R.; Dönnes, P.; Pineda-Torra, I.; et al. Using Serum Metabolomics to Predict Development of Anti-drug Antibodies in Multiple Sclerosis Patients Treated With IFNβ. Front. Immunol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Conti, M.G.; Petrova, B.; Kanarek, N.; Angelidou, A.; Levy, O. Integrative metabolomics to identify molecular signatures of responses to vaccines and infections. Metabolites 2020, 10, 492. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Sarandi, E.; Georgaki, S.; Frima, E.S.; Varvarigou, A.; Tsoukalas, D. Application of metabolomics in pediatric asthma: Prediction, diagnosis and personalized treatment. Metabolites 2021, 11, 251. [Google Scholar] [CrossRef]
- Amberg, A.; Riefke, B.; Schlotterbeck, G.; Ross, A.; Senn, H.; Dieterle, F.; Keck, M. NMR and MS Methods for Metabolomics. Methods Mol. Biol. 2017, 1641, 229–258. [Google Scholar] [CrossRef]
- Emwas, A.H.M. The Strengths and Weaknesses of NMR Spectroscopy and Mass Spectrometry with Particular Focus on Metabolomics Research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Fanos, V. Could metabolomics drive the fate of COVID-19 pandemic? A narrative review on lights and shadows. Clin. Chem. Lab. Med. 2021, 59, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Lawler, N.G.; Gray, N.; Kimhofer, T.; Boughton, B.; Gay, M.; Yang, R.; Morillon, A.C.; Chin, S.T.; Ryan, M.; Begum, S.; et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARS-CoV-2 Infection and Inflammatory Cytokine Responses. J. Proteome Res. 2021, 20, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Don Wai Luu, L.; Chen, S.; Jin, F.; Wang, S.; Huang, X.; Wang, L.; Zhou, X.; Chen, X.; et al. Proteomic and metabolomic signatures associated with the immune response in 2 healthy individuals immunized with an inactivated SARS-CoV-2 vaccine 3 4. medRxiv 2021, 13, 242. [Google Scholar] [CrossRef]
- Takis, P.G.; Takis, P.G.; Jiménez, B.; Jiménez, B.; Sands, C.J.; Sands, C.J.; Chekmeneva, E.; Chekmeneva, E.; Lewis, M.R.; Lewis, M.R. SMolESY: An efficient and quantitative alternative to on-instrument macromolecular 1 H-NMR signal suppression. Chem. Sci. 2020, 11, 6000–6011. [Google Scholar] [CrossRef]
- Fages, A.; Duarte-Salles, T.; Stepien, M.; Ferrari, P.; Fedirko, V.; Pontoizeau, C.; Trichopoulou, A.; Aleksandrova, K.; Tjønneland, A.; Olsen, A.; et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 2015, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Kimhofer, T.; Lodge, S.; Whiley, L.; Gray, N.; Loo, R.L.; Lawler, N.G.; Nitschke, P.; Bong, S.H.; Morrison, D.L.; Begum, S.; et al. Integrative Modeling of Quantitative Plasma Lipoprotein, Metabolic, and Amino Acid Data Reveals a Multiorgan Pathological Signature of SARS-CoV-2 Infection. J. Proteome Res. 2020, 19, 4442–4454. [Google Scholar] [CrossRef]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated with the Host Response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shu, T.; Yang, X.; Song, J.X.; Zhang, M.; Yao, C.; Liu, W.; Huang, M.; Yu, Y.; Yang, Q.; et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [Google Scholar] [CrossRef]
- Caterino, M.; Gelzo, M.; Sol, S.; Fedele, R.; Annunziata, A.; Calabrese, C.; Fiorentino, G.; D’Abbraccio, M.; Dell’Isola, C.; Fusco, F.M.; et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci. Rep. 2021, 11, 2941. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, W.S.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomé, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Nutr. Diabetes 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Boldogh, I.; Popov, V.L.; Saini, S.S.; Chopra, A.K. Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Lab. Investig. 1998, 78, 523–534. [Google Scholar] [PubMed]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demopoulos, C.; Antonopoulou, S.; Theoharides, T.C. COVID-19, microthromboses, inflammation, and platelet activating factor. Biofactors 2020, 46, 927–933. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völlmy, F.; van den Toorn, H.; Chiozzi, R.Z.; Zucchetti, O.; Papi, A.; Volta, C.A.; Marracino, L.; Sega, F.V.D.; Fortini, F.; Demichev, V.; et al. A serum proteome signature to predict mortality in severe COVID-19 patients. Life Sci. Alliance 2021, 4, e202101099. [Google Scholar] [CrossRef]
- Depelchin, A.; Letesson, J.J. Adrenaline influence on the immune response. I. Accelerating or suppressor effects according to the time of application. Immunol. Lett. 1981, 3, 199–205. [Google Scholar] [CrossRef]
- NW, K.; VM, S. It takes nerve to tell T and B cells what to do. J. Leukoc. Biol. 2006, 79, 1093–1104. [Google Scholar] [CrossRef]
- Shi, D.; Yan, R.; Lv, L.; Jiang, H.; Lu, Y.; Sheng, J.; Xie, J.; Wu, W.; Xia, J.; Xu, K.; et al. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism 2021, 118, 154739. [Google Scholar] [CrossRef] [PubMed]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O.; et al. Amino Acid Metabolism is Significantly Altered at the Time of Admission in Hospital for Severe COVID-19 Patients: Findings from Longitudinal Targeted Metabolomics Analysis. Microbiol. Spectr. 2021, 9, e0033821. [Google Scholar] [CrossRef] [PubMed]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, T.C.; Cadirci, E.; Bayrak, B.; Celik, S.; Atila, N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The serum amino acid profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J. Biol. Chem. 2015, 6, 281. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Borku Uysal, B.; Ikitimur, H.; Ozcan, E.; Islamoğlu, M.S.; Aktepe, E.; Yavuzer, H.; Yavuzer, S. Effect of oral l-Glutamine supplementation on COVID-19 treatment. Clin. Nutr. Exp. 2020, 33, 24–31. [Google Scholar] [CrossRef]
- Mohajeri, M.; Horriatkhah, E.; Mohajery, R. Correction to: The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: A case-control study. Inflammopharmacology 2021, 30, 353. [Google Scholar] [CrossRef]
- Matsuyama, T.; Yoshinaga, S.K.; Shibue, K.; Mak, T.W. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 2021, 28, 3199–3213. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.O.; Şenol, O.; Bolat, S.; Yıldız, Ş.N.; Büyüktuna, S.A.; Sarıismailoğlu, R.; Doğan, K.; Hasbek, M.; Hekim, S.N. Understanding the pathophysiological changes via untargeted metabolomics in COVID-19 patients. J. Med. Virol. 2021, 93, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Gorjao, R.; Levada-Pires, A.C.; Masi, L.N.; Hatanaka, E.; Cury-Boaventura, M.F.; da Silva, E.B.; dos Santos-Oliveira, L.C.; Sousa Diniz, V.L.; Duarte Serdan, T.A.; et al. Host cell glutamine metabolism as a potential antiviral target. Clin. Sci. 2021, 135, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Yang, E.J.; Yoo, K.H.; Choi, I.H. Macrophage Differentiation from Monocytes Is Influenced by the Lipid Oxidation Degree of Low Density Lipoprotein. Mediators Inflamm. 2015, 2015, 235797. [Google Scholar] [CrossRef] [Green Version]
- Hubler, M.J.; Kennedy, A.J. Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem. 2016, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Toms, T.E.; Panoulas, V.F.; Kitas, G.D. Dyslipidaemia in rheumatological autoimmune diseases. Open Cardiovasc. Med. J. 2011, 5, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sappati Biyyani, R.S.R.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta. 2020, 510, 105–110. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lu, R.; Dai, M.; Shen, M.; Zhang, J.; Cui, Y.; Liu, B.; Lin, F.; Chen, L.; et al. Lipid metabolism changes in patients with severe COVID-19. Clin. Chim. Acta. 2021, 517, 66–73. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Ye, G.; Cao, X.; Xu, X.; Tan, W.; Zhang, Y. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism 2020, 107, 154243. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jericó, C.; Guerrero, C.; Miret, M.L.; Näf, S.; Pardo, A.; et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.W.; Jumper, C.C.; Ackerman, P.J.; Bracha, D.; Donlic, A.; Kim, H.; Kenney, D.; Castello-Serrano, I.; Suzuki, S.; Tamura, T.; et al. SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation. eLife 2021, 10, e65962. [Google Scholar] [CrossRef]
- Radenkovic, D.; Chawla, S.; Pirro, M.; Sahebkar, A.; Banach, M. Cholesterol in Relation to COVID-19: Should We Care about It? J. Clin. Med. 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Albeituni, S.; Stiban, J.; Albeituni, S.; Stiban, J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv. Exp. Med. Biol. 2019, 1161, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Malisan, F.; Tomassini, B.; Testi, R. Ceramide catabolism critically controls survival of human dendritic cells. J. Leukoc. Biol. 2006, 79, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J. Antigen-Presenting Cells: Professionals and amateurs. Curr. Biol. 1995, 5, 1095–1097. [Google Scholar] [CrossRef] [Green Version]
- Eiz-Vesper, B.; Schmetzer, H.M. Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfus. Med. Hemother. 2020, 47, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; Zanotta, S.; Capitelli, L.; Bocchino, M. A bird’s eye view on the role of dendritic cells in SARS-CoV-2 infection: Perspectives for immune-based vaccines. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 195–197. [Google Scholar] [CrossRef]
- Hivroz, C.; Chemin, K.; Tourret, M.; Bohineust, A. Crosstalk between T Lymphocytes and Dendritic Cells. Crit. Rev. Immunol. 2012, 32, 139–155. [Google Scholar] [CrossRef]
- Sallusto, F.; Nicolò, C.; De Maria, R.; Corinti, S.; Testi, R. Ceramide Inhibits Antigen Uptake and Presentation by Dendritic Cells. J. Exp. Med 1996, 184, 2411–2416. [Google Scholar] [CrossRef]

| High Responders | Low Responders | |
|---|---|---|
| No. Observations | 29 | 29 |
| Gender | ||
| Female | 23 | 17 |
| Male | 6 | 12 |
| Age | ||
| Minimum | 27 | 25 |
| Maximum | 64 | 68 |
| 1st Quartile | 33 | 51 |
| Median | 45 | 58 |
| 3rd Quartile | 54 | 64 |
| Mean | 43.79 | 54.86 |
| Standard deviation | 11.99 | 12.15 |
| NAbs titers, Day 1 | ||
| Minimum | 0 | 0 |
| Maximum | 43.753 | 25.64 |
| 1st Quartile | 5.363 | 4.142 |
| Median | 14.607 | 9.076 |
| 3rd Quartile | 23.955 | 12.762 |
| Mean | 14.888 | 9.55 |
| Standard deviation | 11.474 | 6.916 |
| NAbs titers, Day 22 | ||
| Minimum | 72.027 | 6.971 |
| Maximum | 98.23 | 38.146 |
| 1st Quartile | 76.894 | 22.859 |
| Median | 81.742 | 30.383 |
| 3rd Quartile | 86.179 | 34.703 |
| Mean | 82.9 | 27.531 |
| Standard deviation | 7.412 | 8.663 |
| NAbs titers, 3 Months | ||
| Minimum | 78.478 | 41.33 |
| Maximum | 98.036 | 96.997 |
| 1st Quartile | 93.266 | 78.567 |
| Median | 96.682 | 84.074 |
| 3rd Quartile | 97.328 | 91.331 |
| Mean | 94.349 | 82.04 |
| Standard deviation | 4.9 | 13.909 |
| Metabolite | Peak ppm Range | Index | ppm with Smallest p-Value | p-Value | Partial Correlation r |
|---|---|---|---|---|---|
| l-Histidine | [7.739–7.812] | 3 | 7.772 | 2.49 × 10−6 | 0.5824 |
| l-Histidine | [7.041–7.069] | 3 | 7.052 | 1.71 × 10−6 | 0.5899 |
| l-Phenylalanine | [7.400–7.446] | 5 | 7.422 | 6.03 × 10−5 | 0.5096 |
| l-Phenylalanine | [7.306–7.345] | 5 | 7.333 | 1.12 × 10−4 | 0.4933 |
| l-Phenylalanine | [7.359–7.387] | 5 | 7.38 | 1.56 ×1 0−4 | 0.4842 |
| l-Histidine; l-Phenylalanine | Overlapping area | 3/5 | 3.955 | 1.67 × 10−5 | 0.5410 |
| 3-Methylhistidine | [7.649–7.677] | 4 | 7.653 | 4.40 × 10−4 | 0.4541 |
| 3-Methylhistidine | [6.944–6.996] | 4 | 6.951 | 5.84 × 10−5 | 0.5105 |
| l-Glutamine | [2.092–2.153] * | 14 | 2.136 | 2.66 × 10−5 | 0.5300 |
| l-Glutamine | [2.428–2.470] | 14 | 2.431 | 8.60 × 10−5 | 0.5004 |
| m/z | tR (min) | Theoretical m/z | Δm (ppm) | Name | Molecular Formula | Exact Mass | Trend a | Dataset | Adduct |
|---|---|---|---|---|---|---|---|---|---|
| 622.6114 | 18.92 | 622.6133 | −3.05 | Cer(D18:0/22:0) | C40H81NO3 | 623.6211 | ↑ | ESI (−) | - |
| 646.6098 | 18.81 | 646.6109 | −1.70 | ESI (+) | [M+Na] | ||||
| 634.6120 | 18.99 | 634.6133 | −2.05 | Cer(D18:1/23:0) | C41H81NO3 | 635.6211 | ↑ | ESI (−) | - |
| 658.6120 | 18.96 | 658.6109 | 1.67 | ESI (+) | [M+Na] | ||||
| 594.5802 | 18.28 | 594.5820 | −3.02 | Cer(D18:0/20:0) | C28H77NO3 | 595.5898 | ↑ | ESI (−) | - |
| 684.6241 | 19.39 | 684.6265 | −3.49 | Cer(D18:1/25:0) | C43H85NO3 | 663.6524 | ↑ | ESI (−) | [M+Na-2H] |
| 436.2816 | 15.30 | 436.2823 | −1.60 | LysoPE(P-16:0/0:0) | C21H44NO6P | 437.2901 | ↑ | ESI (−) | - |
| 552.3055 | 14.65 | 552.3085 | −5.33 | LysoPE(0:0/24:6) or LysoPE(24:6/0:0) | C29H48NO7P | 553.3163 | ↑ | ESI (−) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagla, I.; Iliou, A.; Benaki, D.; Gikas, E.; Mikros, E.; Bagratuni, T.; Kastritis, E.; Dimopoulos, M.A.; Terpos, E.; Tsarbopoulos, A. Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response. Cells 2022, 11, 1241. https://doi.org/10.3390/cells11071241
Dagla I, Iliou A, Benaki D, Gikas E, Mikros E, Bagratuni T, Kastritis E, Dimopoulos MA, Terpos E, Tsarbopoulos A. Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response. Cells. 2022; 11(7):1241. https://doi.org/10.3390/cells11071241
Chicago/Turabian StyleDagla, Ioanna, Aikaterini Iliou, Dimitra Benaki, Evagelos Gikas, Emmanuel Mikros, Tina Bagratuni, Efstathios Kastritis, Meletios A. Dimopoulos, Evangelos Terpos, and Anthony Tsarbopoulos. 2022. "Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response" Cells 11, no. 7: 1241. https://doi.org/10.3390/cells11071241
APA StyleDagla, I., Iliou, A., Benaki, D., Gikas, E., Mikros, E., Bagratuni, T., Kastritis, E., Dimopoulos, M. A., Terpos, E., & Tsarbopoulos, A. (2022). Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response. Cells, 11(7), 1241. https://doi.org/10.3390/cells11071241











