Improving Microalgal Biomass Productivity Using Weather-Forecast-Informed Operations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
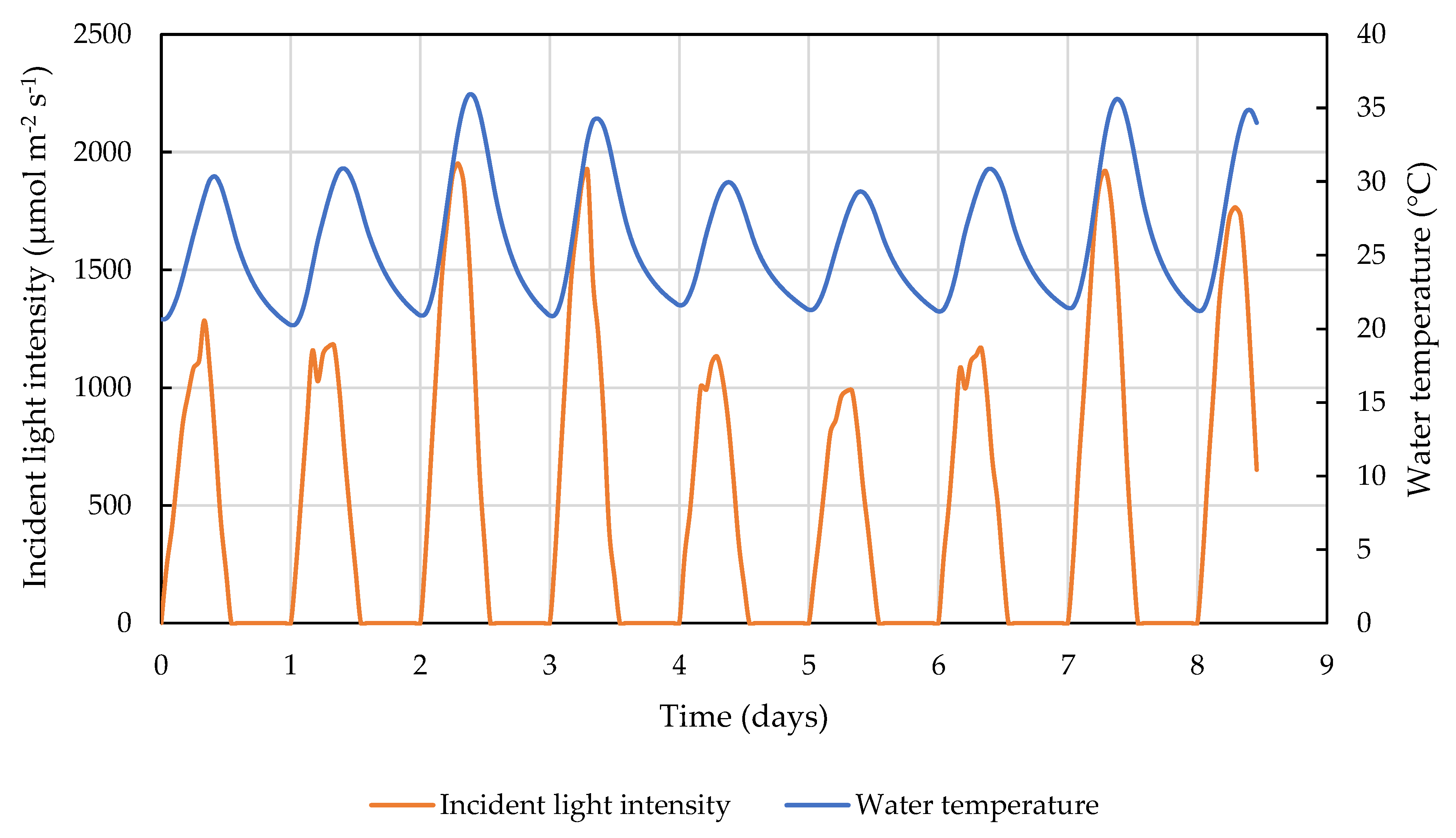
2.2. Outdoor Pond Condition Simulation
2.3. Operational Scenarios
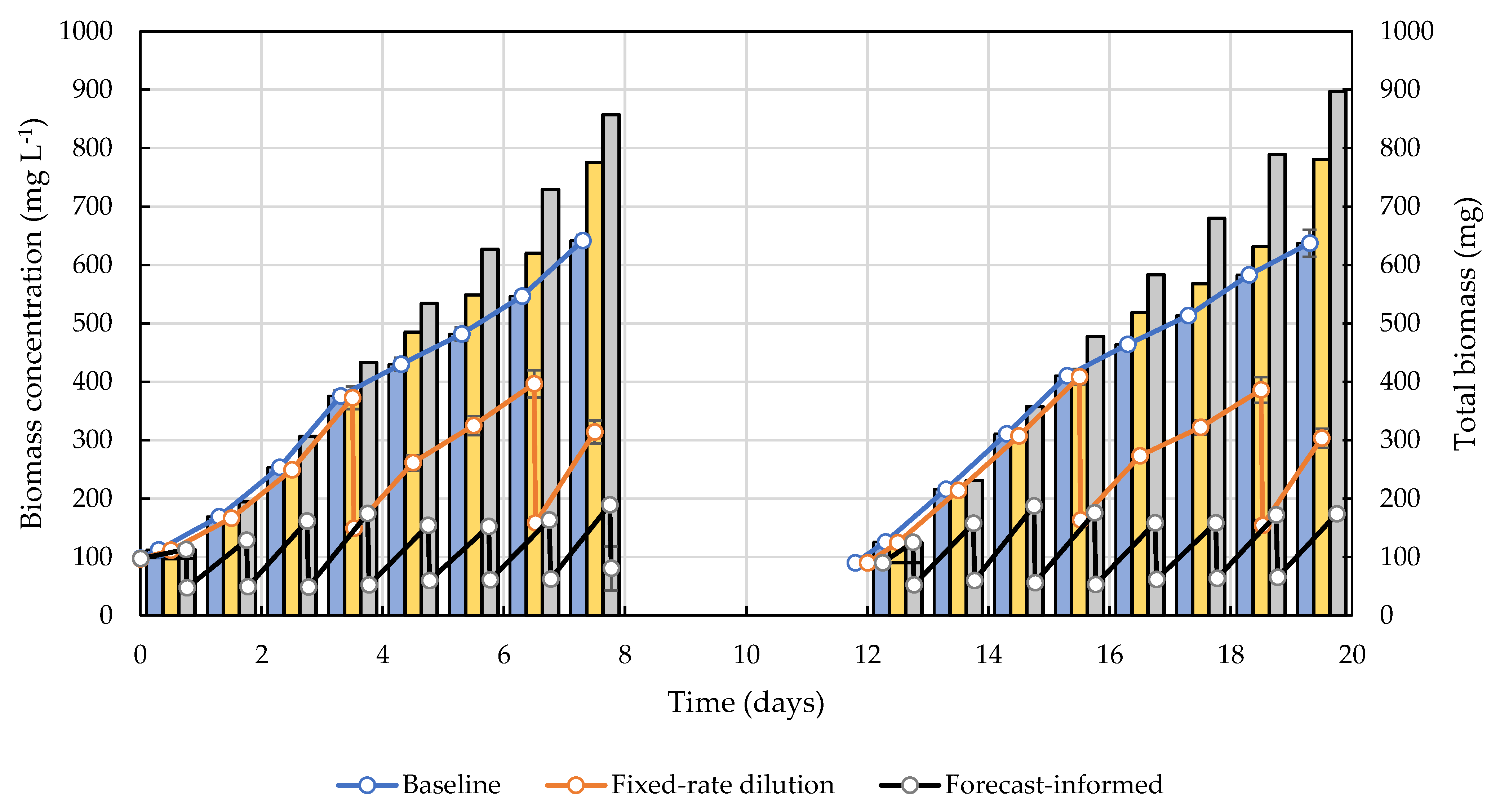
2.3.1. Baseline Scenario
2.3.2. Fixed-Rate Dilution Scenario
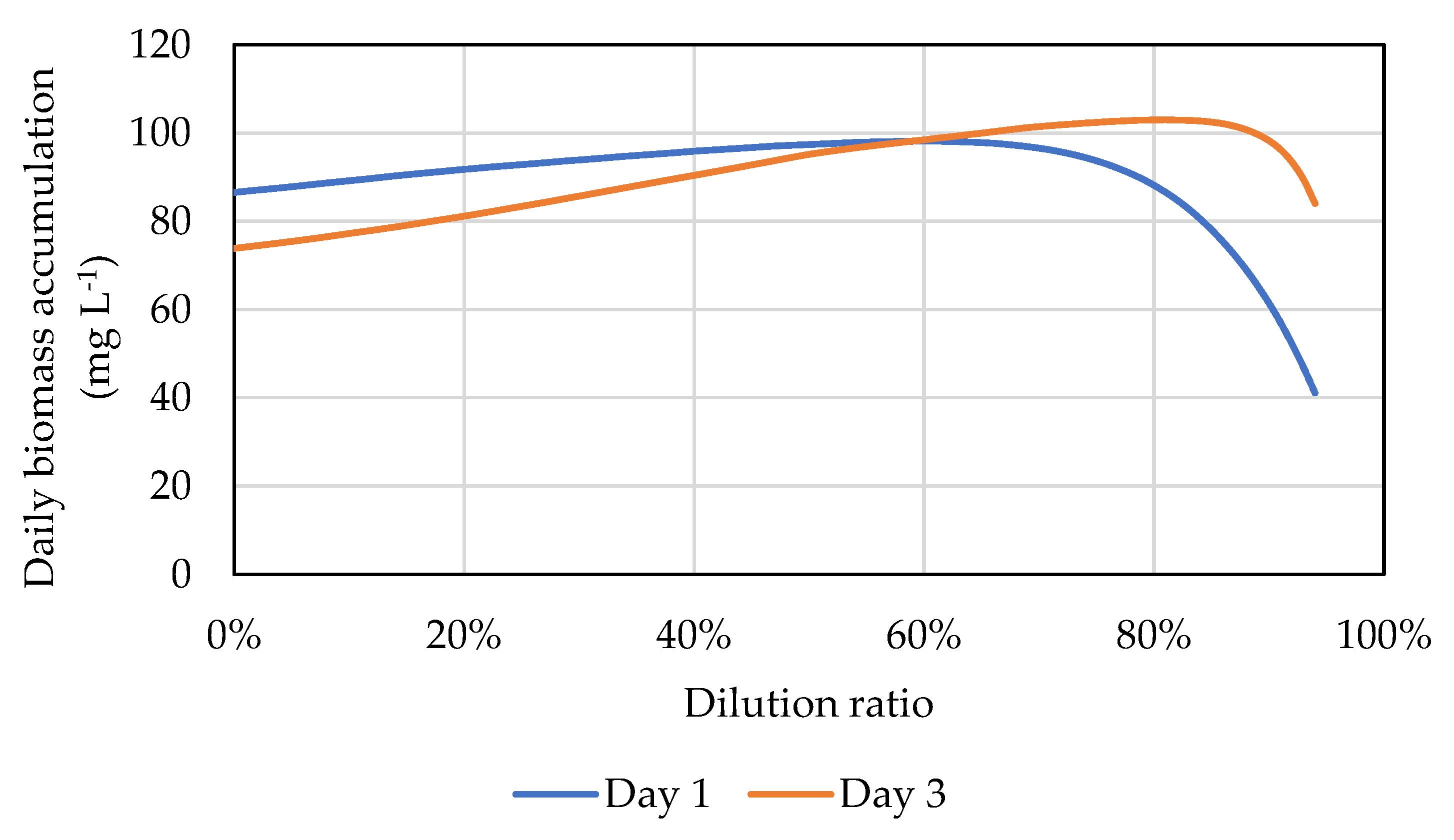
2.3.3. Weather-Forecast-Informed Dilution Scenario
2.4. Data Collection and Processing
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C, Intergovermental Panel on Climate Change. 2018. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_High_Res.pdf (accessed on 20 April 2022).
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Davis, R.; Markham, J.; Kinchin, C.; Grundl, N.; Tan, E.; Humbird, D. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing through Dewatering for Downstream Conversion; The National Renewable Energy Laboratory: Denver, CO, USA, 2016; 119p.
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- De-Luca, R.; Bezzo, F.; Béchet, Q.; Bernard, O. Exploiting meteorological forecasts for the optimal operation of algal ponds. J. Process Control 2017, 55, 55–65. [Google Scholar] [CrossRef]
- De-Luca, R.; Trabuio, M.; Barolo, M.; Bezzo, F. Microalgae growth optimization in open ponds with uncertain weather data. Comput. Chem. Eng. 2018, 117, 410–419. [Google Scholar] [CrossRef]
- De-Luca, R.; Bezzo, F.; Béchet, Q.; Bernard, O. Meteorological Data-Based Optimal Control Strategy for Microalgae Cultivation in Open Pond Systems. Complexity 2019, 2019, 4363895. [Google Scholar] [CrossRef]
- Yan, H.; Wigmosta, M.S.; Sun, N.; Huesemann, M.H.; Gao, S. Real-time ensemble microalgae growth forecasting with data assimilation. Biotechnol. Bioeng. 2021, 118, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.; Crowe, B.; Waller, P.; Chavis, A.; Hobbs, S.; Edmundson, S.; Wigmosta, M. A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Res. 2016, 13, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Wigmosta, M.S.; Coleman, A.M.; Skaggs, R.J.; Huesemann, M.H.; Lane, L.J. National microalgae biofuel production potential and resource demand. Water Resour. Res. 2011, 47, W00H04. [Google Scholar] [CrossRef]
- Coleman, A.M.; Abodeely, J.M.; Skaggs, R.L.; Moeglein, W.A.; Newby, D.T.; Venteris, E.R.; Wigmosta, M.S. An integrated assessment of location-dependent scaling for microalgae biofuel production facilities. Algal Res. 2014, 5, 79–94. [Google Scholar] [CrossRef]
- Xu, H.; Lee, U.; Coleman, A.M.; Wigmosta, M.S.; Wang, M. Assessment of algal biofuel resource potential in the United States with consideration of regional water stress. Algal Res. 2018, 37, 30–39. [Google Scholar] [CrossRef]
- Simionato, D.; Basso, S.; Giacometti, G.M.; Morosinotto, T. Optimization of light use efficiency for biofuel production in algae. Biophys. Chem. 2013, 182, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, Y.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X. Improvement on light penetrability and microalgae biomass production by periodically pre-harvesting Chlorella vulgaris cells with culture medium recycling. Bioresour. Technol. 2016, 216, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Unkefer, C.J.; Sayre, R.; Magnuson, J.; Anderson, D.B.; Baxter, I.; Blaby, I.K.; Brown, J.K.; Carleton, M.; Cattolico, R.A.; Dale, T.; et al. Review of the algal biology program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 2017, 22, 187–215. [Google Scholar] [CrossRef] [Green Version]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Huesemann, M.; Williams, P.; Edmundson, S.; Chen, P.; Kruk, R.; Cullinan, V.; Crowe, B.; Lundquist, T. The laboratory environmental algae pond simulator (LEAPS) photobioreactor: Validation using outdoor pond cultures of Chlorella sorokiniana and Nannochloropsis salina. Algal Res. 2017, 26, 39–46. [Google Scholar] [CrossRef]
- Andersen, R.; Berges, J.; Harrison, P.; Watanabe, M. Recipes for Freshwater and Seawater Media; Elsevier Academic Press: Singapore, 2005; pp. 429–538. [Google Scholar]
- Xia, Y.; Mitchell, K.; Ek, M.; Sheffield, J.; Cosgrove, B.; Wood, E.; Luo, L.; Alonge, C.; Wei, H.; Meng, J.; et al. Continental-scale water and energy flux analysis and validation for the North American Land Data Assimilation System project phase 2 (NLDAS-2): 1. Intercomparison and application of model products. J. Geophys. Res. Atmos. 2012, 117, D03109. [Google Scholar] [CrossRef]
- Perkins, W.A.; Richmond, M.C. MASS2, Modular Aquatic Simulation System in Two Dimensions Theory and Numerical Methods; Pacific Northwest National Laboratory: Richland, WA, USA, 2004.
- Andersson, B.; Salter, A.; Virgin, I.; Vass, I.; Styring, S. Photodamage to photosystem II—Primary and secondary events. J. Photochem. Photobiol. B Biol. 1992, 15, 15–31. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Yan, H.; Beirne, N.; Wigmosta, M.; Huesemann, M. Improving Microalgal Biomass Productivity Using Weather-Forecast-Informed Operations. Cells 2022, 11, 1498. https://doi.org/10.3390/cells11091498
Gao S, Yan H, Beirne N, Wigmosta M, Huesemann M. Improving Microalgal Biomass Productivity Using Weather-Forecast-Informed Operations. Cells. 2022; 11(9):1498. https://doi.org/10.3390/cells11091498
Chicago/Turabian StyleGao, Song, Hongxiang Yan, Nathan Beirne, Mark Wigmosta, and Michael Huesemann. 2022. "Improving Microalgal Biomass Productivity Using Weather-Forecast-Informed Operations" Cells 11, no. 9: 1498. https://doi.org/10.3390/cells11091498






