Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Zebrafish Anesthesia, Pigmentation Prevention, Dechorionation, and Euthanasia
2.3. Virus and Primary Infection
2.4. ZIKV Transmission
2.5. RNA Isolation and qRT-PCR
2.6. Phenotype-Based Screening
2.7. Zebrafish Locomotor Behavior Assessment
2.8. Histology of the Eyes
2.9. Statistical Analysis
3. Results
3.1. ZIKV Infection of One-Cell Stage Embryo Induces Mortality and Abnormal Development, and It Triggers Antiviral Innate Immune Response
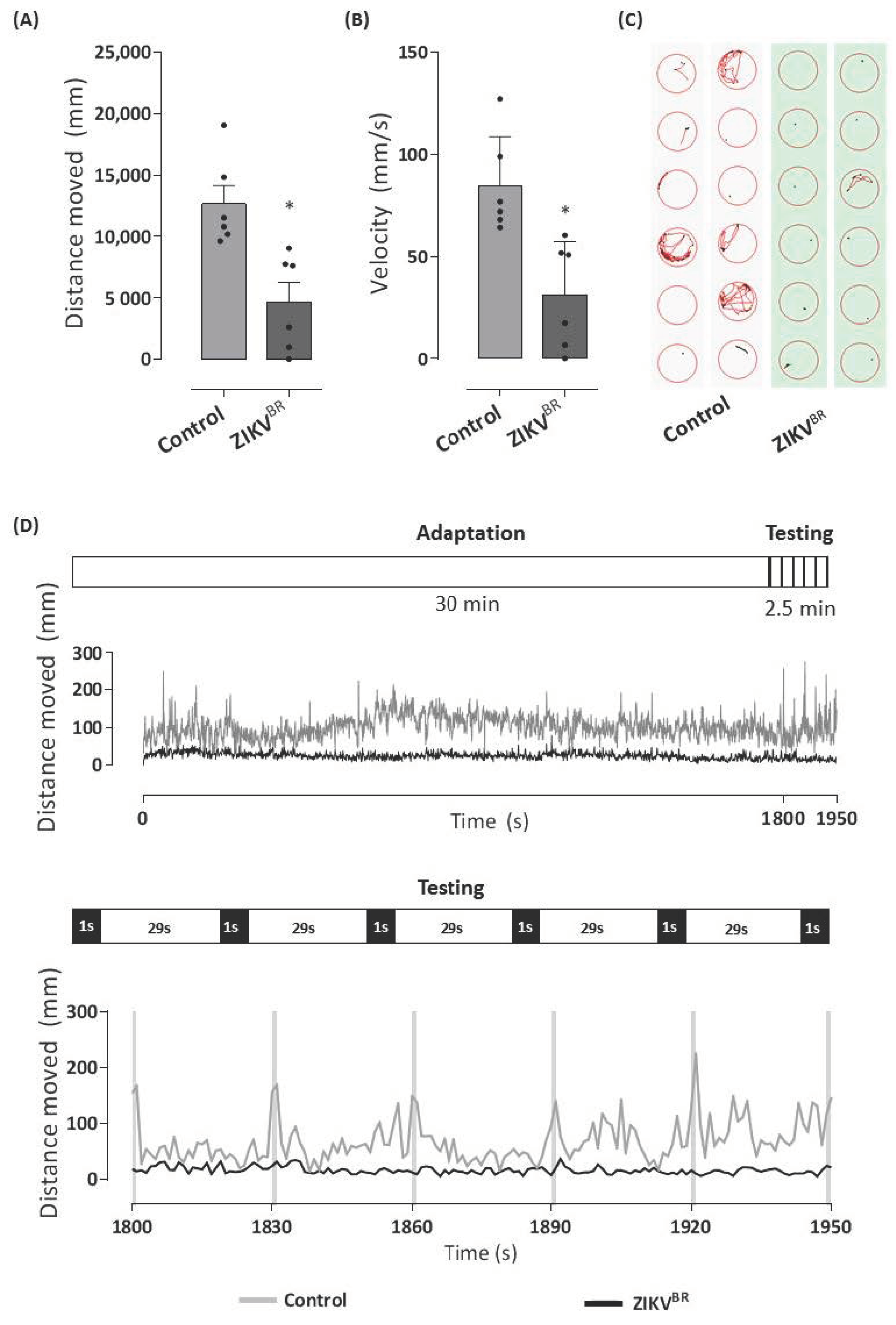
3.2. ZIKV-Infected Zebrafish Larvae Show Motor Disorders and Signs of Visual Impairment
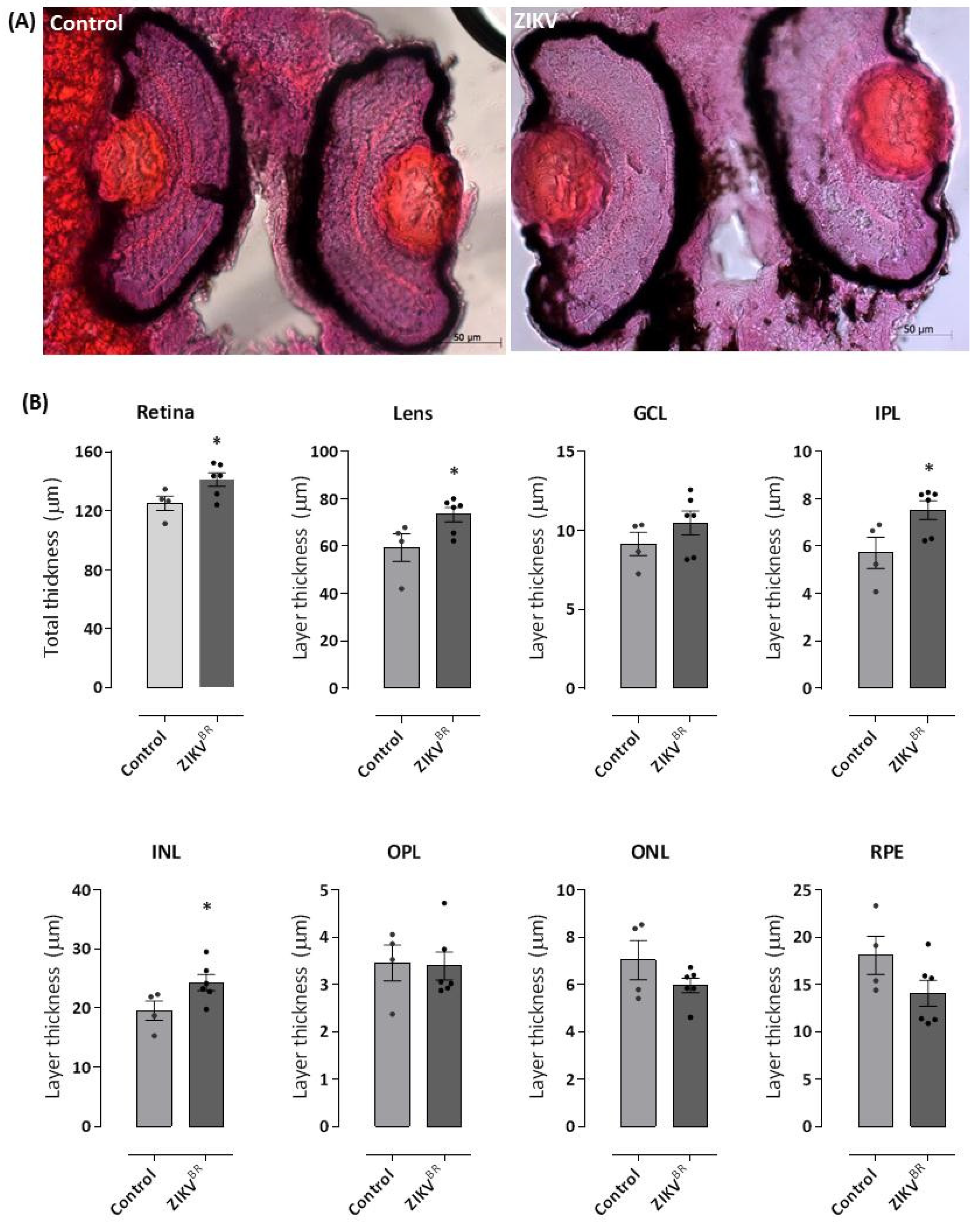
3.3. ZIKV Alters the Thickness of Retinal Cell Layers
3.4. Zebrafish Larvae Serve as Vectors for ZIKV Replication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanluca, C.; dos Santos, C.N.D. Zika virus—An overview. Microbes Infect. 2016, 18, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; da Silva Azevedo, R.D.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell 2017, 169, 610–620.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, K.M.; Song, H.; Ming, G.-L. Pathophysiology and Mechanisms of Zika Virus Infection in the Nervous System. Annu. Rev. Neurosci. 2019, 42, 249–269. [Google Scholar] [CrossRef]
- De Paula Freitas, B.; Zin, A.; Ko, A.; Maia, M.; Ventura, C.V.; Belfort, R., Jr. Anterior-Segment Ocular Findings and Microphthalmia in Congenital Zika Syndrome. Ophthalmology 2017, 124, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Oo, H.H.; Balne, P.K.; Ng, L.; Tong, L.; Leo, Y.S. Zika Virus and the Eye. Ocul. Immunol. Inflamm. 2017, 26, 654–659. [Google Scholar] [CrossRef]
- Lima, D.D.S.; Baran, L.C.P.; Hamer, R.D.; da Costa, M.F.; Vidal, K.S.; Damico, F.M.; Barboni, M.T.S.; França, V.D.C.R.D.M.; Martins, C.M.G.; Tabares, H.S.; et al. Longitudinal visual acuity development in ZIKV-exposed children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 23.e1–23.e6. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Moshfeghi, D.M.; de Miranda, H.A.; Costa, M.C. Zika Virus, Microcephaly, and Ocular Findings. JAMA Ophthalmol. 2016, 134, 945. [Google Scholar] [CrossRef]
- Freitas, B.D.P.; Dias, J.R.D.O.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R. Ocular Findings in Infants with Microcephaly Associated with Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, K. Optical coherence tomography in congenital Zika syndrome. Arq. Bras. Oftalmol. 2017, 80, 138. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Filho, M.C.V.; Ventura, L.O. Ocular Manifestations and Visual Outcome in Children with Congenital Zika Syndrome. Top. Magn. Reson. Imaging 2019, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Farr, D.; Kumar, A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Alcendor, D.J. Zika virus infection of cellular components of the blood-retinal barriers: Implications for viral associated congenital ocular disease. J. Neuroinflamm. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrazzo, P.; Cricca, M.; Nastasi, C. Are the Organoid Models an Invaluable Contribution to ZIKA Virus Research? Pathogens 2021, 10, 1233. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.G.W. Recovering the Principles of Humane Experimental Technique. Sci. Technol. Hum. Values 2018, 43, 622–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, M.; Léonard, M.; Iguchi, T.; Oris, J.; Ryder, K.; Belanger, S.; Braunbeck, T.; Embry, M.R.; Whale, G.; Norberg-King, T.; et al. Regulatory aspects on the use of fish embryos in environmental toxicology. Integr. Environ. Assess. Manag. 2010, 6, 484–491. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; Ventura Fernandes, B.H.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Grejo Setti, P.; Roça, R.D.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
- Burden, N.; Benstead, R.; Benyon, K.; Clook, M.; Green, C.; Handley, J.; Harper, N.; Maynard, S.K.; Mead, C.; Pearson, A.; et al. Key Opportunities to Replace, Reduce, and Refine Regulatory Fish Acute Toxicity Tests. Environ. Toxicol. Chem. 2020, 39, 2076–2089. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–78. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF. (accessed on 24 June 2021).
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye—A paradigm for investigating human ocular genetics. Eye 2016, 31, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Greiling, T.M.S.; Aose, M.; Clark, J.I. Cell Fate and Differentiation of the Developing Ocular Lens. Investig. Opthalmol. Vis. Sci. 2010, 51, 1540–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavodeassi, F.; Wilson, S.W. Looking to the future of zebrafish as a model to understand the genetic basis of eye disease. Qual. Life Res. 2019, 138, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Binesh, C.P. Mortality due to viral nervous necrosis in zebrafish Danio rerio and goldfish Carassius auratus. Dis. Aquat. Org. 2013, 104, 257–260. [Google Scholar] [CrossRef]
- Bermúdez, R.; Losada, A.P.; de Azevedo, A.M.; Guerra-Varela, J.; Pérez-Fernández, D.; Sánchez, L.; Padrós, F.; Nowak, B.; Quiroga, M.I. First description of a natural infection with spleen and kidney necrosis virus in zebrafish. J. Fish Dis. 2018, 41, 1283–1294. [Google Scholar] [CrossRef]
- Altan, E.; Kubiski, S.V.; Boros, Á.; Reuter, G.; Sadeghi, M.; Deng, X.; Creighton, E.K.; Crim, M.J.; Delwart, E. A Highly Divergent Picornavirus Infecting the Gut Epithelia of Zebrafish (Danio rerio) in Research Institutions Worldwide. Zebrafish 2019, 16, 291–299. [Google Scholar] [CrossRef]
- Encinas, P.; Rodriguez-Milla, M.A.; Novoa, B.; Estepa, A.; Figueras, A.; Coll, J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. A proteomic and transcriptomic approach. BMC Genom. 2010, 11, 518. [Google Scholar] [CrossRef] [Green Version]
- López-Muñoz, A.; Roca, F.J.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Zebrafish larvae are unable to mount a protective antiviral response against waterborne infection by spring viremia of carp virus. Dev. Comp. Immunol. 2010, 34, 546–552. [Google Scholar] [CrossRef]
- Ludwig, M.; Palha, N.; Torhy, C.; Briolat, V.; Colucci-Guyon, E.; Brémont, M.; Herbomel, P.; Boudinot, P.; Levraud, J.-P. Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae. PLoS Pathog. 2011, 7, e1001269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojzesz, M.; Widziolek, M.; Adamek, M.; Orzechowska, U.; Podlasz, P.; Prajsnar, T.K.; Pooranachandran, N.; Pecio, A.; Michalik, A.; Surachetpong, W.; et al. Tilapia Lake Virus-Induced Neuroinflammation in Zebrafish: Microglia Activation and Sickness Behavior. Front. Immunol. 2021, 12, 760882. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.S.; Ripoll-Gomez, J.; Alfaro, J.M.; Sastre, I.; Valdivieso, F. Zebrafish as a New Model for Herpes Simplex Virus Type 1 Infection. Zebrafish 2008, 5, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.E.; Jones, K.S.; Dale, R.M.; Shukla, D.; Tiwari, V. Zebrafish: Modeling for Herpes Simplex Virus Infections. Zebrafish 2014, 11, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shieh, Y.-S.; Chang, Y.-S.; Hong, J.-R.; Chen, L.-J.; Jou, L.-K.; Hsu, C.-C.; Her, G.M. Increase of hepatic fat accumulation by liver specific expression of Hepatitis B virus X protein in zebrafish. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.-B.; Zhang, J.-P.; Zhao, Y.; Peng, Z.-G.; Song, D.-Q.; Jiang, J.-D. Zebrafish as a Potential Model Organism for Drug Test Against Hepatitis C Virus. PLoS ONE 2011, 6, e22921. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.-B.; Zhao, Y.; Zhang, J.-P.; Peng, Z.-G.; Song, D.-Q.; Jiang, J.-D. A zebrafish model for subgenomic hepatitis C virus replication. Int. J. Mol. Med. 2015, 35, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.-H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-Time Whole-Body Visualization of Chikungunya Virus Infection and Host Interferon Response in Zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef] [Green Version]
- Gabor, K.A.; Goody, M.F.; Mowel, W.; Breitbach, M.E.; Gratacap, R.L.; Witten, P.E.; Kim, C.H. Influenza a virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. Dis. Model. Mech. 2014, 7, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Passoni, G.; Langevin, C.; Palha, N.; Mounce, B.C.; Briolat, V.; Affaticati, P.; De Job, E.; Joly, J.-S.; Vignuzzi, M.; Saleh, M.-C.; et al. Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes. Dis. Model. Mech. 2017, 10, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Varela, J.; Baz-Martínez, M.; Da Silva-Álvarez, S.; Losada, A.P.; Quiroga, M.I.; Collado, M.; Rivas, C.; Sánchez, L. Susceptibility of Zebrafish to Vesicular Stomatitis Virus Infection. Zebrafish 2018, 15, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Van Dycke, J.; Ny, A.; Conceição-Neto, N.; Maes, J.; Hosmillo, M.; Cuvry, A.; Goodfellow, I.; Nogueira, T.C.; Verbeken, E.; Matthijnssens, J.; et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019, 15, e1008009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkrishna, A.; Solleti, S.K.; Verma, S.; Varshney, A. Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati. Biomolecules 2020, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Rückert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Guest, J.-M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.-S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2, e92340. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, M.; Azar, S.R.; Soong, L.; Weaver, S.; Sun, J.; Chen, Y.; Rossi, S.L.; Cai, J. Viral Retinopathy in Experimental Models of Zika Infection. Investig. Opthalmology Vis. Sci. 2017, 58, 4355–4365. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Kielczewski, J.L.; Sen, H.N.; Xu, B.C.; Ireland, D.D.C.; McWilliams, I.L.; Chan, C.-C.; Caspi, R.R.; Verthelyi, D. ZIKA virus infection causes persistent chorioretinal lesions. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.; Rengifo, A.C.; Santamaría, G.; Corchuelo, S.; Alvarez-Diaz, D.A.; Parra, E.A.; Naizaque, J.; Gomez, J.A.M.; Torres-Fernández, O. Inmunorreacción de la infección por el virus de Zika en retina de ratones. Biomédica 2019, 39, 8–10. [Google Scholar] [CrossRef]
- Singh, P.K.; Kasetti, R.B.; Zode, G.S.; Goyal, A.; Juzych, M.S.; Kumar, A. Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes. mSphere 2019, 4, e00173-19. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, S.; Xia, F.; Shan, C.; Ha, Y.; Zou, J.; Adam, A.; Zhang, M.; Wang, T.; Liu, H.; et al. Zika virus induces neuronal and vascular degeneration in developing mouse retina. Acta Neuropathol. Commun. 2021, 9, 97. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Wahba, M.; Yu, L.; Achari, G.; Habibi, H.R. Health Impact Assessment of Sulfolane on Embryonic Development of Zebrafish (Danio rerio). Toxics 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, C.A.; Marsden, A.N.; Slusarski, D.C. Automated, high-throughput, in vivo analysis of visual function using the zebrafish. Dev. Dyn. 2016, 245, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.L.; Shive, H.R. Sequential Immunofluorescence and Immunohistochemistry on Cryosectioned Zebrafish Embryos. J. Vis. Exp. 2019, 14, e59344. [Google Scholar] [CrossRef] [Green Version]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Q.; Tiwari, S.K.; Lichinchi, G.; Yau, E.H.; Hui, H.; Li, W.; Furnari, F.; Rana, T.M. Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Rep. 2020, 30, 969–983.e4. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Zou, P.F.; Nie, P. Innate Antiviral Immunity. In Methods in Molecular Biology; Mossman, K., Ed.; Springer: New York, NY, USA, 2017; Volume 1656, ISBN 978-1-4939-7236-4. [Google Scholar]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.-P. The Two Groups of Zebrafish Virus-Induced Interferons Signal via Distinct Receptors with Specific and Shared Chains. J. Immunol. 2009, 183, 3924–3931. [Google Scholar] [CrossRef] [Green Version]
- Hamming, O.J.; Lutfalla, G.; Levraud, J.-P.; Hartmann, R. Crystal Structure of Zebrafish Interferons I and II Reveals Conservation of Type I Interferon Structure in Vertebrates. J. Virol. 2011, 85, 8181–8187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Navarro, F.J.; Martínez-Morcillo, F.J.; de Oliveira, S.; Candel, S.; Cabas, I.; García-Ayala, A.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-Del-Castillo, P.; Cayuela, M.L.; et al. Hydrogen peroxide in neutrophil inflammation: Lesson from the zebrafish. Dev. Comp. Immunol. 2020, 105, 103583. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.; Mulero, V.; Calado, A. Cxcl8 (IL-8) Mediates Neutrophil Recruitment and Behavior in the Zebrafish Inflammatory Response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kono, T.; Monte, M.M.; Kuse, H.; Costa, M.M.; Korenaga, H.; Maehr, T.; Husain, M.; Sakai, M.; Secombes, C.J. Identification of IL-34 in teleost fish: Differential expression of rainbow trout IL-34, MCSF1 and MCSF2, ligands of the MCSF receptor. Mol. Immunol. 2013, 53, 398–409. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.; Flores, M.V.; Oehlers, S.; Sanderson, L.E.; Ni Lam, E.Y.; Crosier, K.E.; Crosier, P.S. Infection-Responsive Expansion of the Hematopoietic Stem and Progenitor Cell Compartment in Zebrafish Is Dependent upon Inducible Nitric Oxide. Cell Stem Cell 2012, 10, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Padilla, S.; Hunter, D.; Padnos, B.; Frady, S.; MacPhail, R. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef]
- Lee, S.-B.; Choe, Y.; Chon, T.-S.; Kang, H.Y. Analysis of zebrafish (Danio rerio) behavior in response to bacterial infection using a self-organizing map. BMC Vet. Res. 2015, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; de Brito, T.M.; Colmanetti, R.; Pontes, A.A.A.; de Castro, H.M.; de Lacerda, R.I.T.; Morato, S.; Gouveia, A. Parametric analyses of anxiety in zebrafish scototaxis. Behav. Brain Res. 2010, 210, 1–7. [Google Scholar] [CrossRef]
- Midttun, H.L.E.; Vindas, M.A.; Nadler, L.E.; Øverli, Ø.; Johansen, I.B. Behavioural effects of the common brain-infecting parasite Pseudoloma neurophilia in laboratory zebrafish (Danio rerio). Sci. Rep. 2020, 10, 8083. [Google Scholar] [CrossRef]
- Thomas, J.L.; Ranski, A.H.; Morgan, G.W.; Thummel, R. Reactive gliosis in the adult zebrafish retina. Exp. Eye Res. 2016, 143, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular–Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Vorontsova, I.; Hoshino, M.; Uesugi, K.; Yagi, N.; Hall, J.E.; Schilling, T.F.; Pierscionek, B.K. Optical development in the zebrafish eye lens. FASEB J. 2020, 34, 5552–5562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maucourant, C.; Queiroz, G.A.N.; Samri, A.; Grassi, M.F.R.; Yssel, H.; Vieillard, V. Zika virus in the eye of the cytokine storm. Eur. Cytokine Netw. 2019, 30, 74–81. [Google Scholar] [PubMed]
- Simonin, Y.; Erkilic, N.; Damodar, K.; Clé, M.; Desmetz, C.; Bolloré, K.; Taleb, M.; Torriano, S.; Barthelemy, J.; Dubois, G.; et al. Zika virus induces strong inflammatory responses and impairs homeostasis and function of the human retinal pigment epithelium. eBioMedicine 2019, 39, 315–331. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleski, A.L.A.; Rosa, J.G.S.; Bernardo, J.T.G.; Astray, R.M.; Walker, C.I.B.; Lopes-Ferreira, M.; Lima, C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells 2022, 11, 1457. https://doi.org/10.3390/cells11091457
Maleski ALA, Rosa JGS, Bernardo JTG, Astray RM, Walker CIB, Lopes-Ferreira M, Lima C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells. 2022; 11(9):1457. https://doi.org/10.3390/cells11091457
Chicago/Turabian StyleMaleski, Adolfo Luis Almeida, Joao Gabriel Santos Rosa, Jefferson Thiago Gonçalves Bernardo, Renato Mancini Astray, Cristiani Isabel Banderó Walker, Monica Lopes-Ferreira, and Carla Lima. 2022. "Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus" Cells 11, no. 9: 1457. https://doi.org/10.3390/cells11091457
APA StyleMaleski, A. L. A., Rosa, J. G. S., Bernardo, J. T. G., Astray, R. M., Walker, C. I. B., Lopes-Ferreira, M., & Lima, C. (2022). Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells, 11(9), 1457. https://doi.org/10.3390/cells11091457








