Evolutionary Transition in the Regulation of Vertebrate Pronephros Development: A New Role for Retinoic Acid
Abstract
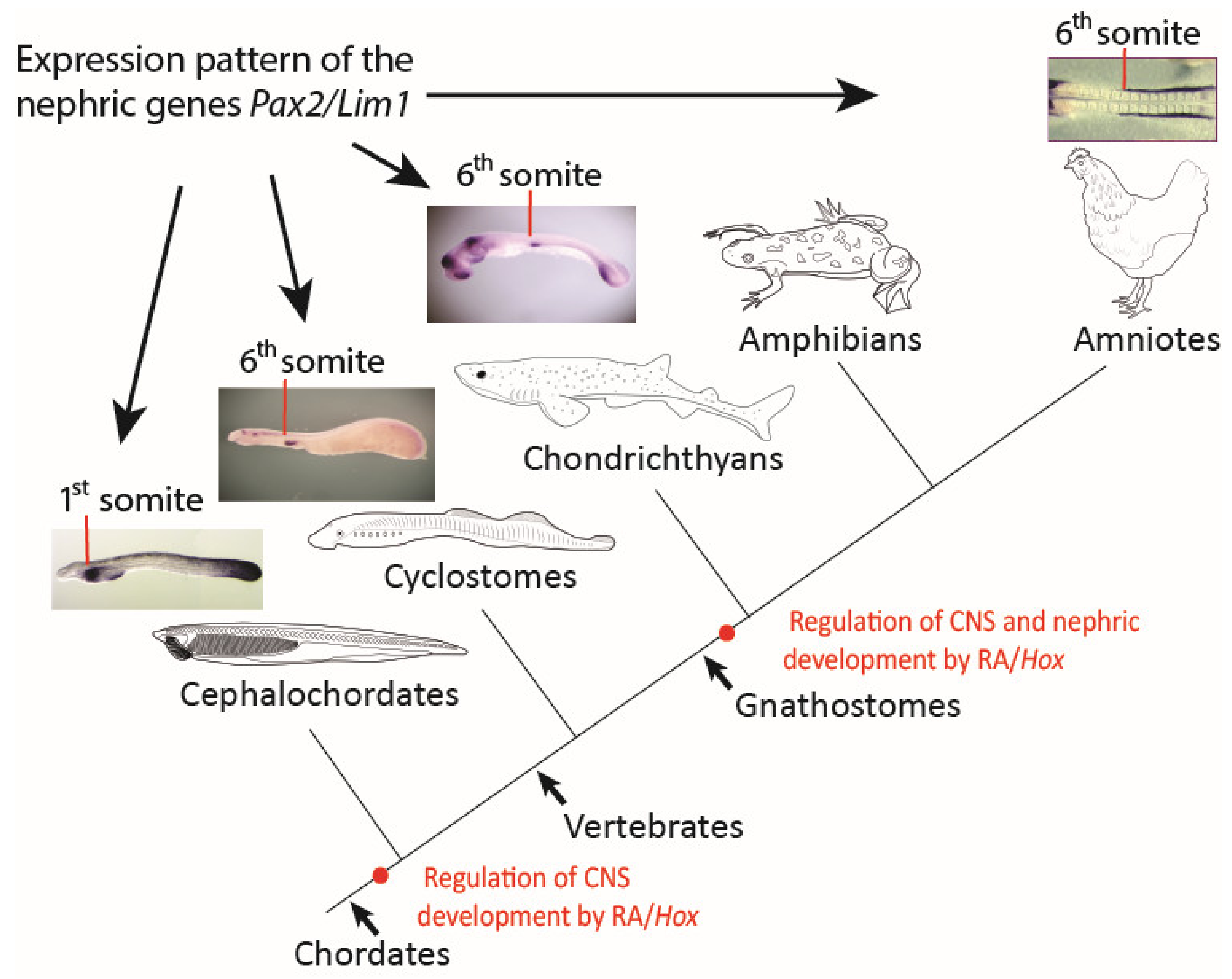
:1. Introduction
2. Materials and Methods
2.1. Embryos
2.2. Pharmacological Treatments
2.3. Whole Mount In Situ Hybridization
2.4. Histology
3. Results
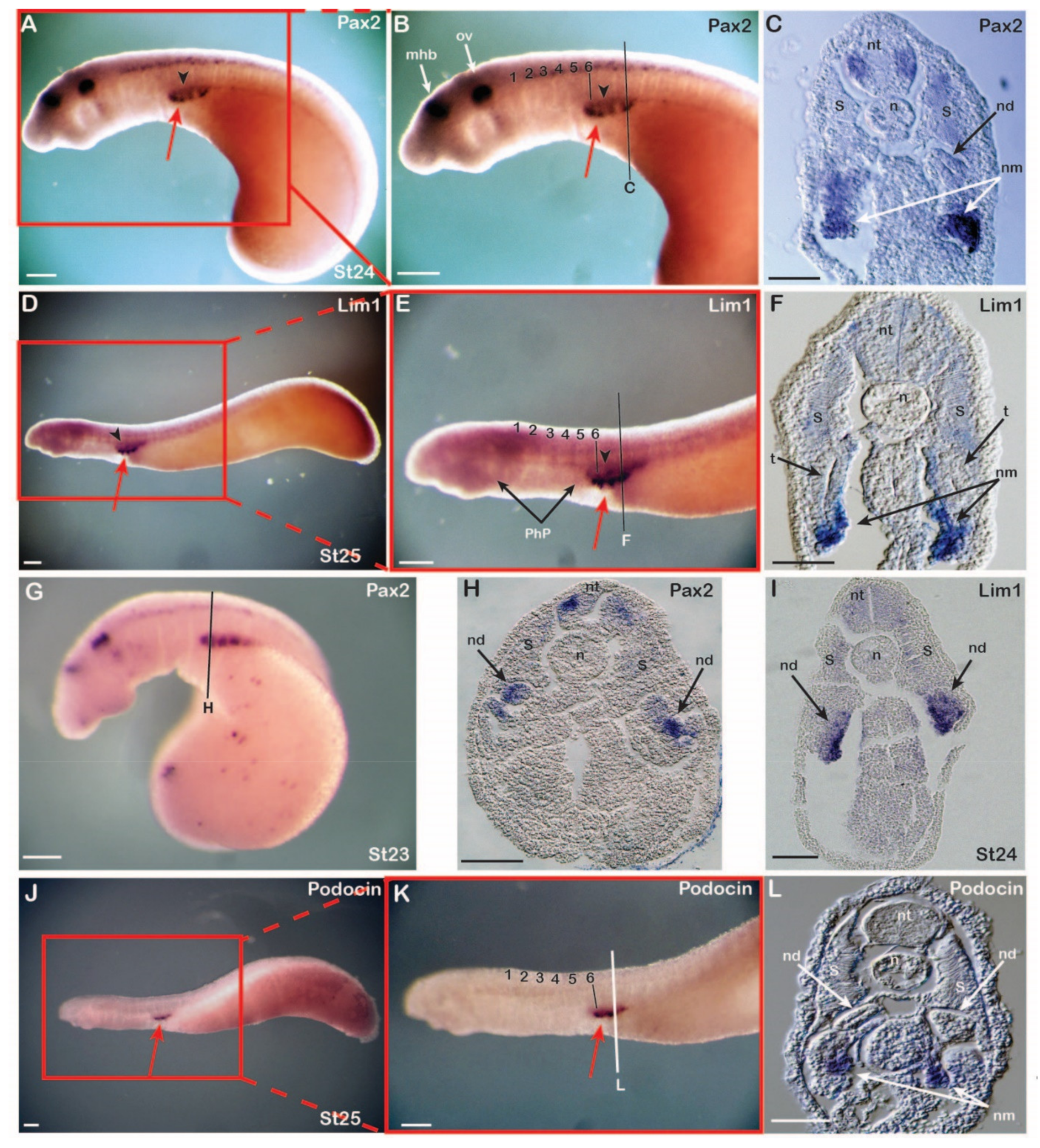
3.1. Expression of Nephric Markers in Catsharks
3.2. Expression of Nephric Markers in Lampreys
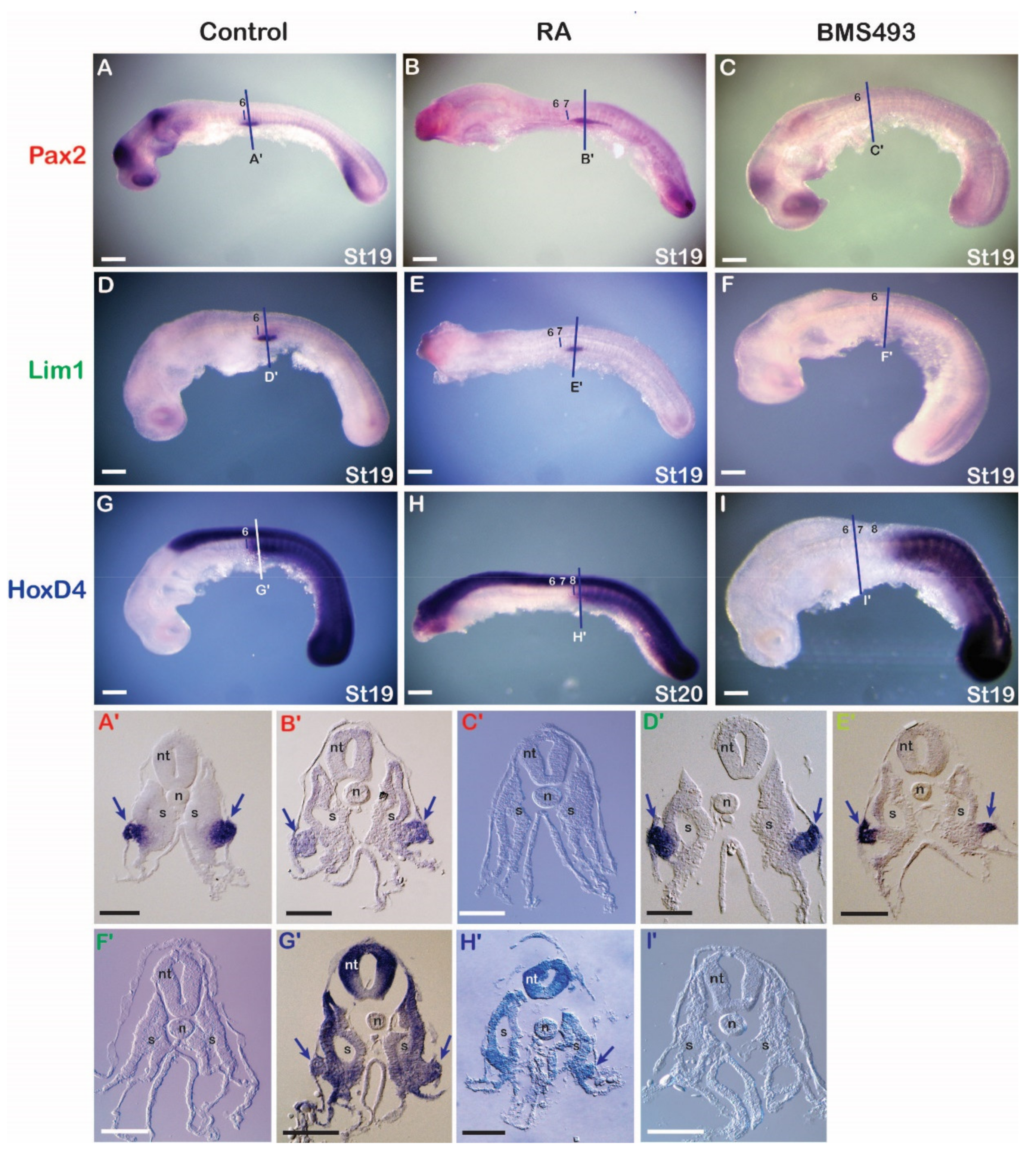
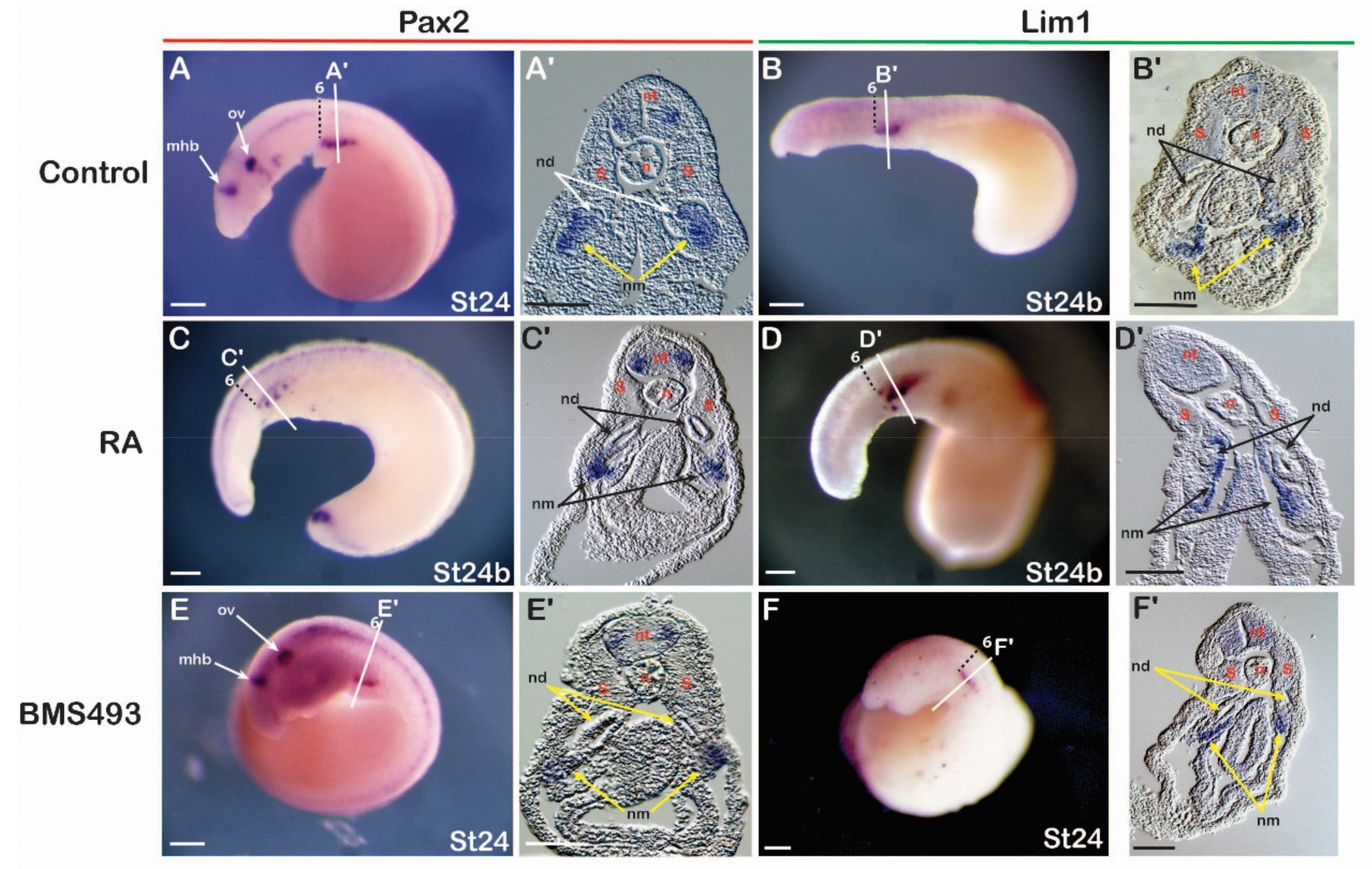
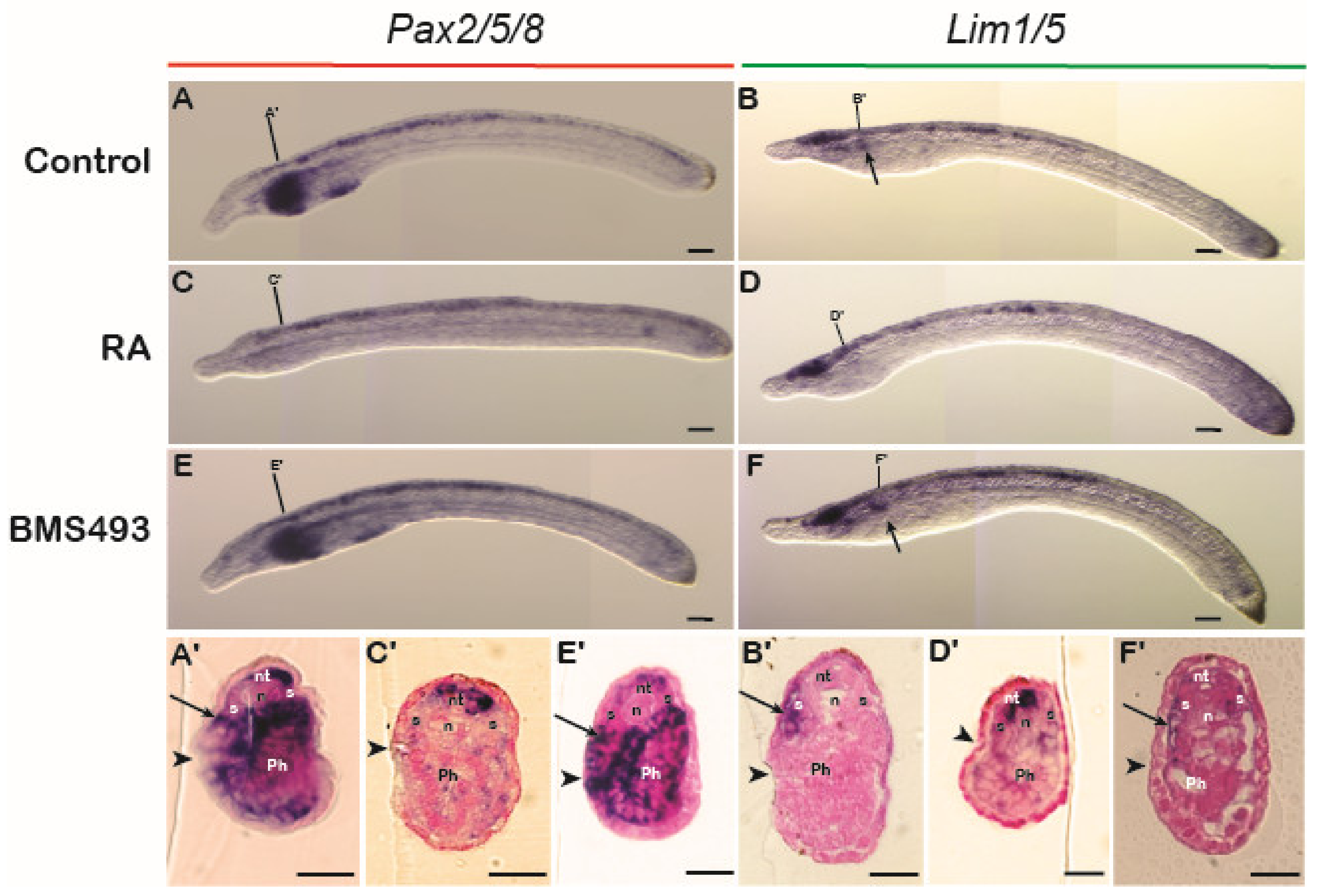
3.3. Retinoic Acid and the Regulation of Nephric Genes in Catshark, Lamprey, and Amphioxus
3.3.1. Catshark
3.3.2. Lamprey
3.3.3. Amphioxus
4. Discussion
4.1. Defining the Anterior Boundary of Pronephros Formation
4.2. The Role of RA in the Regulation of Pronephros Formation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallo, M.; Vinagre, T.; Carapuço, M. The road to the vertebral formula. Int. J. Dev. Biol. 2009, 53, 1469–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koop, D.; Holland, N.D.; Sémon, M.; Alvarez, S.; de Lera, A.R.; Laudet, V.; Holland, L.Z.; Schubert, M. Retinoic acid signaling targets Hox genes during the amphioxus gastrula stage: Insights into early anterior-posterior patterning of the chordate body plan. Dev. Biol. 2010, 338, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, C.; De Kumar, B.; Krumlauf, R. Hox genes: Downstream “effectors” of retinoic acid signaling in vertebrate embryogenesis. Genesis 2019, 57, e23306. [Google Scholar] [CrossRef] [PubMed]
- Saxen, L. Organogenesis of the Kidney; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar] [CrossRef]
- Price, G.C. Development of the excretory organs of a myxinoid, Bdellostoma stouti, Lockington. Zool. Jahrb. Abt. Anat. Ontog. 1897, 10, 205–226. [Google Scholar]
- Davidson, A.J.; Lewis, P.; Przepiorski, A.; Sander, V. Turning mesoderm into kidney. Semin. Cell Dev. Biol. 2019, 91, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Barak, H.; Rosenfelder, L.; Schultheiss, T.M.; Reshef, R. Cell fate specification along the anterior–posterior axis of the intermediate mesoderm. Dev. Dyn. 2005, 232, 901–914. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Dressler, G.R.; Deutsch, U.; Chowdhury, K.; Nornes, H.O.; Gruss, P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 1990, 109, 787–795. [Google Scholar] [CrossRef]
- Fujii, T.; Pichel, J.G.; Taira, M.; Toyama, R.; Dawid, I.B.; Westphal, H. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 1994, 199, 73–83. [Google Scholar] [CrossRef]
- Burrow, C.R. Regulatory molecules in kidney development. Pediatr. Nephrol. 2000, 14, 240–253. [Google Scholar] [CrossRef]
- James, R.G.; Schultheiss, T.M. Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev. Biol. 2003, 253, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiruma, T.; Nakamura, H. Origin and development of the pronephros in the chick embryo. J. Anat. 2003, 203, 539. [Google Scholar] [CrossRef] [PubMed]
- Barak, H.; Preger-Ben Noon, E.; Reshef, R. Comparative spatiotemporal analysis of Hox gene expression in early stages of intermediate mesoderm formation. Dev. Dyn. 2012, 241, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Seufert, D.W.; Brennan, H.C.; Deguire, J.; Jones, E.A.; Vize, P.D. Developmental basis of pronephric defects in Xenopus body plan phenotypes. Dev. Biol. 1999, 215, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Obara-Ishihara, T.; Kuhlman, J.; Niswander, L.; Herzlinger, D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 1999, 126, 1103–1108. [Google Scholar] [CrossRef]
- Mauch, T.J.; Yang, G.; Wright, M.; Smith, D.; Schoenwolf, G.C. Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev. Biol. 2000, 220, 62–75. [Google Scholar] [CrossRef] [Green Version]
- James, R.G.; Schultheiss, T.M. Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev. Biol. 2005, 288, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Taira, M.; Jamrich, M.; Good, P.J.; Dawid, I.B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992, 6, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Moriya, N.; Uchiyama, H.; Asashima, M. Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev. Growth Differ. 1993, 35, 123–128. [Google Scholar] [CrossRef]
- Taira, M.; Otani, H.; Jamrich, M.; Dawid, I.B. Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 1994, 120, 1525–1536. [Google Scholar] [CrossRef]
- Rebbert, M.L.; Dawid, I.B. Transcriptional regulation of the Xlim-1 gene by activin is mediated by an element in intron I. Proc. Natl. Acad. Sci. USA 1997, 94, 9717–9722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osafune, K.; Nishinakamura, R.; Komazaki, S.; Asashima, M. In vitro induction of the pronephric duct in Xenopus explants. Dev. Growth Differ. 2002, 44, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Rebbert, M.L.; Andreazzoli, M.; Takahashi, N.; Toyama, R.; Zimmerman, S.; Whitman, M.; Dawid, I.B. Regulation of the Lim-1 gene is mediated through conserved FAST-1/FoxH1 sites in the first intron. Dev. Dyn. 2002, 225, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Haldin, C.E.; Nijjar, S.; Masse, K.; Barnett, M.W.; Jones, E.A. Isolation and growth factor inducibility of the Xenopus laevis Lmx1b gene. Int. J. Dev. Biol. 2004, 47, 253–262. [Google Scholar] [CrossRef]
- Kim, D.; Dressler, G.R. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J. Am. Soc. Nephrol. 2005, 16, 3527–3534. [Google Scholar] [CrossRef] [Green Version]
- Cartry, J.; Nichane, M.; Ribes, V.; Colas, A.; Riou, J.F.; Pieler, T.; Dollé, P.; Bellefroid, E.J.; Umbhauer, M. Retinoic acid signaling is required for specification of pronephric cell fate. Dev. Biol. 2006, 299, 35–51. [Google Scholar] [CrossRef]
- Preger-Ben Noon, E.; Barak, H.; Guttmann-Raviv, N.; Reshef, R. Interplay between activin and Hox genes determines the formation of the kidney morphogenetic field. Development 2009, 136, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [Green Version]
- Fraser, E.A. The development of the vertebrate excretory system. Biol. Rev. 1950, 25, 159–187. [Google Scholar] [CrossRef]
- Escrivá, H. My favorite animal, amphioxus: Unparalleled for studying early vertebrate evolution. BioEssays 2018, 40, e1800130. [Google Scholar] [CrossRef]
- Kozmik, Z.; Holland, N.D.; Kalousova, A.; Paces, J.; Schubert, M.; Holland, L.Z. Characterization of an amphioxus paired box gene, AmphiPax2/5/8: Developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain-hindbrain boundary region. Development 1999, 126, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Langeland, J.A.; Holland, L.Z.; Chastain, R.A.; Holland, N.D. An amphioxus LIM-homeobox gene, AmphiLim1/5, expressed early in the invaginating organizer region and later in differentiating cells of the kidney and central nervous system. Int. J. Biol. Sci. 2006, 2, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppert, E.E. Morphology of Hatschek’s nephridium in larval and juvenile stages of Branchiostoma virginiae (Cephalochordata). Isr. J. Zool. 1996, 42, S161–S182. [Google Scholar] [CrossRef]
- Ballard, W.W.; Mellinger, J.; Lechenault, H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J. Exp. Zool. 1993, 267, 318–336. [Google Scholar] [CrossRef]
- Sauka-Spengler, T.; Le Mentec, C.; Lepage, M.; Mazan, S. Embryonic expression of Tbx1, a DiGeorge syndrome candidate gene, in the lamprey Lampetra fluviatilis. Gene Expr. Patterns 2002, 2, 99–103. [Google Scholar] [CrossRef]
- Tahara, Y. Normal stages of development in the lamprey, Lampetra reissneri (Dybowski). Zoolog. Sci. 1988, 5, 109–118. [Google Scholar]
- Fuentes, M.; Schubert, M.; Dalfo, D.; Candiani, S.; Benito, E.; Gardenyes, J.; Godoy, L.; Moret, F.; Illas, M.; Patten, I.; et al. Preliminary observations on the spawning conditions of the European amphioxus (Branchiostoma lanceolatum) in captivity. J. Exp. Zool. B 2004, 302, 384–391. [Google Scholar] [CrossRef]
- Holland, L.Z.; Yu, J.K. Cephalochordate (amphioxus) embryos: Procurement, culture, and basic methods. Methods Cell Biol. 2004, 74, 195–215. [Google Scholar] [CrossRef]
- Carvalho, J.E.; Lahaye, F.; Yong, L.W.; Croce, J.C.; Escrivá, H.; Yu, J.K.; Schubert, M. An updated staging system for cephalochordate development: One table suits them all. Front. Cell Dev. Biol. 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Zieger, E.; Candiani, S.; Garbarino, G.; Croce, J.C.; Schubert, M. Roles of retinoic acid signaling in shaping the neuronal architecture of the developing amphioxus nervous system. Mol. Neurobiol. 2018, 55, 5210–5229. [Google Scholar] [CrossRef]
- Yu, J.K.; Holland, L.Z. Amphioxus whole-mount in situ hybridization. Cold Spring Harb. Protoc. 2009, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.; McCole, R.B.; Baker, C.V.H. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev. Biol. 2007, 304, 156–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coolen, M.; Sauka-Spengler, T.; Nicolle, D.; Le-Mentec, C.; Lallemand, Y.; Da Silva, C.; Plouhinec, J.L.; Robert, B.; Wincker, P.; Shi, D.L.; et al. Evolution of axis specification mechanisms in jawed vertebrates: Insights from a chondrichthyan. PLoS ONE 2007, 2, e374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oulion, S.; Debiais-Thibaud, M.; D’Aubenton-Carafa, Y.; Thermes, C.; Da Silva, C.; Bernard-Samain, S.; Gavory, F.; Wincker, P.; Mazan, S.; Casane, D. Evolution of Hox gene clusters in gnathostomes: Insights from a survey of a shark (Scyliorhinus canicula) transcriptome. Mol. Biol. Evol. 2010, 27, 2829–2838. [Google Scholar] [CrossRef] [Green Version]
- McCauley, D.W.; Bronner-Fraser, M. Conservation of Pax gene expression in ectodermal placodes of the lamprey. Gene 2002, 287, 129–139. [Google Scholar] [CrossRef]
- Osorio, J.; Mazan, S.; Rétaux, S. Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: Insights from LIM-homeodomain, Pax and hedgehog genes. Dev. Biol. 2005, 288, 100–112. [Google Scholar] [CrossRef]
- Carvalho, J.E.; Lahaye, F.; Croce, J.C.; Schubert, M. CYP26 function is required for the tissue-specific modulation of retinoic acid signaling during amphioxus development. Int. J. Dev. Biol. 2017, 61, 733–747. [Google Scholar] [CrossRef]
- Takio, Y.; Kuraku, S.; Murakami, Y.; Pasqualetti, M.; Rijli, F.M.; Narita, Y.; Kuratani, S.; Kusakabe, R. Hox gene expression patterns in Lethenteron japonicum embryos—Insights into the evolution of the vertebrate Hox code. Dev. Biol. 2007, 308, 606–620. [Google Scholar] [CrossRef]
- Boute, N.; Gribouval, O.; Roselli, S.; Benessy, F.; Lee, H.; Fuchshuber, A.; Dahan, K.; Gubler, M.C.; Niaudet, P.; Antignac, C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 2000, 24, 349–354. [Google Scholar] [CrossRef]
- Roselli, S.; Heidet, L.; Sich, M.; Henger, A.; Kretzler, M.; Gubler, M.C.; Antignac, C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol. Cell. Biol. 2004, 24, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Kuratani, S.; Ueki, T.; Hirano, S.; Aizawa, S. Rostral truncation of a cyclostome, Lampetra japonica, induced by all-trans retinoic acid defines the head/trunk interface of the vertebrate body. Dev. Dyn. 1998, 211, 35–51. [Google Scholar] [CrossRef]
- Escriva, H.; Holland, N.D.; Gronemeyer, H.; Laudet, V.; Holland, L.Z. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development 2002, 129, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Yu, J.-K.; Holland, N.D.; Escriva, H.; Laudet, V.; Holland, L.Z. Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development 2005, 132, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Zhi, Q.; Patel, K.; Wilting, J.; Christ, B. Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anat. Embryol. 2000, 202, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Asamoto, K. The developmental fate of the rostral/caudal half of a somite for vertebra and rib formation: Experimental confirmation of the resegmentation theory using chick-quail chimeras. Mech. Dev. 2000, 99, 71–82. [Google Scholar] [CrossRef]
- Drummond, I.A.; Majumdar, A.; Hentschel, H.; Elger, M.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Stemple, D.L.; Zwartkruis, F.; Rangini, Z.; et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 1998, 125, 4655–4667. [Google Scholar] [CrossRef]
- Wingert, R.A.; Selleck, R.; Yu, J.; Song, H.-D.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.P.; et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007, 3, e189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vize, P.D.; Seufert, D.W.; Carroll, T.J.; Wallingford, J.B. Model systems for the study of kidney development: Use of the pronephros in the analysis of organ induction and patterning. Dev. Biol. 1997, 188, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.K.; Admiraal, J.; Wright, G.M. Developmental anatomy of lampreys. Biol. Rev. 2010, 85, 1–33. [Google Scholar] [CrossRef]
- Kaji, T.; Reimer, J.D.; Morov, A.R.; Kuratani, S.; Yasui, K. Amphioxus mouth after dorso-ventral inversion. Zool. Lett. 2016, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Holland, N.D. Formation of the initial kidney and mouth opening in larval amphioxus studied with serial blockface scanning electron microscopy (SBSEM). EvoDevo 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E. Evolutionary origin of the vertebrate nephron. Am. Zool. 1994, 34, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Holland, N.D. The long and winding path to understanding kidney structure in amphioxus—A review. Int. J. Dev. Biol. 2017, 61, 683–688. [Google Scholar] [CrossRef]
- Nylor, R.W.; Qubisi, S.S.; Davidson, A.J. Zebrafish pronephros development. Results Probl. Cell Differ. 2017, 60, 27–53. [Google Scholar] [CrossRef]
- Lee, L.M.Y.; Leung, C.Y.; Tang, W.W.C.; Choi, H.L.; Leung, Y.C.; McCaffery, P.J.; Wang, C.C.; Woolf, A.S.; Shum, A.S.W. A paradoxical teratogenic mechanism for retinoic acid. Proc. Natl. Acad. Sci. USA 2012, 109, 13668–13673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, S.; Aldea, D.; Oulion, S.; Subirana, L.; de Lera, A.R.; Somorjai, I.; Escrivá, H. Evolution of the role of RA and FGF signals in the control of somitogenesis in chordates. PLoS ONE 2015, 10, e0136587. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Holland, N.D.; Laudet, V.; Holland, L.Z. A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev. Biol. 2006, 296, 190–202. [Google Scholar] [CrossRef]
- Smith, J.J.; Timoshevskaya, N.; Ye, C.; Holt, C.; Keinath, M.C.; Parker, H.J.; Cook, M.E.; Hess, J.E.; Narum, S.R.; Lamanna, F.; et al. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 2018, 50, 270–277. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, P.; Leman, E.; Lagadec, R.; Schubert, M.; Mazan, S.; Reshef, R. Evolutionary Transition in the Regulation of Vertebrate Pronephros Development: A New Role for Retinoic Acid. Cells 2022, 11, 1304. https://doi.org/10.3390/cells11081304
Schmidt P, Leman E, Lagadec R, Schubert M, Mazan S, Reshef R. Evolutionary Transition in the Regulation of Vertebrate Pronephros Development: A New Role for Retinoic Acid. Cells. 2022; 11(8):1304. https://doi.org/10.3390/cells11081304
Chicago/Turabian StyleSchmidt, Pascal, Eva Leman, Ronan Lagadec, Michael Schubert, Sylvie Mazan, and Ram Reshef. 2022. "Evolutionary Transition in the Regulation of Vertebrate Pronephros Development: A New Role for Retinoic Acid" Cells 11, no. 8: 1304. https://doi.org/10.3390/cells11081304
APA StyleSchmidt, P., Leman, E., Lagadec, R., Schubert, M., Mazan, S., & Reshef, R. (2022). Evolutionary Transition in the Regulation of Vertebrate Pronephros Development: A New Role for Retinoic Acid. Cells, 11(8), 1304. https://doi.org/10.3390/cells11081304






