Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Bergamot Polyphenolic Fraction (BPF)
2.3. Preparation of the Mitochondrial Fractions
2.4. Mitochondrial F-ATPase Activity Assays
2.5. Evaluation of Oxidative Phosphorylation
2.6. MPTP Evaluation
2.7. Cell Cultures
2.8. Cellular Metabolism
2.9. Cell Viability
2.10. Statistical Analysis
3. Results
3.1. Effect of the BPF on Isolated Mitochondria
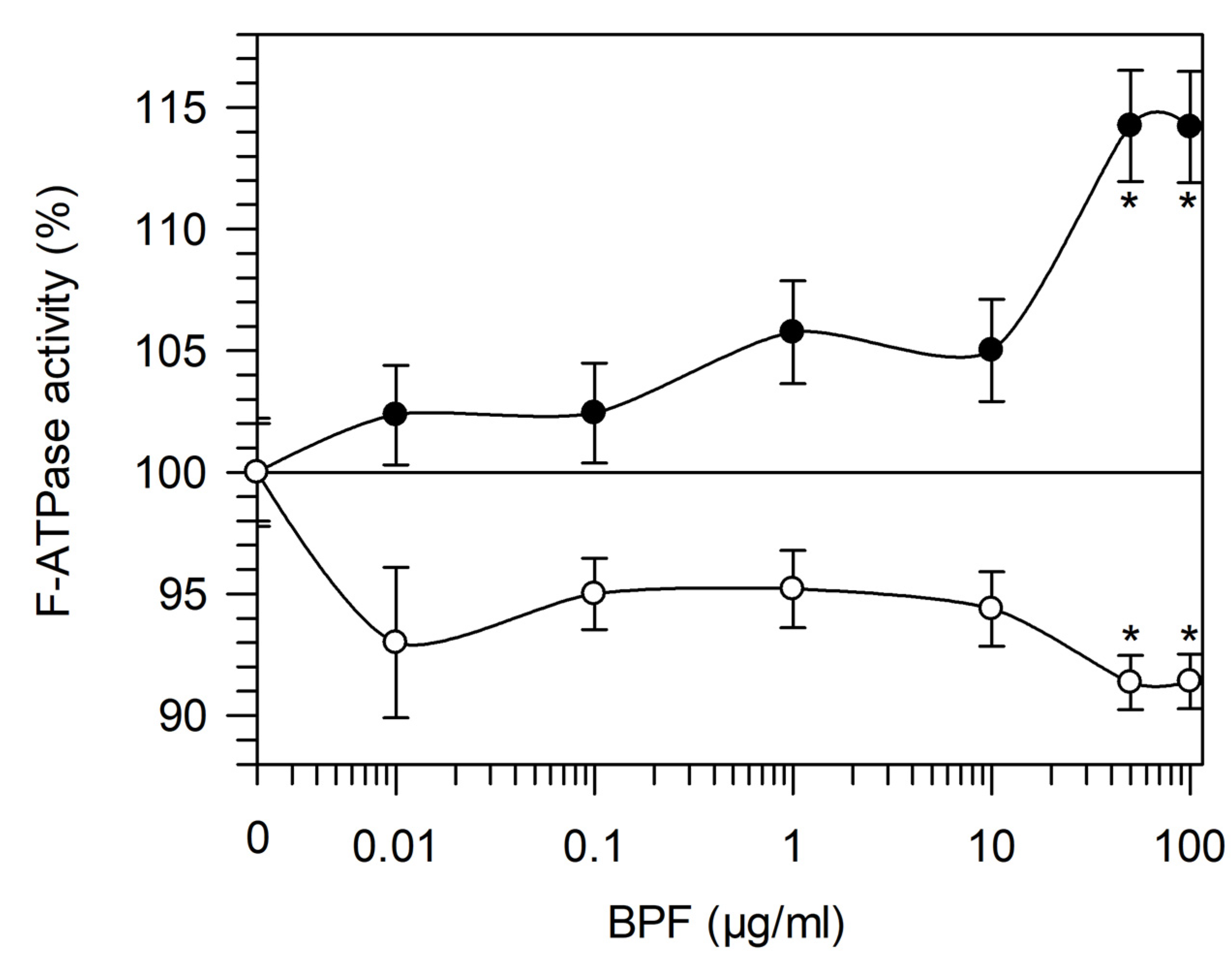
3.1.1. Mg2+- and Ca2+-activated F1FO-ATPase Activity in the Presence of the BPF
3.1.2. BPF Effect on Oxidative Phosphorylation
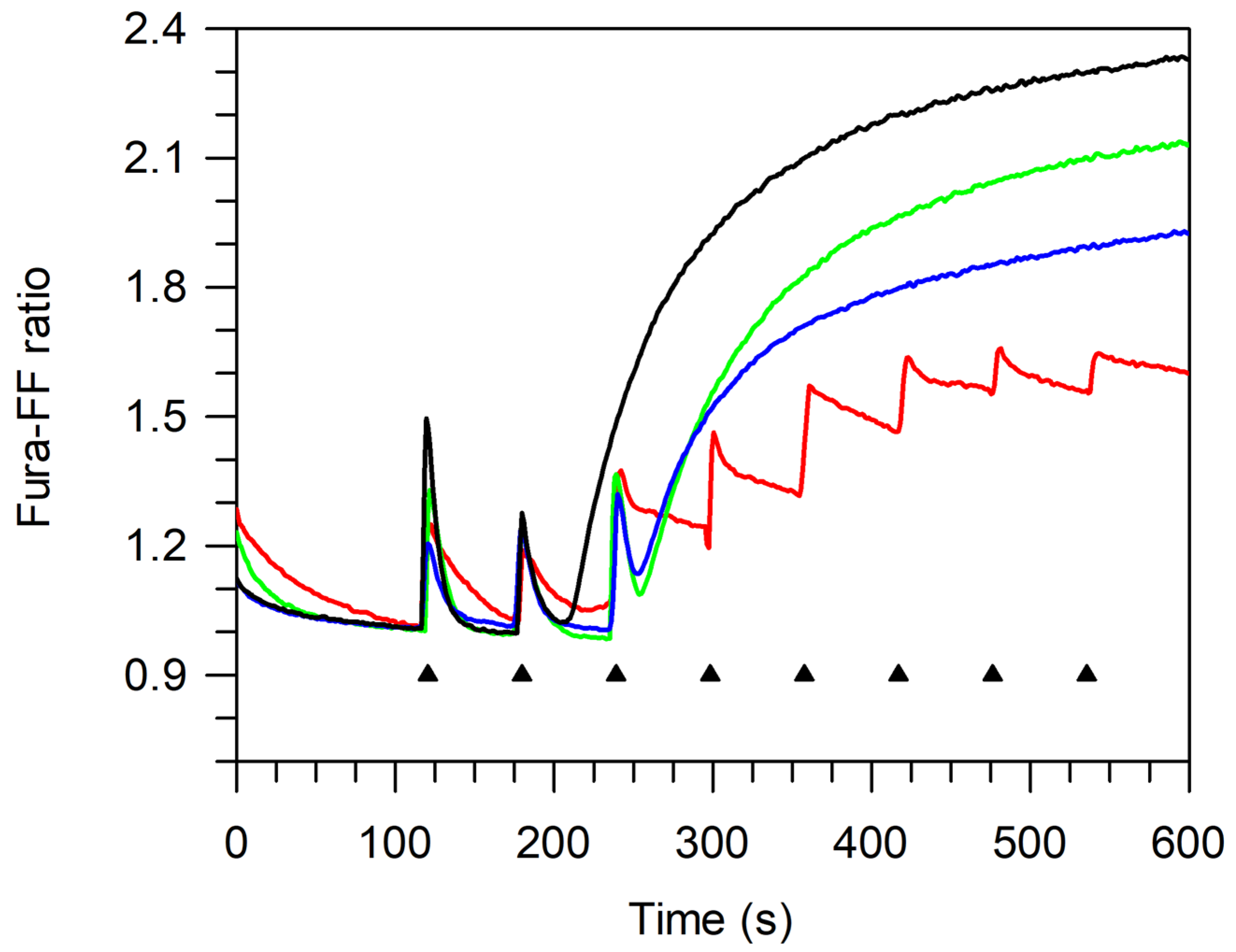
3.1.3. MPTP Desensitization to Ca2+
3.2. Effect of BPF on pAECs
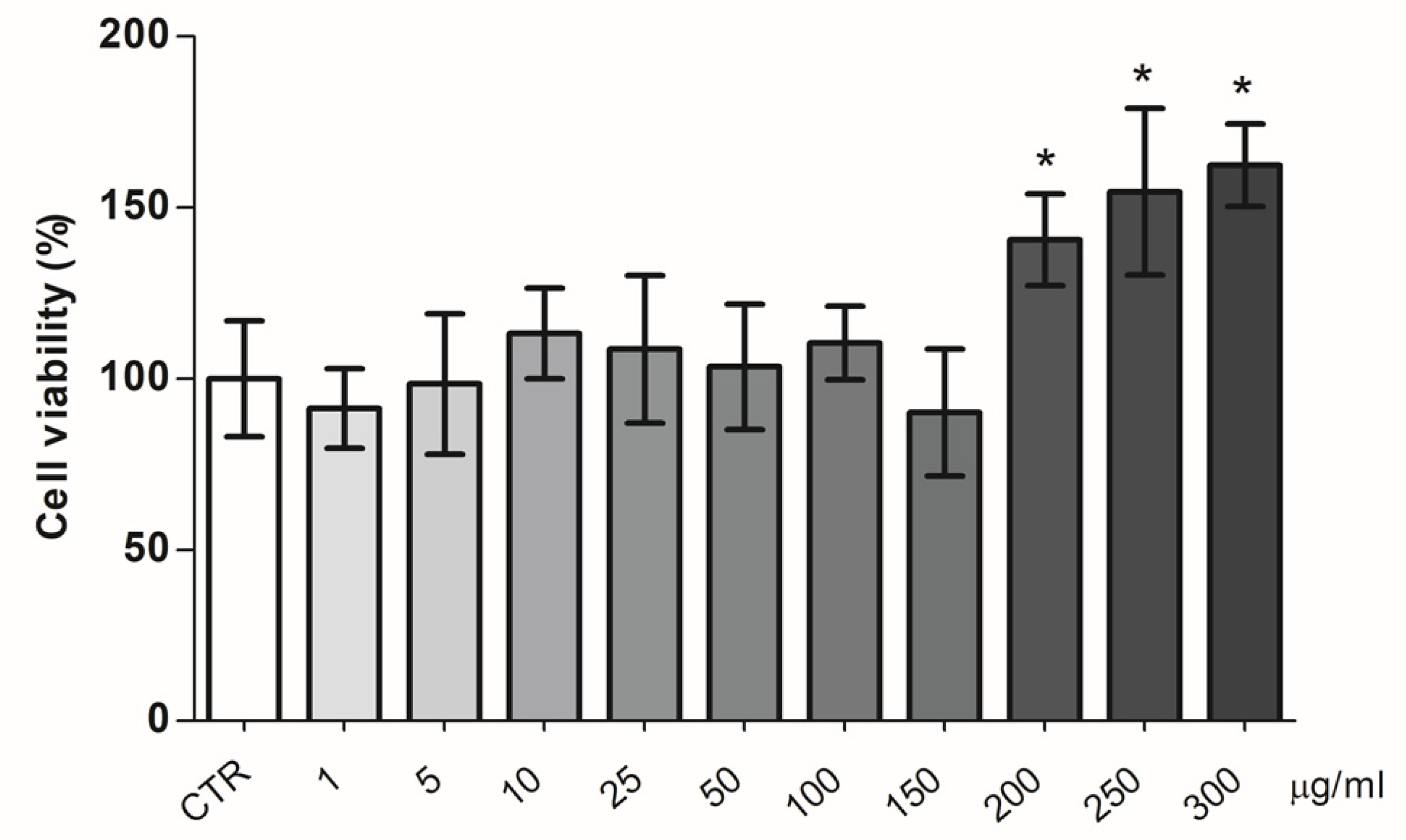
BPF Effect on pAECs’ Cellular Metabolism and Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nesci, S.; Pagliarani, A.; Algieri, C.; Trombetti, F. Mitochondrial F-Type ATP Synthase: Multiple Enzyme Functions Revealed by the Membrane-Embedded FO Structure. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Ishmukhametov, R.; Spetzler, D.; Hornung, T.; Frasch, W.D. Elastic Coupling Power Stroke Mechanism of the F1-ATPase Molecular Motor. Proc. Natl. Acad. Sci. USA 2018, 115, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Moghimyfiroozabad, S.; Safaie, S.; Yang, Y.; Jonas, E.A.; Alavian, K.N. Phylogenetic Profiling of Mitochondrial Proteins and Integration Analysis of Bacterial Transcription Units Suggest Evolution of F1Fo ATP Synthase from Multiple Modules. J. Mol. Evol. 2017, 85, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, S.; Frasch, W.D. Protonation-Dependent Stepped Rotation of the F-Type ATP Synthase c-Ring Observed by Single-Molecule Measurements. J. Biol. Chem. 2017, 292, 17093–17100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junge, W.; Nelson, N. ATP Synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.M.; Anselmi, C.; Wittig, I.; Faraldo-Gómez, J.D.; Kühlbrandt, W. Structure of the Yeast F1Fo-ATP Synthase Dimer and Its Role in Shaping the Mitochondrial Cristae. Proc. Natl. Acad. Sci. USA 2012, 109, 13602–13607. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Moyle, J. Respiration-Driven Proton Translocation in Rat Liver Mitochondria. Biochem. J. 1967, 105, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Pinke, G.; Zhou, L.; Sazanov, L.A. Cryo-EM Structure of the Entire Mammalian F-Type ATP Synthase. Nat. Struct. Mol. Biol. 2020, 27, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Alavian, K.N.; Beutner, G.; Lazrove, E.; Sacchetti, S.; Park, H.-A.; Licznerski, P.; Li, H.; Nabili, P.; Hockensmith, K.; Graham, M.; et al. An Uncoupling Channel within the C-Subunit Ring of the F1FO ATP Synthase Is the Mitochondrial Permeability Transition Pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10580–10585. [Google Scholar] [CrossRef] [Green Version]
- Bonora, M.; Bononi, A.; De Marchi, E.; Giorgi, C.; Lebiedzinska, M.; Marchi, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; Wojtala, A.; et al. Role of the c Subunit of the FO ATP Synthase in Mitochondrial Permeability Transition. Cell Cycle 2013, 12, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Nesci, S.; Pagliarani, A. Incoming News on the F-Type ATPase Structure and Functions in Mammalian Mitochondria. BBA Adv. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Izzo, V.; Bravo-San Pedro, J.M.; Sica, V.; Kroemer, G.; Galluzzi, L. Mitochondrial Permeability Transition: New Findings and Persisting Uncertainties. Trends Cell Biol. 2016, 26, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S. The Mitochondrial Permeability Transition Pore in Cell Death: A Promising Drug Binding Bioarchitecture. Med. Res. Rev. 2020, 40, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Algieri, C.; Pagliarani, A. A Therapeutic Role for the F1FO-ATP Synthase. SLAS Discov. 2019, 24, 893–903. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients 2021, 13, 2476. [Google Scholar] [CrossRef]
- Nesci, S.; Palma, E.; Mollace, V.; Romeo, G.; Oppedisano, F. Enjoy Your Journey: The Bergamot Polyphenols from the Tree to the Cell Metabolism. J. Transl. Med. 2021, 19, 457. [Google Scholar] [CrossRef]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of Flavonoids in Citrus Bergamia (Bergamot) Polyphenolic Fraction by Liquid Chromatography–High Resolution Mass Spectrometry (LC/HRMS). PharmaNutrition 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Mollace, V. The Effect of Bergamot Polyphenolic Fraction in Patients with Non Alcoholic Liver Steato-Hepatitis and Metabolic Syndrome. PharmaNutrition 2016, 4, S27–S31. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara Cardunculus, and Olea Europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Pandolfo, G.; Crucitti, M.; Cedro, C.; Zoccali, R.A.; Muscatello, M.R.A. Bergamot Polyphenolic Fraction Supplementation Improves Cognitive Functioning in Schizophrenia: Data From an 8-Week, Open-Label Pilot Study. J. Clin. Psychopharmacol. 2017, 37, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-Oxidant Effect of Bergamot Polyphenolic Fraction Counteracts Doxorubicin-Induced Cardiomyopathy: Role of Autophagy and c-KitposCD45negCD31neg Cardiac Stem Cell Activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Ventrella, V.; Trombetti, F.; Pirini, M.; Pagliarani, A. Thiol Oxidation Is Crucial in the Desensitization of the Mitochondrial F1FO-ATPase to Oligomycin and Other Macrolide Antibiotics. Biochim. Biophys. Acta 2014, 1840, 1882–1891. [Google Scholar] [CrossRef]
- Nesci, S.; Ventrella, V.; Trombetti, F.; Pirini, M.; Pagliarani, A. Preferential Nitrite Inhibition of the Mitochondrial F1FO-ATPase Activities When Activated by Ca(2+) in Replacement of the Natural Cofactor Mg(2+). Biochim. Biophys. Acta 2016, 1860, 345–353. [Google Scholar] [CrossRef]
- Nesci, S.; Ventrella, V.; Trombetti, F.; Pirini, M.; Pagliarani, A. The Mitochondrial F1FO-ATPase Desensitization to Oligomycin by Tributyltin Is Due to Thiol Oxidation. Biochimie 2014, 97, 128–137. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbar, Y.S. The Colorimetric Determination of Phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory Enzymes in Oxidative Phosphorylation. III. The Steady State. J. Biol. Chem. 1955, 217, 409–427. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R.; Holmes, W.F.; Higgins, J. Respiratory Enzymes in Oxidative Phosphorylation: V. A Mechanism for Oxidative Phosphorylation. J. Biol. Chem. 1955, 217, 439–451. [Google Scholar] [CrossRef]
- Nesci, S.; Algieri, C.; Trombetti, F.; Ventrella, V.; Fabbri, M.; Pagliarani, A. Sulfide Affects the Mitochondrial Respiration, the Ca2+-Activated F1FO-ATPase Activity and the Permeability Transition Pore but Does Not Change the Mg2+-Activated F1FO-ATPase Activity in Swine Heart Mitochondria. Pharmacol. Res. 2021, 166, 105495. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Fabbri, M.; Nesci, S. The Inhibition of Gadolinium Ion (Gd3+) on the Mitochondrial F1FO-ATPase Is Linked to the Modulation of the Mitochondrial Permeability Transition Pore. Int. J. Biol. Macromol. 2021, 184, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; Zannoni, A.; Turba, M.E.; Fantinati, P.; Tamanini, C.; Bacci, M.L.; Forni, M. Heat Shock Protein 70, Heat Shock Protein 32, and Vascular Endothelial Growth Factor Production and Their Effects on Lipopolysaccharide-Induced Apoptosis in Porcine Aortic Endothelial Cells. Cell Stress Chaperones 2005, 10, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardini, C.; Algieri, C.; La Mantia, D.; Zannoni, A.; Salaroli, R.; Trombetti, F.; Forni, M.; Pagliarani, A.; Nesci, S. Relationship between Serum Concentration, Functional Parameters and Cell Bioenergetics in IPEC-J2 Cell Line. Histochem. Cell Biol. 2021, 156, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Algieri, C.; Tioli, G.; Lenaz, G. Molecular and Supramolecular Structure of the Mitochondrial Oxidative Phosphorylation System: Implications for Pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial Disorders of the OXPHOS System. FEBS Lett. 2021, 595, 1062–1106. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Nesci, S.; Trombetti, F.; Ventrella, V.; Pirini, M.; Pagliarani, A. Kinetic Properties of the Mitochondrial F1FO-ATPase Activity Elicited by Ca(2+) in Replacement of Mg(2+). Biochimie 2017, 140, 73–81. [Google Scholar] [CrossRef]
- Nesci, S.; Pagliarani, A. Ca2+ as Cofactor of the Mitochondrial H+-Translocating F1FO-ATP(Hydrol)Ase. Proteins Struct. Funct. Bioinform. 2021, 89, 477–482. [Google Scholar] [CrossRef]
- Urbani, A.; Giorgio, V.; Carrer, A.; Franchin, C.; Arrigoni, G.; Jiko, C.; Abe, K.; Maeda, S.; Shinzawa-Itoh, K.; Bogers, J.F.M.; et al. Purified F-ATP Synthase Forms a Ca2+-Dependent High-Conductance Channel Matching the Mitochondrial Permeability Transition Pore. Nat. Commun. 2019, 10, 4341. [Google Scholar] [CrossRef] [Green Version]
- Mnatsakanyan, N.; Llaguno, M.C.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.J.; Jonas, E.A. A Mitochondrial Megachannel Resides in Monomeric F1FO ATP Synthase. Nat. Commun. 2019, 10, 5823. [Google Scholar] [CrossRef]
- Nesci, S.; Lenaz, G. The Mitochondrial Energy Conversion Involves Cytochrome c Diffusion into the Respiratory Supercomplexes. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148394. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Bernardini, C.; Fabbri, M.; Forni, M.; Nesci, S. Mitochondrial Ca2+-Activated F1 FO-ATPase Hydrolyzes ATP and Promotes the Permeability Transition Pore. Ann. N. Y. Acad. Sci. 2019, 1457, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Ventrella, V.; Trombetti, F.; Pirini, M.; Pagliarani, A. Multi-Site TBT Binding Skews the Inhibition of Oligomycin on the Mitochondrial Mg-ATPase in Mytilus Galloprovincialis. Biochimie 2011, 93, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Nesci, S. The Mitochondrial F1FO-ATPase Exploits the Dithiol Redox State to Modulate the Permeability Transition Pore. Arch. Biochem. Biophys. 2021, 712, 109027. [Google Scholar] [CrossRef]
- Algieri, V.; Algieri, C.; Maiuolo, L.; De Nino, A.; Pagliarani, A.; Tallarida, M.A.; Trombetti, F.; Nesci, S. 1,5-Disubstituted-1,2,3-Triazoles as Inhibitors of the Mitochondrial Ca2+-Activated F1 FO-ATP(Hydrol)Ase and the Permeability Transition Pore. Ann. N. Y. Acad. Sci. 2021, 1485, 43–55. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Nesci, S. Phenylglyoxal Inhibition of the Mitochondrial F1FO-ATPase Activated by Mg2+ or by Ca2+ Provides Clues on the Mitochondrial Permeability Transition Pore. Arch. Biochem. Biophys. 2020, 681, 108258. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Jonas, E.A. ATP Synthase C-Subunit Ring as the Channel of Mitochondrial Permeability Transition: Regulator of Metabolism in Development and Degeneration. J. Mol. Cell. Cardiol. 2020, 144, 109–118. [Google Scholar] [CrossRef]
- Casadio, R.; Melandri, B.A. CaATP Inhibition of the MgATP-Dependent Proton Pump (H+-ATPase) in Bacterial Photosynthetic Membranes with a Mechanism of Alternative Substrate Inhibition. JBIC 1996, 1, 284–291. [Google Scholar] [CrossRef]
- Giorgio, V.; Burchell, V.; Schiavone, M.; Bassot, C.; Minervini, G.; Petronilli, V.; Argenton, F.; Forte, M.; Tosatto, S.; Lippe, G.; et al. Ca(2+) Binding to F-ATP Synthase β Subunit Triggers the Mitochondrial Permeability Transition. EMBO Rep. 2017, 18, 1065–1076. [Google Scholar] [CrossRef]
- Garone, C.; Pietra, A.; Nesci, S. From the Structural and (Dys)Function of ATP Synthase to Deficiency in Age-Related Diseases. Life 2022, 12, 401. [Google Scholar] [CrossRef]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, e2100670. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Nesci, S. Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells 2022, 11, 1401. https://doi.org/10.3390/cells11091401
Algieri C, Bernardini C, Oppedisano F, La Mantia D, Trombetti F, Palma E, Forni M, Mollace V, Romeo G, Nesci S. Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells. 2022; 11(9):1401. https://doi.org/10.3390/cells11091401
Chicago/Turabian StyleAlgieri, Cristina, Chiara Bernardini, Francesca Oppedisano, Debora La Mantia, Fabiana Trombetti, Ernesto Palma, Monica Forni, Vincenzo Mollace, Giovanni Romeo, and Salvatore Nesci. 2022. "Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction" Cells 11, no. 9: 1401. https://doi.org/10.3390/cells11091401
APA StyleAlgieri, C., Bernardini, C., Oppedisano, F., La Mantia, D., Trombetti, F., Palma, E., Forni, M., Mollace, V., Romeo, G., & Nesci, S. (2022). Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells, 11(9), 1401. https://doi.org/10.3390/cells11091401








