The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk of the Peripheral Nervous System
Abstract
:1. Introduction
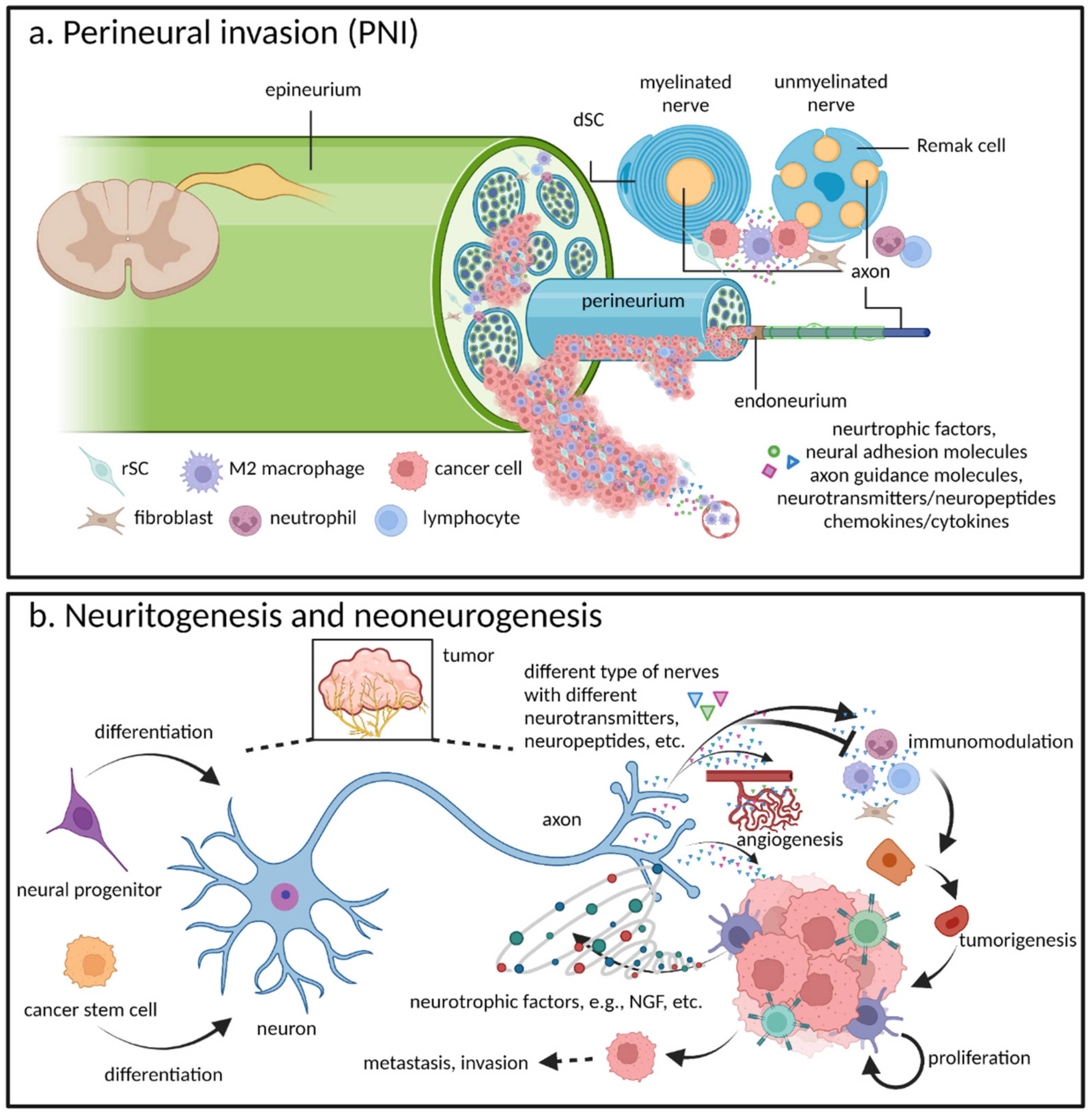
- Neuritogenesis, axonogenesis, and innervation all describe the process by which newly formed axons (or neurites) actively project themselves onto cancer cells and thereby support cancer progression via multiple neurotransmitters or neuropeptides [9,15,16]. Neuritogenesis is the initial and foremost event involved in neuronal morphogenesis before transforming into both axonogenesis and dendritogenesis. Nevertheless, in all of the literature we reviewed on cancer–nerve crosstalk, as well as that of nerve injury and regeneration, it appears as if the term neuritogenesis is frequently being used to convey a broader concept. In other words, whether in vitro or in vivo, when inducing the neurite outgrowth of neurons or DRGs, researchers may actually be observing dendritogenesis, axonogenesis, or the narrower definition of neuritogenesis, but will simply use the latter term as a catchall. For the purposes of this article, we shall do the same. With this understood, as regards the more narrow-focused definition of neuritogenesis, it is important to understand that dendritogenesis is referring to the outgrowth of dendrites and which, to date, no connection to cancer–nerve crosstalk has been found. Axonogenesis emphasizes the outgrowth of axons [17], which is more specifically descriptive of the actual process of in vivo cancer–nerve crosstalk (as well as that of nerve injury and regeneration) that results from newly formed neural twigs (axons) sprouting toward target cells. The concept of innervation focuses more on nerve-derived functions, where their influence on target (innervated) cells depends on different types of nerves and target cells, as well as the several neurotransmitters or neuropeptides that are released [9,10,16]. As such, when necessary, to emphasize neural functionality, we will use the term innervation.
- Neurogenesis or neoneurogenesis sometimes convey broad meanings, which refer not only to the new formation of neurons, but also to neuritogenesis or axonogenesis. Accordingly, the exact meaning of these concepts is context-dependent [10,18,19]. Nevertheless, in cancer–nerve crosstalk literature, neoneurogenesis best describes the increasing number of neurons in the TME from neural progenitors [18] and is the term we shall use in this review.
2. Cancer–Nerve Crosstalk and Its Relationships with Neural Physiology, Inflammation, Injury, and Regeneration
2.1. Perineural Invasion: A Critical Pathway for Tumor Spread
2.2. Neuritogenesis and Neoneurogenesis: Additional Components of Cancer–Nerve Crosstalk Pathogenesis
3. The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk in the Peripheral Nervous System
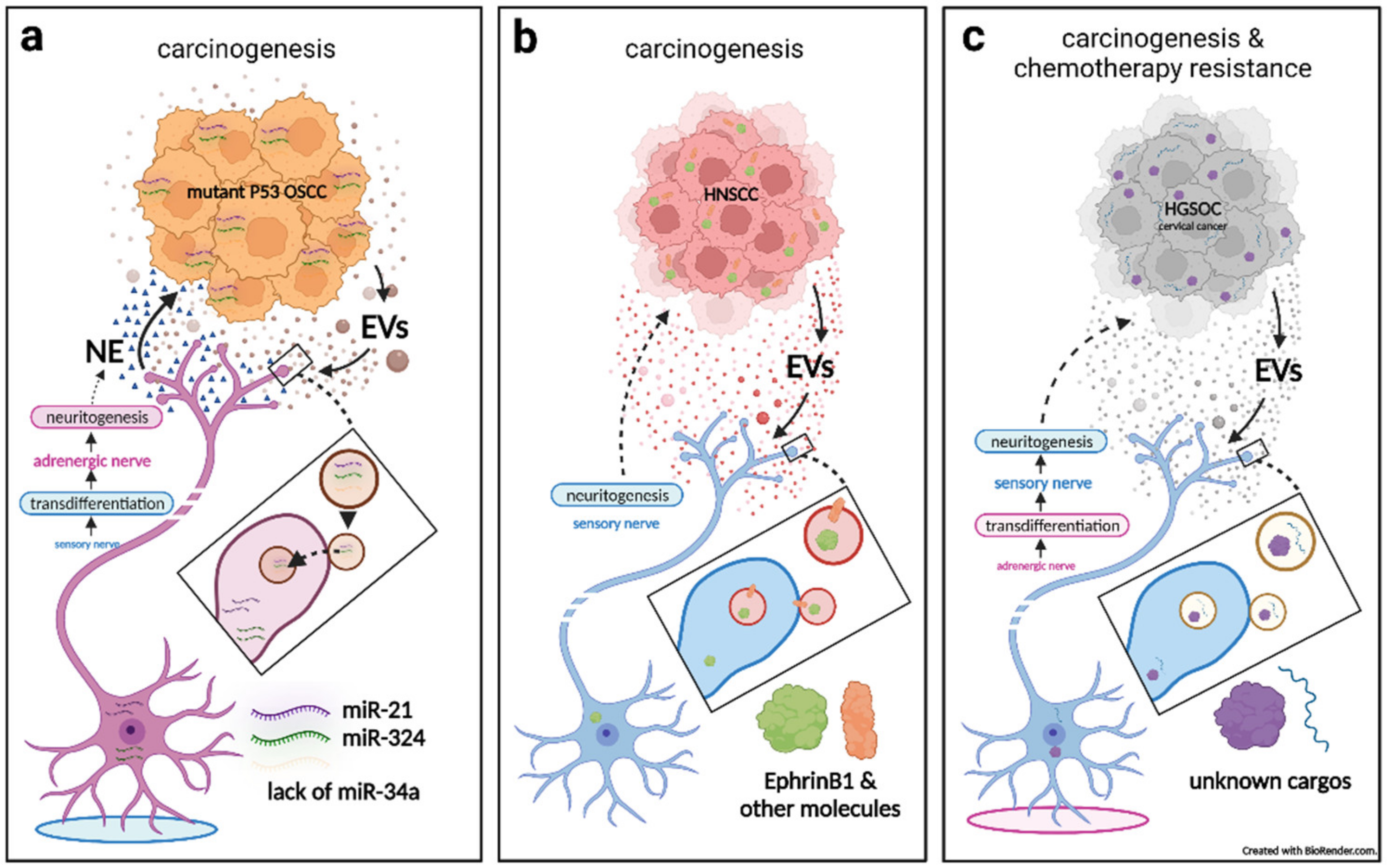
3.1. Cancerous Extracellular Vesicle-Delivered miRNAs Induce Reprogramming and Neuritogenesis of Nerves, Which in Turn Facilitates Tumorigenesis
3.2. Cancerous Extracellular Vesicle-Delivered Axon Guidance Proteins Promote Neuritogenesis of Sensory Nerves, Which in Turn Facilitates Tumorigenesis
3.3. Sensory Neuritogenesis Promotion by Cancer Extracellular Vesicles Facilitates Tumorigenesis, Tumor-Associated Pain, and Chemotherapy Resistance
3.4. Artificial Nanovesicles Containing Peripheral Nerve Blockers Can Inhibit Cancer Progression by Targeting Cancer–Nerve Crosstalk
4. The Role of Extracellular Vesicles in Peripheral Nerve Physiology, Inflammation, Injury, and Regeneration, and How They May Be Hijacked by Cancer–Nerve Crosstalk
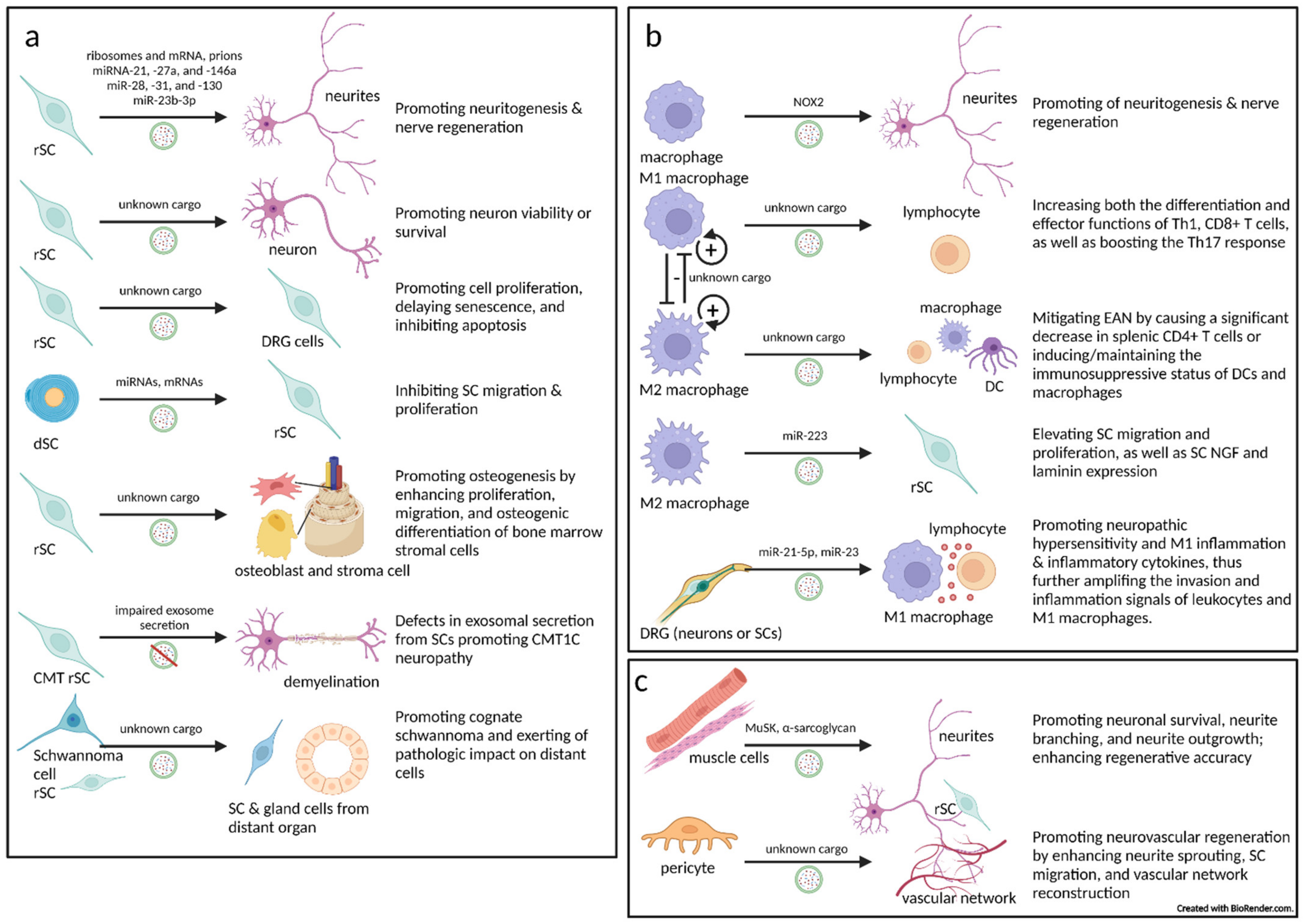
4.1. The Critical Role Played by Schwann Cell-Derived Extracellular Vesicles in the Peripheral Nervous System
4.1.1. Schwann Cells Transfer Extracellular Vesicles to Axons
4.1.2. Extracellular Vesicles from Schwann Cells to Neurons
4.1.3. Extracellular Vesicles from Schwann Cells to Schwann Cells
4.1.4. Extracellular Vesicles from Schwann Cells to Innervated (Target) Tissue
4.1.5. Inherited Peripheral Demyelinating Disease Can Be Largely Contributed to by Extracellular Vesicle Secretion Defects in Schwann Cells
4.1.6. Schwannoma or Schwann Cell-Derived Extracellular Vesicles May Promote Cognate Schwannoma and Exert Pathologic Impact on Distant Cells
4.2. Macrophage-Associated Extracellular Vesicles in the Peripheral Nervous System
4.2.1. Extracellular Vesicles from Macrophages to Nerves (Axons/Neurons, and Peri/Intraneural Immunocytes)
4.2.2. Extracellular Vesicles from Macrophages to Schwann Cells
4.2.3. Extracellular Vesicles from Dorsal Root Ganglia Cells (Neurons or Schwann Cells) to Macrophages
4.3. Extracellular Vesicles from Innervated (Target) Tissues to Nerves (Neurons, Axons, or Schwann Cells)
4.3.1. Extracellular Vesicles from Muscles to Motor Neurons
4.3.2. Extracellular Vesicles from Pericytes to Nerves
5. Conclusions
- Define the pathogenesis of cancer–nerve crosstalk (e.g., PNI, neoneurogenesis) in primary and metastatic tumor sites.
- Consider the distinctiveness of cancer–nerve crosstalk when mapping potential mechanisms to those of neural physiology and pathology.
- Unearth other molecular EV cargo types (e.g., lncRNAs, circRNAs, metabolites, etc.) since current research is mainly concentrating on miRNAs and proteins.
- Investigate if tumor cells which metastasize to organs other than those of the nervous system can also perform cancer–nerve interactions with nerves inside of these organs (particularly by the means of EVs).
- Examine the multiple intercellular communication components that exist within neural microenvironments. In peripheral tumors, this should center on SCs, macrophages, fibroblasts, endothelial cells, mast cells, and other immune or stroma cells in nerves or the perineural niche.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willms, E.; Cabanas, C.; Mager, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; le Bleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, P.D. Exosomal induction of tumor innervation. Cancer Res. 2019, 79, 3529–3535. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Demir, I.E.; Reyes, C.M.; Alrawashdeh, W.; Ceyhan, G.O.; Deborde, S.; Friess, H.; Gorgulu, K.; Istvanffy, R.; Jungwirth, D.; Kuner, R.; et al. Clinically actionable strategies for studying neural influences in cancer. Cancer Cell 2020, 38, 11–14. [Google Scholar] [CrossRef]
- Monje, M.; Borniger, J.C.; D’Silva, N.J.; Deneen, B.; Dirks, P.B.; Fattahi, F.; Frenette, P.S.; Garzia, L.; Gutmann, D.H.; Hanahan, D.; et al. Roadmap for the emerging field of cancer neuroscience. Cell 2020, 181, 219–222. [Google Scholar] [CrossRef]
- Demir, I.E.; Reyes, C.M.; Alrawashdeh, W.; Ceyhan, G.O.; Deborde, S.; Friess, H.; Gorgulu, K.; Istvanffy, R.; Jungwirth, D.; Kuner, R.; et al. Future directions in preclinical and translational cancer neuroscience research. Nat. Cancer 2021, 1, 1027–1031. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the tumor microenvironment: Origin and effects. Front. Cell Dev. Biol. 2020, 8, 601738. [Google Scholar] [CrossRef]
- Boilly, B.; Faulkner, S.; Jobling, P.; Hondermarck, H. Nerve dependence: From regeneration to cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural invasion in pancreatic cancer: The past, present and future. Cancers 2010, 2, 1513–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.; Monje, M. The neural regulation of cancer. Annu. Rev. Cancer Biol. 2020, 4, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Flynn, K.C. The cytoskeleton and neurite initiation. Bioarchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef] [Green Version]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Entschladen, F.; Palm, D.; Lang, K.; Drell, T.L.T.; Zaenker, K.S. Neoneurogenesis: Tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 2006, 67, 33–35. [Google Scholar] [CrossRef]
- Kovacs, A.; Vermeer, D.W.; Madeo, M.; Reavis, H.D.; Vermeer, S.J.; Williamson, C.S.; Rickel, A.; Stamp, J.; Lucido, C.T.; Cain, J.; et al. Tumor-infiltrating nerves create an electro-physiologically active microenvironment and contribute to treatment resistance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.N.; Tian, D.P.; Gong, Q.Y.; Huang, H.; Yang, P.; Chen, S.B.; Billan, S.; He, J.Y.; Huang, H.H.; Xiong, P.; et al. Perineural invasion is a better prognostic indicator than lymphovascular invasion and a potential adjuvant therapy indicator for pN0M0 esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2020, 27, 4371–4381. [Google Scholar] [CrossRef]
- Bowe, C.M.; Godhania, B.; Whittaker, M.; Walsh, S. Pleomorphic dermal sarcoma: A clinical and histological review of 49 cases. Br. J. Oral Maxillofac. Surg. 2021, 59, 460–465. [Google Scholar] [CrossRef]
- Chatzistefanou, I.; Lubek, J.; Markou, K.; Ord, R.A. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J. Craniomaxillofac. Surg. 2017, 45, 821–825. [Google Scholar] [CrossRef]
- Said-Al-Naief, N.; Sciandra, K.; Gnepp, D.R. Moderately differentiated neuroendocrine carcinoma (atypical carcinoid) of the parotid gland: Report of three cases with contemporary review of salivary neuroendocrine carcinomas. Head Neck Pathol. 2013, 7, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Santos-Briz, A.; Garcia-Gavin, J.; Pastushenko, I.; Sayagues, J.M.; Rodriguez-Peralto, J.L.; Requena, L. Perineural invasion as a clue to malignant behavior in a dermatofibroma. Am. J. Dermatopathol. 2020, 42, 533–538. [Google Scholar] [CrossRef]
- Porto, A.C.; Pinto Blumetti, T.; Oliveira Santos Filho, I.D.A.; Calsavara, V.F.; Duprat Neto, J.P.; Tavoloni Braga, J.C. Primary cutaneous melanoma of the scalp: Patterns of clinical, histological and epidemiological characteristics in Brazil. PLoS ONE 2020, 15, e0240864. [Google Scholar] [CrossRef]
- Yilmaz, A.; Duyar, S.S.; Cakir, E.; Aydin, E.; Demirag, F.; Karakaya, J.; Yazici, U.; Erdogan, Y. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2011, 40, 664–670. [Google Scholar] [CrossRef]
- Perez-Perez, M.; Umbria-Jimenez, S.; Mora-Cabeza, M.; Fernandez-Orland, A.; Rios-Martin, J.J. Basal cell carcinoma colonized by lentigo maligna melanoma, with perineural invasion by both neoplasms. J. Cutan. Pathol. 2020, 47, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Mesologites, T.; Homsi, S.; Ichim, T.E.; Alexandrescu, D.T. Chronic lymphocytic leukemia presenting with cholecystitis-like symptoms and gallbladder wall invasion. South Med. J. 2010, 103, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.W.; Chen, Y.L. Perineural invasion of sinonasal lymphoma: A rare cause of trigeminal neuropathy. Headache 2007, 47, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, X.; Shi, M.; Chen, Y.; Yu, D.; Zhao, C.; Gu, Y.; Yang, B.; Guo, S.; Ding, G.; et al. CD13(hi) Neutrophil-like myeloid-derived suppressor cells exert immune suppression through Arginase 1 expression in pancreatic ductal adenocarcinoma. Oncoimmunology 2017, 6, e1258504. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef] [Green Version]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Saricaoglu, O.C.; Pfitzinger, P.L.; Teller, S.; Wang, K.; Waldbaur, C.; Kurkowski, M.U.; Wormann, S.M.; et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 2016, 65, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The success and failure of the Schwann cell response to nerve injury. Front Cell Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammat. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kang, R.; Tang, D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun. 2021, 41, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Mills, S. Histology for Pathologists; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Deborde, S.; Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell Mol. Life Sci 2017, 74, 4405–4420. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Leider-Trejo, L.; Binenbaum, Y.; Kulish, N.; Fridman, E.; Shabtai-Orbach, A.; Wong, R.J.; Gil, Z. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene 2017, 36, 3232–3239. [Google Scholar] [CrossRef]
- Gil, Z.; Cavel, O.; Kelly, K.; Brader, P.; Rein, A.; Gao, S.P.; Carlson, D.L.; Shah, J.P.; Fong, Y.; Wong, R.J. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J. Natl. Cancer Inst. 2010, 102, 107–118. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Fridman, E.; Vladovski, E.; Wasserman, T.; Milman, N.; Gil, Z. RET, a targetable driver of pancreatic adenocarcinoma. Int. J. Cancer 2019, 144, 3014–3022. [Google Scholar] [CrossRef]
- Bakst, R.L.; Xiong, H.; Chen, C.H.; Deborde, S.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.; Lee, S.Y.; Olson, O.C.; et al. Inflammatory monocytes promote perineural invasion via CCL2-mediated recruitment and cathepsin B expression. Cancer Res. 2017, 77, 6400–6414. [Google Scholar] [CrossRef] [Green Version]
- Cavel, O.; Shomron, O.; Shabtay, A.; Vital, J.; Trejo-Leider, L.; Weizman, N.; Krelin, Y.; Fong, Y.; Wong, R.J.; Amit, M.; et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012, 72, 5733–5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Chen, C.H.; Chernichenko, N.; He, S.; Bakst, R.L.; Barajas, F.; Deborde, S.; Allen, P.J.; Vakiani, E.; Yu, Z.; et al. GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E2008–E2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; He, S.; Chen, C.H.; Deborde, S.; Bakst, R.L.; Chernichenko, N.; McNamara, W.F.; Lee, S.Y.; Barajas, F.; Yu, Z.; et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol. Cancer Res. 2015, 13, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na’ara, S.; Amit, M.; Gil, Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene 2019, 38, 596–608. [Google Scholar] [CrossRef]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Landers, M.; Altenburger, P. Peripheral nerve injury. Adv. Physiother. 2003, 5, 67–82. [Google Scholar] [CrossRef]
- Demir, I.E.; Schorn, S.; Schremmer-Danninger, E.; Wang, K.; Kehl, T.; Giese, N.A.; Algul, H.; Friess, H.; Ceyhan, G.O. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS ONE 2013, 8, e60529. [Google Scholar] [CrossRef]
- Kulkarni, S.; Micci, M.A.; Leser, J.; Shin, C.; Tang, S.C.; Fu, Y.Y.; Liu, L.; Li, Q.; Saha, M.; Li, C.; et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E3709–E3718. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Schafer, K.H. Enteric neurogenesis during life span under physiological and pathophysiological conditions. Anat. Rec. 2019, 302, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct. Target Ther. 2017, 2, 16036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galvan, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; de Berry, J.J.; Bielefeldt, K.; Rhim, A.D.; de Pinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef] [Green Version]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 2018, 33, 75–90.e77. [Google Scholar] [CrossRef] [Green Version]
- Zahalka, A.H.; Arnal-Estape, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agur, A.M.R.; Moore, K.L.; Dalley, A.F. Moore’s Essential Clinical Anatomy; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Chu, C.; Artis, D.; Chiu, I.M. Neuro-immune Interactions in the Tissues. Immunity 2020, 52, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.; Rolls, A. Editorial overview: Brain, gut and immune system interactions. Curr. Opin. Neurobiol. 2020, 62, 3–5. [Google Scholar] [CrossRef]
- Yang, M.W.; Tao, L.Y.; Jiang, Y.S.; Yang, J.Y.; Huo, Y.M.; Liu, D.J.; Li, J.; Fu, X.L.; He, R.; Lin, C.; et al. Perineural invasion reprograms the immune microenvironment through cholinergic signaling in pancreatic ductal adenocarcinoma. Cancer Res. 2020, 80, 1991–2003. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 2018, 9, 2723. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, E.M.; Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 2009, 10, 713–723. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; de Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Awadasseid, A.; Wu, Y.; Zhang, W. Extracellular vesicles (exosomes) as immunosuppressive mediating variables in tumor and chronic inflammatory microenvironments. Cells 2021, 10, 2533. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Whiteside, T.L. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin. Ther. Targets 2018, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Court, F.A. Transfer of vesicles from Schwann cells to axons: A novel mechanism of communication in the peripheral nervous system. Front. Physiol. 2012, 3, 205. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Moisoiu, V.; Manaila, R.; Pardini, B.; Knutsen, E.; Anfossi, S.; Amit, M.; Calin, G.A. A Holistic perspective: Exosomes Shuttle between nerves and immune cells in the tumor microenvironment. J. Clin. Med. 2020, 9, 3529. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor innervation: Cancer has some nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef]
- Hunt, P.J.; Amit, M. Head and neck cancer exosomes drive microRNA-mediated reprogramming of local neurons. Extracell. Vesicles Circ. Nucl. Acids 2020, 1, 57–62. [Google Scholar] [CrossRef]
- Kim, Y.J.; Munsell, M.F.; Park, J.C.; Meyer, L.A.; Sun, C.C.; Brown, A.J.; Bodurka, D.C.; Williams, J.L.; Chase, D.M.; Bruera, E.; et al. Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol. Oncol. 2015, 139, 553–558. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Thomas, A.; Kannan, S.; Deodhar, K.; Shrivastava, S.K.; Mahantshetty, U.; Mulherkar, R. Human papillomavirus (HPV) genome status & cervical cancer outcome—A retrospective study. Indian J. Med. Res. 2015, 142, 525–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koneva, L.A.; Zhang, Y.; Virani, S.; Hall, P.B.; McHugh, J.B.; Chepeha, D.B.; Wolf, G.T.; Carey, T.E.; Rozek, L.S.; Sartor, M.A. HPV Integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol. Cancer Res. 2018, 16, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef] [Green Version]
- Honegger, A.; Leitz, J.; Bulkescher, J.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int. J. Cancer 2013, 133, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sultmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Kaduri, M.; Sela, M.; Kagan, S.; Poley, M.; Abumanhal-Masarweh, H.; Mora-Raimundo, P.; Ouro, A.; Dahan, N.; Hershkovitz, D.; Shklover, J.; et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci. Adv. 2021, 7, eabj5435. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Health Mater. 2021, 11, e2100639. [Google Scholar] [CrossRef]
- Kim, M.W.; Kwon, S.H.; Choi, J.H.; Lee, A. A promising biocompatible platform: Lipid-based and bio-inspired smart drug delivery systems for cancer therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef] [Green Version]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and their MicroRNA cargo: New players in peripheral nerve regeneration. Neurorehabil. Neural Repair 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Leal, R.; Alvarez, J.; Court, F.A. Origin of axonal proteins: Is the axon-schwann cell unit a functional syncytium? Cytoskeleton 2016, 73, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, T.E.; Tytell, M. Transfer of molecules from glia to axon in the squid may be mediated by glial vesicles. J. Neurobiol. 1992, 23, 217–230. [Google Scholar] [CrossRef]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Court, F.A.; Midha, R.; Cisterna, B.A.; Grochmal, J.; Shakhbazau, A.; Hendriks, W.T.; van Minnen, J. Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia 2011, 59, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Court, F.A.; Hendriks, W.T.; Mac Gillavry, H.D.; Alvarez, J.; van Minnen, J. Schwann cell to axon transfer of ribosomes: Toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 2008, 28, 11024–11029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Leal, R.; Diaz-Viraque, F.; Catalan, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef]
- Strickland, I.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.F. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Fan, B.; Ding, H.; Liu, Y.; Tang, H.; Pan, D.; Shi, J.; Zheng, P.; Shi, H.; Wu, H.; et al. Proteomics analysis of Schwann cell-derived exosomes: A novel therapeutic strategy for central nervous system injury. Mol. Cell Biochem. 2019, 457, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Chopp, M.; Wang, L.; Lu, X.; Szalad, A.; Zhang, Z.G. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. FASEB J. 2018, 32, fj201800597R. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes derived from schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy—A review. Nat. Clin. Pract. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Fan, B.; Szalad, A.; Jia, L.; Wang, L.; Wang, X.; Pan, W.; Zhang, L.; Zhang, R.; Hu, J.; et al. MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes 2017, 66, 3111–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Gao, J.; Li, S.; Huang, L.; Zhu, L.; Ma, T.; Zhao, L.; Yang, Y.; Luo, K.; Shi, X.; et al. Mechanical stimulation of Schwann cells promote peripheral nerve regeneration via extracellular vesicle-mediated transfer of microRNA 23b-3p. Theranostics 2020, 10, 8974–8995. [Google Scholar] [CrossRef]
- Peng, D.; Reed-Maldonado, A.B.; Zhou, F.; Tan, Y.; Yuan, H.; Banie, L.; Wang, G.; Tang, Y.; He, L.; Lin, G.; et al. Exosome released from Schwann cells may be involved in microenergy acoustic pulse-associated cavernous nerve regeneration. J. Sex Med. 2020, 17, 1618–1628. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef]
- Stratton, J.A.; Shah, P.T.; Kumar, R.; Stykel, M.G.; Shapira, Y.; Grochmal, J.; Guo, G.F.; Biernaskie, J.; Midha, R. The immunomodulatory properties of adult skin-derived precursor Schwann cells: Implications for peripheral nerve injury therapy. Eur. J. Neurosci. 2016, 43, 365–375. [Google Scholar] [CrossRef]
- Cong, M.; Shen, M.; Wu, X.; Li, Y.; Wang, L.; He, Q.; Shi, H.; Ding, F. Improvement of sensory neuron growth and survival via negatively regulating PTEN by miR-21-5p-contained small extracellular vesicles from skin precursor-derived Schwann cells. Stem Cell Res. Ther. 2021, 12, 80. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Cong, M.; Shen, M.; He, Q.; Ding, F.; Shi, H. Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann. Transl. Med. 2020, 8, 1640. [Google Scholar] [CrossRef]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021, 134, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Hyung, S.; Kim, J.; Yu, C.; Jung, H.; Hong, J. Neuroprotective effect of glial cell-derived exosomes on neurons. Immunotherapy 2019, 5, 156. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Han, S.; Li, X.; Gao, Y.; Zhu, J.; Yang, X.; Xu, L. Paeoniflorin effect of schwann cell-derived exosomes ameliorates dorsal root ganglion neurons apoptosis through IRE1alpha pathway. Evid. Based Complement Alternat. Med. 2021, 2021, 6079305. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hu, M.; Lu, D.; Hong, L. Protective effect and potential mechanism of Schwann cell-derived exosomes on mechanical damage of rat dorsal root ganglion cells. J. Obstet. Gynaecol. Res. 2021, 47, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hu, M.; He, S.; Li, B.; Liu, C.; Min, J.; Hong, L. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med. Sci. Monit. 2018, 24, 7841–7849. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Hong, L.; Liu, C.; Hong, S.; He, S.; Zhou, M.; Huang, G.; Chen, Q. Electrical stimulation enhances neuronal cell activity mediated by Schwann cell derived exosomes. Sci. Rep. 2019, 9, 4206. [Google Scholar] [CrossRef]
- Sohn, E.J.; Park, H.T.; Shin, Y.K. Exosomes derived from differentiated Schwann cells inhibit Schwann cell migration via microRNAs. Neuroreport 2020, 31, 515–522. [Google Scholar] [CrossRef]
- Grassel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.C.; Ye, L.; Demir, I.E.; Kahlert, C. Schwann cell-derived exosomes: Janus-faced mediators of regeneration and disease. Glia 2021, 70, 20–34. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, K.; Lupski, J.R. Charcot-Marie-tooth disease. Eur. J. Hum. Genet. 2009, 17, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Guariglia, S.; Yu, R.Y.; Li, W.; Brancho, D.; Peinado, H.; Lyden, D.; Salzer, J.; Bennett, C.; Chow, C.W. Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes. Mol. Biol. Cell 2013, 24, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Latour, P.; Gonnaud, P.M.; Ollagnon, E.; Chan, V.; Perelman, S.; Stojkovic, T.; Stoll, C.; Vial, C.; Ziegler, F.; Vandenberghe, A.; et al. SIMPLE mutation analysis in dominant demyelinating Charcot-Marie-Tooth disease: Three novel mutations. J. Peripher. Nerv. Syst. 2006, 11, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Prabhakar, S.; Balaj, L.; Lai, C.P.; Cerione, R.A.; Breakefield, X.O. Delivery of therapeutic proteins via extracellular vesicles: Review and potential treatments for Parkinson’s disease, glioma, and Schwannoma. Cell Mol. Neurobiol. 2016, 36, 417–427. [Google Scholar] [CrossRef]
- Soares, V.Y.; Atai, N.A.; Fujita, T.; Dilwali, S.; Sivaraman, S.; Landegger, L.D.; Hochberg, F.H.; Oliveira, C.A.; Bahmad, F.; Breakefield, X.O.; et al. Extracellular vesicles derived from human vestibular schwannomas associated with poor hearing damage cochlear cells. Neuro Oncol. 2016, 18, 1498–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, L.; Ryan, Y.; Hilton, D.A.; Lyons-Rimmer, J.; Dave, F.; Maze, E.A.; Adams, C.L.; Rigby-Jones, R.; Ammoun, S.; Hanemann, C.O. Cellular prion protein (PrP(C)) in the development of Merlin-deficient tumours. Oncogene 2017, 36, 6132–6142. [Google Scholar] [CrossRef] [Green Version]
- Chignon-Sicard, B.; Hofman, V.; Chevallier, D.; Cucchi, J.M.; Ilie, M.; Dadone-Montaudie, B.; Paul, F.; Carpentier, X.; Quintens, H.; Bence-Gauchiez, C.; et al. Age-related schwannomatosis with potential exosome-mediated contribution to prostate hyperplasia: A case report and mini-review. Ther. Adv. Urol. 2019, 11, 1756287219875578. [Google Scholar] [CrossRef]
- Peyre, M.; Goutagny, S.; Imbeaud, S.; Bozorg-Grayeli, A.; Felce, M.; Sterkers, O.; Kalamarides, M. Increased growth rate of vestibular schwannoma after resection of contralateral tumor in neurofibromatosis type 2. Neuro Oncol. 2011, 13, 1125–1132. [Google Scholar] [CrossRef]
- Von Eckardstein, K.L.; Beatty, C.W.; Driscoll, C.L.; Link, M.J. Spontaneous regression of vestibular schwannomas after resection of contralateral tumor in neurofibromatosis Type 2. J. Neurosurg. 2010, 112, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Sceneay, J.; Smyth, M.J.; Moller, A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Erkan, E.P.; Senfter, D.; Madlener, S.; Jungwirth, G.; Strobel, T.; Saydam, N.; Saydam, O. Extracellular vesicle-mediated suicide mRNA/protein delivery inhibits glioblastoma tumor growth in vivo. Cancer Gene Ther. 2017, 24, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Strobel, T.; Breakefield, X.O.; Saydam, O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013, 21, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, C.; Mai, C.; Hu, X.; Cheng, N.; Chen, W.; Peng, D.; Wang, L.; Ji, Z.; Xie, Y. The biogenesis, biological functions, and applications of macrophage-derived exosomes. Front. Mol. Biosci. 2021, 8, 715461. [Google Scholar] [CrossRef]
- Hervera, A.; de Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.; Santos, C.X.C.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Du, T.; Yang, C.L.; Ge, M.R.; Liu, Y.; Zhang, P.; Li, H.; Li, X.L.; Li, T.; Liu, Y.D.; Dou, Y.C.; et al. M1 macrophage derived exosomes aggravate experimental autoimmune neuritis via modulating Th1 response. Front. Immunol. 2020, 11, 1603. [Google Scholar] [CrossRef]
- Lu, M.O.; Zhu, J. The role of cytokines in Guillain-Barre syndrome. J. Neurol. 2011, 258, 533–548. [Google Scholar] [CrossRef]
- Brunn, A.; Mihelcic, M.; Carstov, M.; Hummel, L.; Geier, F.; Schmidt, A.; Saupe, L.; Utermohlen, O.; Deckert, M. IL-10, IL-4, and STAT6 promote an M2 milieu required for termination of P0(106-125)-induced murine experimental autoimmune neuritis. Am. J. Pathol. 2014, 184, 2627–2640. [Google Scholar] [CrossRef]
- Peng, P.; Yu, H.; Xing, C.; Tao, B.; Li, C.; Huang, J.; Ning, G.; Zhang, B.; Feng, S. Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Biomed. Pharmacother. 2021, 144, 112311. [Google Scholar] [CrossRef]
- Zhan, C.; Ma, C.B.; Yuan, H.M.; Cao, B.Y.; Zhu, J.J. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem. Biophys. Res. Commun. 2015, 468, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, J.; Wang, X.; Zhang, J.; Xie, C. Extracellular vesicle-encapsulated microRNA-23a from dorsal root ganglia neurons binds to A20 and promotes inflammatory macrophage polarization following peripheral nerve injury. Aging 2021, 13, 6752–6764. [Google Scholar] [CrossRef]
- Von Bartheld, C.S.; Altick, A.L. Multivesicular bodies in neurons: Distribution, protein content, and trafficking functions. Prog. Neurobiol. 2011, 93, 313–340. [Google Scholar] [CrossRef] [Green Version]
- Madison, R.D.; Robinson, G.A. Muscle-derived extracellular vesicles influence motor neuron regeneration accuracy. Neuroscience 2019, 419, 46–59. [Google Scholar] [CrossRef]
- Madison, R.D.; McGee, C.; Rawson, R.; Robinson, G.A. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34). J. Extracell. Vesicles 2014, 3, 22865. [Google Scholar] [CrossRef] [Green Version]
- Geranmayeh, M.H.; Rahbarghazi, R.; Farhoudi, M. Targeting pericytes for neurovascular regeneration. Cell Commun. Signal 2019, 17, 26. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Gaceb, A.; Barbariga, M.; Ozen, I.; Paul, G. The pericyte secretome: Potential impact on regeneration. Biochimie 2018, 155, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.N.; Park, S.H.; Ock, J.; Choi, M.J.; Limanjaya, A.; Ghatak, K.; Song, K.M.; Kwon, M.H.; Kim, D.K.; Gho, Y.S.; et al. Pericyte-Derived extracellular vesicle-mimetic nanovesicles restore erectile function by enhancing neurovascular regeneration in a mouse model of cavernous nerve injury. J. Sex Med. 2020, 17, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Gil, Z. The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk of the Peripheral Nervous System. Cells 2022, 11, 1294. https://doi.org/10.3390/cells11081294
Guo Y, Gil Z. The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk of the Peripheral Nervous System. Cells. 2022; 11(8):1294. https://doi.org/10.3390/cells11081294
Chicago/Turabian StyleGuo, Yuanning, and Ziv Gil. 2022. "The Role of Extracellular Vesicles in Cancer–Nerve Crosstalk of the Peripheral Nervous System" Cells 11, no. 8: 1294. https://doi.org/10.3390/cells11081294





