Nuclear Lipid Droplet Birth during Replicative Stress
Abstract
:1. Introduction
2. Materials and Methods
| Simplified Name | Detailed Information | Source |
|---|---|---|
| pNLS-Q2 | pRS316-SacI-SacII-CYC1promoter (truncated)-XbaI-NUP60NLS(1-24)-XbaI-Opi1 Q2 -BamHI-mCherry-XmaI-NUP1 terminator-XhoI | [4] |
| pQ2 | pRS316-SacI-SacII-CYC1promoter (truncated)- XbaI-Opi1 Q2 -BamHI-mCherry-XmaI-NUP1 terminator-XhoI | This study |
| pEmpty | pRS424 | [12] |
| pexo1-D173AOE | pSM638 (pRS424-exo1-D173A) | [13] |
| pEXO1OE | pSM502 (pRS424-EXO1) | [13] |
| pmCherry-PUS1 | YIplac211-mCherry-PUS1 | Symeon Siniossoglou |
3. Results
3.1. The Formation of Nuclear Lipid Droplets Is Not a General Response to Genotoxins
3.2. nLD Form in Response to Replication Stress
3.3. Replication Stress-Associated nLD Birth Is Not Accompanied by Biosensor-Detectable Phosphatidic Acid Changes at the Nuclear Membrane
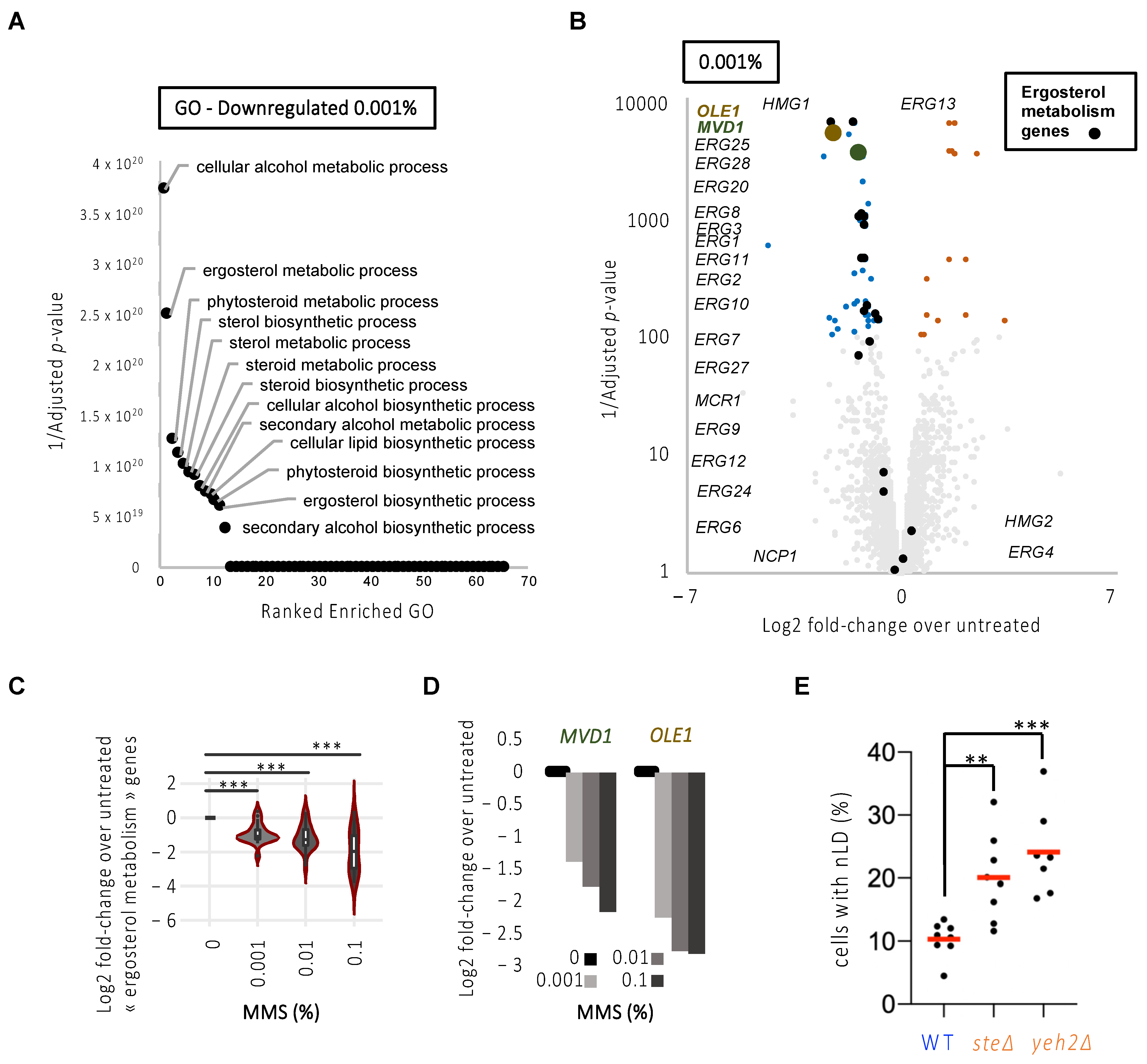
3.4. The Membrane Phospholipid Unsaturation and Sterol Profile Conditions nLD Birth in Response to Replicative Stress
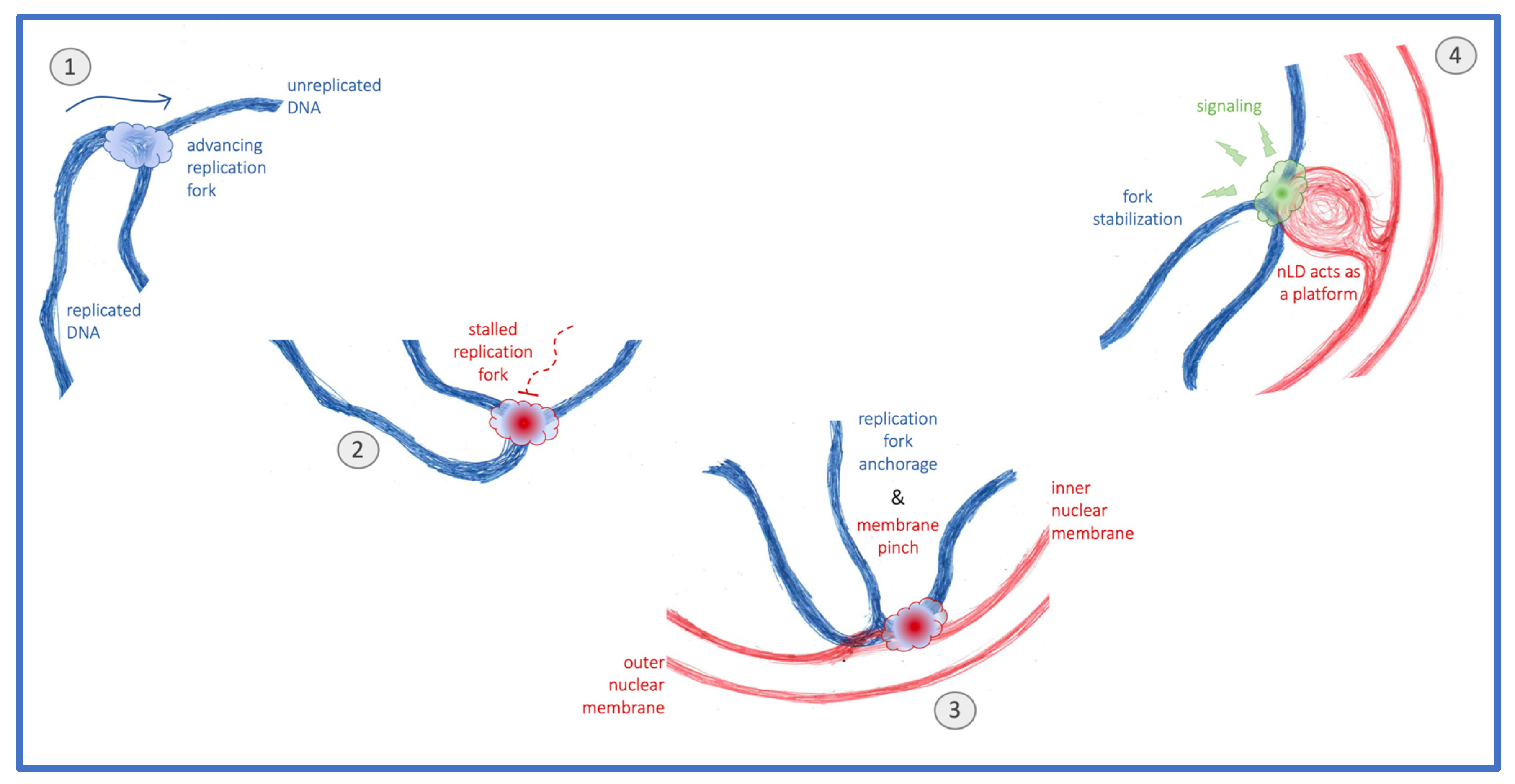
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPT | camptothecin |
| c/nLD | cytoplasmic/nuclear lipid droplets |
| GO | gene ontology |
| HU | hydroxyurea |
| INM | inner nuclear membrane |
| MMS | methylmethane sulfonate |
| NLS | nuclear localization signal |
| PA | phosphatidic acid |
| SEM | standard error of the mean |
| WT | wild type |
| 4-NQO | 4-nitroquinoline-1-oxide |
References
- Srivastava, N.; de Nader, G.P.F.; Williart, A.; Rollin, R.; Cuvelier, D.; Lomakin, A.; Piel, M. Nuclear fragility, blaming the blebs. Curr. Opin. Cell Biol. 2021, 70, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.D.; Siniossoglou, S. New kid on the block: Lipid droplets in the nucleus. FEBS J. 2020, 287, 4838–4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilfling, F.; Haas, J.T.; Walther, T.C.; Farese, R.V. Lipid Droplet Biogenesis. Curr. Opin. Cell Biol. 2014, 29, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanauska, A.; Köhler, A. The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell 2018, 174, 700–715. [Google Scholar] [CrossRef] [Green Version]
- Ohsaki, Y.; Kawai, T.; Yoshikawa, Y.; Cheng, J.; Jokitalo, E.; Fujimoto, T. PML isoform II plays a critical role in nuclear lipid droplet formation. J. Cell Biol. 2016, 212, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, H.; García-Muse, T.; Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 2015, 15, 276–289. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Benton, M.G.; Somasundaram, S.; Glasner, J.D.; Palecek, S.P. Analyzing the dose-dependence of the Saccharomyces cerevisiae global transcriptional response to methyl methanesulfonate and ionizing radiation. BMC Genom. 2006, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Holik, A.Z.; Su, S.; Jansz, N.; Chen, K.; Leong, H.S.; Blewitt, M.E.; Asselin-Labat, M.-L.; Smyth, G.K.; Ritchie, M.E. Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res. 2015, 43, e97. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Lord, S.J.; Velle, K.B.; Dyche Mullins, R.; Fritz-Laylin, L.K. SuperPlots: Communicating reproducibility and variability in cell biology. J. Cell Biol. 2020, 219, e202001064. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.; Morgan, E.A.; Symington, L.S. Overlapping Functions of the Saccharomyces cerevisiae Mre11, Exo1 andRad27 Nucleases in DNA Metabolism. Genetics 2001, 159, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, B.R.; Binns, D.D.; Hilton, C.L.; Han, S.; Gao, Q.; Goodman, J.M. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 2015, 26, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Moriel-Carretero, M.; Vicat, T.; Aguilera, A.; Pasero, P. Homologous recombination and Mus81 promote replication completion in response to replication fork blockage. EMBO Rep. 2020, 21, e49367. [Google Scholar] [CrossRef]
- Su, X.A.; Dion, V.; Gasser, S.M.; Freudenreich, C.H. Regulation of recombination at yeast nuclear pores controls repair and triplet repeat stability. Genes Dev. 2015, 29, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Schirmeisen, K.; Lambert, S.A.E.; Kramarz, K. SUMO-based regulation of nuclear positioning to spatially regulate homologous recombination activities at replication stress sites. Genes 2021, 12, 10. [Google Scholar] [CrossRef]
- Kramarz, K.; Schirmeisen, K.; Boucherit, V.; Ait Saada, A.; Lovo, C.; Palancade, B.; Freudenreich, C.; Lambert, S.A.E. The nuclear pore primes recombination-dependent DNA synthesis at arrested forks by promoting SUMO removal. Nat. Commun. 2020, 11, 5643. [Google Scholar] [CrossRef]
- Whalen, J.M.; Freudenreich, C.H. Location, location, location: The role of nuclear positioning in the repair of collapsed forks and protection of genome stability. Genes 2020, 11, 635. [Google Scholar] [CrossRef]
- Male, G.; Deolal, P.; Manda, N.K.; Yagnik, S.; Mazumder, A.; Mishra, K. Nucleolar size regulates nuclear envelope shape in Saccharomyces cerevisiae. J. Cell Sci. 2021, 133, jcs242172. [Google Scholar] [CrossRef]
- Bozler, J.; Nguyen, H.Q.; Rogers, G.C.; Bosco, G. Condensins exert force on chromatin-nuclear envelope tethers to mediate nucleoplasmic reticulum formation in Drosophila melanogaster. G3 Genes Genomes Genet. 2015, 5, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, J.A.; Bjergbaek, L.; Shimada, K.; Frei, C.; Gasser, S.M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003, 22, 4325–4336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotta-Ramusino, C.; Fachinetti, D.; Lucca, C.; Doksani, Y.; Lopes, M.; Sogo, J.; Foiani, M. Exo1 Processes Stalled Replication Forks and Counteracts Fork Reversal in Checkpoint-Defective Cells. Mol. Cell 2005, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Erdeniz, N.; Dudley, S.; Liskay, R.M. Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst.) 2002, 1, 895–912. [Google Scholar] [CrossRef]
- Romanauska, A.; Köhler, A. Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat. Dev. Cell 2021, 56, 2562–2578.e3. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.R.; Gazpar, M.L.; Jesch, S.A.; Delon, C.; Ktistakis, N.T.; Henry, S.A.; Levine, T.P. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science 2004, 304, 1644–1647. [Google Scholar] [CrossRef]
- Martin, C.E.; Oh, C.-S.; Jiang, Y. Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 271–285. [Google Scholar] [CrossRef]
- Yang, H.; Bard, M.; Bruner, D.A.; Gleeson, A.; Deckelbaum, R.J.; Aljinovic, G.; Pohl, T.M.; Rothstein, R.; Sturley, S.L. Sterol Esterification in Yeast: A Two-Gene Process. Science 1996, 272, 1353–1356. [Google Scholar] [CrossRef]
- Müllner, H.; Deutsch, G.; Leitner, E.; Ingolic, E.; Daum, G. YEH2/YLR020c encodes a novel steryl ester hydrolase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 13321–13328. [Google Scholar] [CrossRef] [Green Version]
- Towbin, B.D.; Meister, P.; Gasser, S.M. The nuclear envelope—A scaffold for silencing? Curr. Opin. Genet. Dev. 2009, 19, 180–186. [Google Scholar] [CrossRef]
- Hirano, Y.; Asakawa, H.; Sakuno, T.; Haraguchi, T.; Hiraoka, Y. Nuclear Envelope Proteins Modulating the Heterochromatin Formation and Functions in Fission Yeast. Cells 2020, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gong, K.; Denholtz, M.; Chandra, V.; Kamps, M.P.; Alber, F.; Murre, C. Comprehensive characterization of neutrophil genome topology. Genes Dev. 2017, 31, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, S.; Dubrana, K.; Tsai-Pflugfelder, M.; Davidson, M.B.; Roberts, T.M.; Brown, G.W.; Varela, E.; Hediger, F.; Gasser, S.M.; Krogan, N.J. Functional Targeting of DNA Damage to a Nuclear Pore-Associated SUMO-Dependent Ubiquitn Ligase. Science 2008, 322, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whale, A.J.; King, M.; Hull, R.M.; Krueger, F.; Houseley, J. Stimulation of adaptive gene amplification by origin firing under replication fork constraint. Nucleic Acids Res. 2022, 50, 915–936. [Google Scholar] [CrossRef]
- Hwang, S.; Williams, J.F.; Kneissig, M.; Lioudyno, M.; Rivera, I.; Helguera, P.; Busciglio, J.; Storchova, Z.; King, M.C.; Torres, E.M. Suppressing Aneuploidy-Associated Phenotypes Improves the Fitness of Trisomy 21 Cells. Cell Rep. 2019, 29, 2473–2488.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovejero, S.; Soulet, C.; Moriel-Carretero, M. The alkylating agent methyl methanesulfonate triggers lipid alterations at the inner nuclear membrane that are independent from its dna-damaging ability. Int. J. Mol. Sci. 2021, 22, 7461. [Google Scholar] [CrossRef]
- Mosquera, J.V.; Bacher, M.C.; Priess, J.R. Nuclear lipid droplets and nuclear damage in caenorhabditis elegans. PLoS Genet. 2021, 17, e1009602. [Google Scholar] [CrossRef]
- Moriel-Carretero, M. The many faces of lipids in genome stability (and how to unmask them). Int. J. Mol. Sci. 2021, 22, 2930. [Google Scholar] [CrossRef]
- Fugger, K.; Chu, W.K.; Haahr, P.; Nedergaard Kousholt, A.; Beck, H.; Payne, M.J.; Hanada, K.; Hickson, I.D.; Sørensen, C.S. FBH1 co-operates with MUS81 in inducing DNA double-strand breaks and cell death following replication stress. Nat. Commun. 2013, 4, 1423. [Google Scholar] [CrossRef] [Green Version]
- Azzi-Martin, L.; He, W.; Péré-Védrenne, C.; Korolik, V.; Alix, C.; Prochazkova-Carlotti, M.; Morel, J.L.; Le Roux-Goglin, E.; Lehours, P.; Djavaheri-Mergny, M.; et al. Cytolethal distending toxin induces the formation of transient messenger-rich ribonucleoprotein nuclear invaginations in surviving cells. PLoS Pathog. 2019, 15, e1007921. [Google Scholar] [CrossRef]
- Layerenza, J.P.; González, P.; García De Bravo, M.M.; Polo, M.P.; Sisti, M.S.; Ves-Losada, A. Nuclear lipid droplets: A novel nuclear domain. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Kumanski, S.; Elías-villalobos, A.; Soulet, C.; Moriel-Carretero, M. Negative curvature-promoting lipids instruct nuclear ingression of low autophagic potential vacuoles. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ovejero, S.; Soulet, C.; Kumanski, S.; Moriel-Carretero, M. Coordination between phospholipid pools and DNA damage sensing. Biol. Cell 2022. minor revisions completed. [Google Scholar]
- Okada, A.K.; Teranishi, K.; Ambroso, M.R.; Isas, J.M.; Vazquez-sarandeses, E.; Lee, J.; Melo, A.A.; Pandey, P.; Merken, D.; Berndt, L.; et al. Lysine acetylation regulates the interaction between proteins and membranes. Nat. Commun. 2021, 12, 6466. [Google Scholar] [CrossRef] [PubMed]
- Sołtysik, K.; Ohsaki, Y.; Tatematsu, T.; Cheng, J.; Fujimoto, T. Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat. Commun. 2019, 10, 473. [Google Scholar] [CrossRef]
- Lee, J.; Salsman, J.; Foster, J.; Dellaire, G.; Ridgway, N.D. Lipid-associated PML structures assemble nuclear lipid droplets containing CCTα and Lipin1. Life Sci. Alliance 2020, 3, e202000751. [Google Scholar] [CrossRef]



| Simplified Genotype | Full Genotype | Source |
|---|---|---|
| WT (W303) | MAT a, ade2, his3, can1, leu2, trp1, ura3, GAL+, psi+, RAD5 | PP870, Philippe Pasero |
| sgs1∆ | MAT a, ade2, his3, can1, leu2, trp1, ura3, sgs1∆LEU2 mCherry-PUS1::URA3 | MM-113, Philippe Pasero |
| sgs1∆ exo1∆ | MAT a, ade2, his3, can1, leu2, trp1, ura3, sgs1∆LEU2 exo1∆ natR mCherry-PUS1::URA3 | MM-119, Philippe Pasero |
| exo1∆ | MAT a, ade2, his3, leu2, trp1, ura3, exo1∆natR mCherry-PUS1::URA3 | MM-110, Philippe Pasero |
| ste∆ | MAT alpha, ade2, his3, can1, leu2, trp1, ura3, are1∆HIS3, are2∆LEU2, mCherry-PUS1::URA3 | MM-55 |
| yeh2∆ | MAT a, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, yeh2ΔkanMX6, mCherry-PUS1::URA3 | MM-60 |
| WT (BY) mCherry-Pus1 | MAT a, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, mCherry-PUS1::URA3 | MM-98 |
| WT (W303) mCherry-Pus1 | MAT a, ade2, his3, can1, leu2, trp1, ura3, GAL+, psi+, RAD5?, mCherry-PUS1::URA3 | MM-102 |
| Rank | Gene | Function | logFC_0.001 | logFC_0.01 | logFC_0.1 | Average |
|---|---|---|---|---|---|---|
| 1 | HMG1 | 3 Hydroxy 3 MethylGlutaryl coenzyme a reductase | −2.2 | −2.8 | −3.0 | −2.7 |
| 2 | OLE1 | OLEic acid requiring | −2.2 | −2.8 | −2.8 | −2.6 |
| 3 | NDE1 | NADH Dehydrogenase, External | −2.1 | −2.5 | −2.5 | −2.4 |
| 4 | COX7 | Cytochrome c OXidase | −1.5 | −1.7 | −3.4 | −2.2 |
| 5 | ERG28 | ERGosterol biosynthesis | −1.2 | −1.8 | −3.5 | −2.2 |
| 6 | ERG3 | ERGosterol biosynthesis | −1.1 | −1.7 | −3.7 | −2.2 |
| 7 | IZH1 | Implicated in Zinc Homeostasis | −1.7 | −2.3 | −2.4 | −2.1 |
| 8 | COX4 | Cytochrome c OXidase | −1.2 | −1.5 | −3.7 | −2.1 |
| 9 | COX5A | Cytochrome c OXidase | −1.5 | −2.0 | −2.6 | −2.0 |
| 10 | ERG25 | ERGosterol biosynthesis | −1.2 | −1.5 | −3.3 | −2.0 |
| 11 | ERG2 | ERGosterol biosynthesis | −1.2 | −1.8 | −3.0 | −2.0 |
| 12 | ERG13 | ERGosterol biosynthesis | −1.5 | −1.6 | −2.7 | −1.9 |
| 13 | COX12 | Cytochrome c OXidase | −1.4 | −1.7 | −2.6 | −1.9 |
| 14 | ERG11 | ERGosterol biosynthesis | −1.1 | −1.7 | −2.8 | −1.9 |
| 15 | HYP2 | HYPusine containing protein | −1.1 | −1.7 | −2.7 | −1.8 |
| 16 | ACS2 | Acetyl CoA Synthetase | −1.3 | −1.7 | −2.5 | −1.8 |
| 17 | MED1 | MEDiator complex | −1.7 | −2.1 | −1.6 | −1.8 |
| 18 | MVD1 | MeValonate pyrophosphate Decarboxylase | −1.4 | −1.8 | −2.1 | −1.8 |
| 19 | ERG1 | ERGosterol biosynthesis | −1.1 | −1.4 | −2.7 | −1.7 |
| 20 | MTC7 | Maintenance of Telomere Capping | −1.1 | −1.6 | −2.4 | −1.7 |
| 21 | SCW11 | Soluble Cell Wall protein | −1.1 | −1.9 | −2.0 | −1.7 |
| 22 | CYT1 | CYTochrome c1 | −1.2 | −1.9 | −1.7 | −1.6 |
| 23 | QCR7 | ubiQuinol cytochrome C oxidoReductase | −1.0 | −1.3 | −2.0 | −1.4 |
| 24 | QCR9 | ubiQuinol cytochrome C oxidoReductase | −1.1 | −1.2 | −2.0 | −1.4 |
| 25 | NOP10 | NucleOlar Protein | −1.1 | −1.2 | −1.7 | −1.3 |
| 26 | ERG7 | ERGosterol biosynthesis | −1.1 | −1.2 | −1.7 | −1.3 |
| 27 | ERG10 | ERGosterol biosynthesis | −1.1 | −1.2 | −1.4 | −1.2 |
| 28 | MSC7 | Meiotic Sister Chromatid recombination | −0.9 | −1.2 | −1.6 | −1.2 |
| 29 | ERG20 | ERGosterol biosynthesis | −1.1 | −1.3 | −1.2 | −1.2 |
| 30 | ERG8 | ERGosterol biosynthesis | −1.3 | −1.4 | −0.8 | −1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumanski, S.; Forey, R.; Cazevieille, C.; Moriel-Carretero, M. Nuclear Lipid Droplet Birth during Replicative Stress. Cells 2022, 11, 1390. https://doi.org/10.3390/cells11091390
Kumanski S, Forey R, Cazevieille C, Moriel-Carretero M. Nuclear Lipid Droplet Birth during Replicative Stress. Cells. 2022; 11(9):1390. https://doi.org/10.3390/cells11091390
Chicago/Turabian StyleKumanski, Sylvain, Romain Forey, Chantal Cazevieille, and María Moriel-Carretero. 2022. "Nuclear Lipid Droplet Birth during Replicative Stress" Cells 11, no. 9: 1390. https://doi.org/10.3390/cells11091390
APA StyleKumanski, S., Forey, R., Cazevieille, C., & Moriel-Carretero, M. (2022). Nuclear Lipid Droplet Birth during Replicative Stress. Cells, 11(9), 1390. https://doi.org/10.3390/cells11091390







