Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis
Abstract
1. Background
1.1. Hepatic Scarring and Extracellular Matrix Leading to Fibrosis
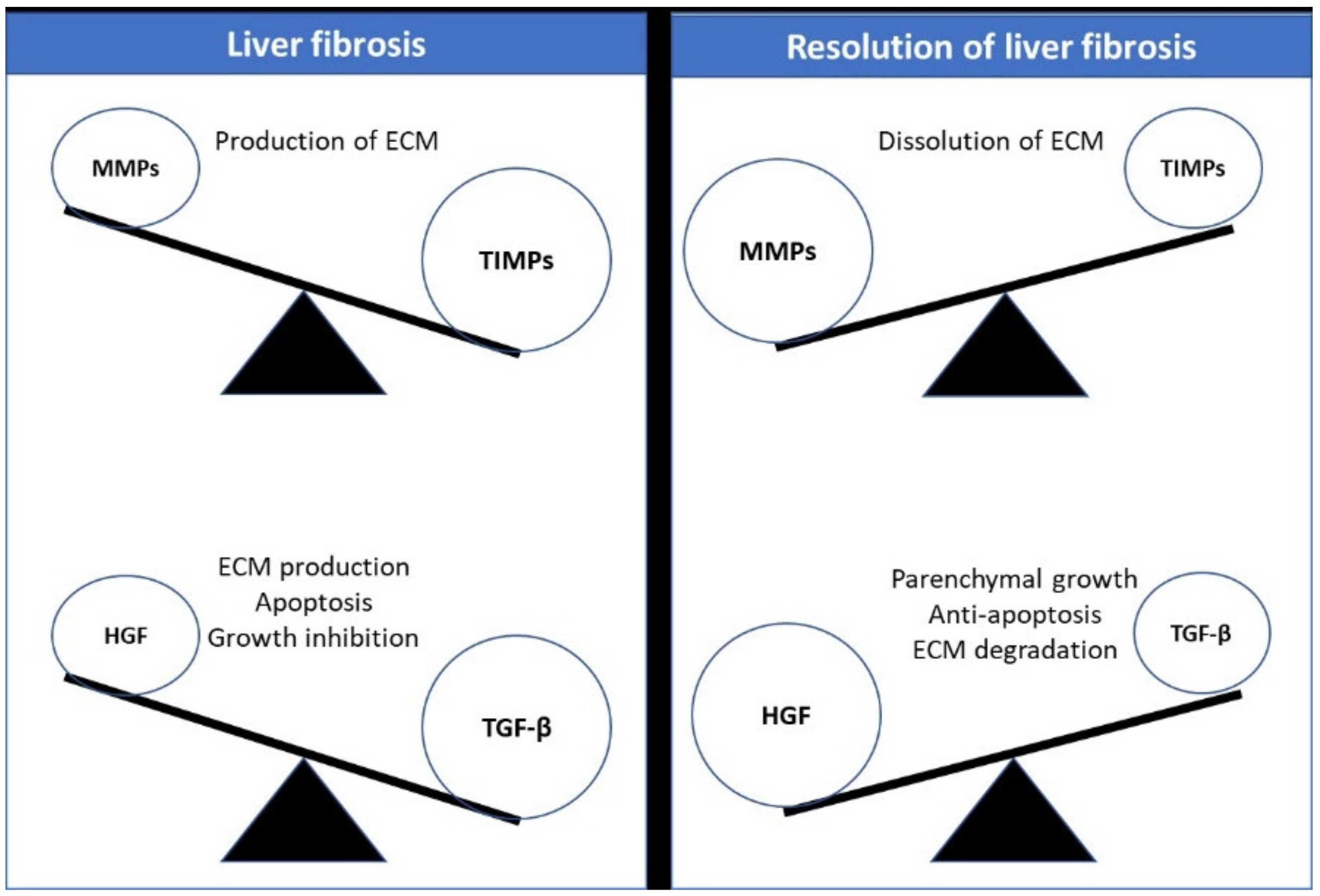
1.2. Fibrosis Reversibility and Possible Regression
1.3. Potential Targets for Cellular Therapy
2. Designing the Framework for Developing Antifibrotic Therapies
2.1. Role of Non-Invasive Biomarkers
2.1.1. Evolving Biomarker Candidates for Liver Fibrosis
2.1.2. Role of Gut Microbiota as a Biomarker in Liver Fibrosis
2.2. Role of Metabolic Agents
2.2.1. Farnesoid X Receptor Agonist
2.2.2. PPAR Agonist
2.2.3. Insulin-Based Targets
2.2.4. Renin–Angiotensin System Inhibitor
2.2.5. Inhibition of HMG-CoA Reductase
2.3. Cellular Target-Specific Fibrosis Resolution
| Target | Cells | Drug | Clinical Trial Stage | Clinical Trial Details | References |
|---|---|---|---|---|---|
| TGFβ | HSCs, cholangiocytes, inflammatory cells, endothelia | Soluble type II, anti-TGFβ antibody; | Phase 2 | FG-3019 for HBV infection, anti-CTGF monoclonal antibody; | [119,120,121] |
| Lerdelimumab; | Phase 3 | Monoclonal antibody, Cambridge antibody technology; | |||
| Metelimumab | Phase 1/2 | Monoclonal antibody, Cambridge antibody technology | |||
| TIMP1 | HSCs, human endothelial cells, lymphoma, and breast carcinoma | MMP antagonist | Serum levels of TIMP-I in 268 patients with liver diseases | [122] | |
| Integrin αvβ6 | Activated epithelia | Small-molecule antagonist, blocking AB | Phase 2 | Monoclonal antibody (STX-100) for idiopathic pulmonary fibrosis | [125] |
| TLR-4 | Macrophages, HSC | Small-molecule antagonists and downstream targets | Preclinical trial | TLR4-deficient mice protected against hepatic injury; vaccine development for hepatitis B (GlaxoSmithKline/Dynavax) | [130,131] |
| HNF4α | Hepatocytes, pancreatic beta cells | HNF4α agonists | Preclinical trial | (CureVac) restoration of HNF4α via mRNA delivery using paraoxonase 1 as a therapeutic target | [132] |
| LPA | HSC | LPA receptor and small-molecule antagonist | Phase 2 | Idiopathic pulmonary fibrosis treatment using the LPA1 pathway | [133] |
2.4. Role of Growth Factors in Liver Fibrosis
2.5. Reduction in Inflammation and Immune Response
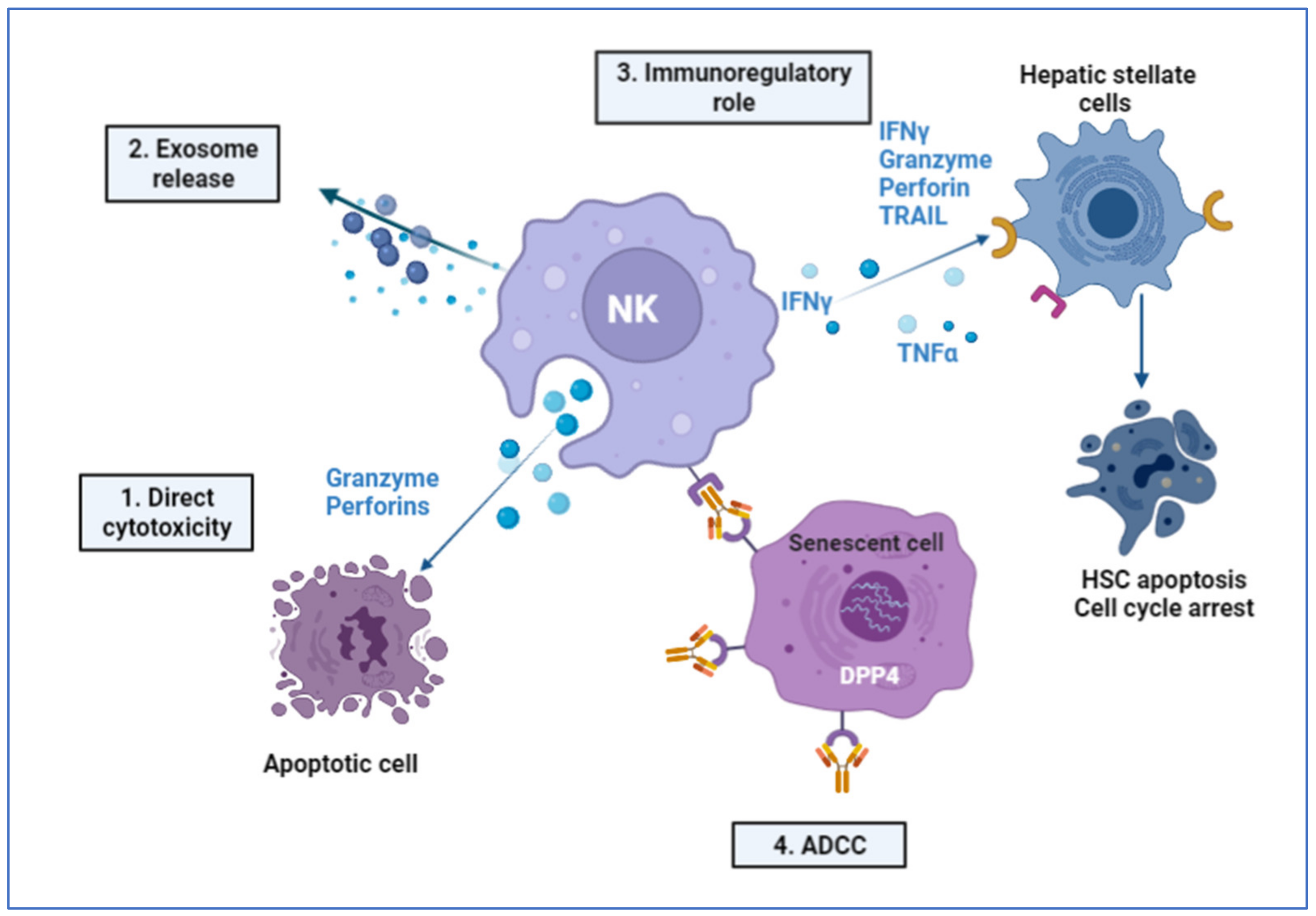
2.6. Immune-Mediated Role of NK Cells
2.7. Role of Cytokines in Liver Fibrosis
2.8. Role of miRNA Family
2.9. Role of Mesenchymal Stromal Cells in Liver Tissues
2.10. Current Challenges in Clinical Trials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| ABCB4 | ATP-binding cassette subfamily B member 4 |
| ACSL1 | Acyl-CoA synthetase long-chain family member |
| ADCC | Antibody-dependent cytotoxicity |
| AKT | AKT serine/threonine kinase 3 |
| ALD | Alcoholic liver disease |
| ALDOB | Aldolase, fructose-bisphosphate B |
| APAP | N-acetyl-p-aminophenol |
| APO-1 | Apoptosis antigen 1 |
| ASH | Alcoholic steatohepatitis |
| ASL | Argininosuccinate lyase |
| BDL | Bile duct ligation |
| CCL4 | Carbon tetrachloride |
| CCN2 | Cellular communication network factor 2 |
| CD1 | Cluster of differentiation 1 |
| CREB3L1 | CAMP-responsive element-binding protein 3-like 1 |
| CTGF | Connective tissue growth factor |
| DPP4 | Dipeptidyl peptidase 4 |
| ECM | Extracellular matrix |
| EVs | Extracellular vesicles |
| FAH | Fumarylacetoacetate hydrolase |
| FXR | Farnesoid X receptor |
| GBE1 | 1,4-alpha-glucan branching enzyme 1 |
| HDAC4 | Histone deacetylase 4 |
| HGF | Hepatocyte growth factor |
| hMSCs | Human mesenchymal stem cells |
| HNF4 | Hepatocyte nuclear factor 4 |
| HSCs, qHSCs | Hepatic stellate cells, quiescent hepatic stellate cells |
| HSP47 | Heat shock protein 47 |
| IGF | Insulin-like growth factor |
| IHH | Indian Hedgehog |
| IKK, NF-κB | Inhibitor of nuclear factor-κB (IκB) kinase, nuclear factor-kappa B |
| ILs | Interleukins |
| KCs | Kupffer cells |
| LPA | Lysophosphatidic acid |
| LSECs | Liver sinusoidal endothelium cells |
| MAITs | Mucosa-associated invariant T cells |
| MAPK | Mitogen-activated protein kinase |
| MFGE-8 | Milk factor globule EGF8 |
| MHC | Major histocompatibility complex |
| miRNAs | microRNAs |
| MMP | Matrix metalloprotein |
| MRE | Magnetic resonance elastography |
| NASH | Non-alcoholic steatohepatitis |
| NKs | Natural killer cells |
| PD-1 | Programmed cell death protein 1 |
| PDGF | Platelet-derived growth factor |
| PI3k | Phosphatidylinositol 3-kinase |
| PPARϒ | Peroxisome proliferators–activated receptor γ |
| SAM | Scar-associated macrophages |
| SERPINA | Serpin family A member 1 |
| SLC25A13 | Solute carrier family 25-member 13 |
| SMAD | Fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, mothers against decapentaplegic |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAA | Thioacetamide |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TE | Transient elastography |
| TGF-β | Transforming growth factor β |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR4 | Toll-like receptor-4 |
| TNF | Tumor necrosis factor |
| TNF-α | Tumor Necrosis Factor α |
| Tregs | Regulatory T cells |
| TSG-6 | TNF-α-stimulated gene 6 |
| UCB | Umbilical cord blood |
| uPA, uPAR | Urokinase plasminogen activator, urokinase plasminogen activator receptor |
| Wnt | Wingless-related integration site |
| WPBs | Weibel–Palade bodies |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef]
- Makarev, E.; Izumchenko, E.; Aihara, F.; Wysocki, P.T.; Zhu, Q.; Buzdin, A.; Sidransky, D.; Zhavoronkov, A.; Atala, A. Common pathway signature in lung and liver fibrosis. Cell Cycle 2016, 15, 1667–1673. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical states of cirrhosis and competing risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Groszmann, R.J. Pathophysiology of portal hypertension. Variceal Hemorrhage 2014, 18, 281–291. [Google Scholar]
- Sauerbruch, T.; Trebicka, J. Future therapy of portal hypertension in liver cirrhosis–a guess. F1000prime Rep. 2014, 6, 95. [Google Scholar] [CrossRef]
- Khanam, A.; Saleeb, P.G.; Kottilil, S. Pathophysiology and Treatment Options for Hepatic Fibrosis: Can It Be Completely Cured? Cells 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Tátrai, P.; Egedi, K.; Somorácz, Á.; Van Kuppevelt, T.H.; Dam, G.t.; Lyon, M.; Deakin, J.A.; Kiss, A.; Schaff, Z.; Kovalszky, I. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J. Histochem. Cytochem. 2010, 58, 429–441. [Google Scholar] [CrossRef]
- Trautwein, C.; Friedman, S.L.; Schuppan, D.; Pinzani, M. Hepatic fibrosis: Concept to treatment. J. Hepatol. 2015, 62, S15–S24. [Google Scholar] [CrossRef]
- Magee, N.; Zou, A.; Zhang, Y. Pathogenesis of nonalcoholic steatohepatitis: Interactions between liver parenchymal and nonparenchymal cells. BioMed Res. Int. 2016, 2016, 5170402. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef]
- Tanwar, S.; Rhodes, F.; Srivastava, A.; Trembling, P.M.; Rosenberg, W.M. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J. Gastroenterol. 2020, 26, 109. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Chien, R.-N.; Liaw, Y.-F. Hepatitis B virus-related decompensated liver cirrhosis: Benefits of antiviral therapy. J. Hepatol. 2012, 57, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019, 70, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512. [Google Scholar] [CrossRef] [PubMed]
- Sanz-García, C.; Fernández-Iglesias, A.; Gracia-Sancho, J.; Arráez-Aybar, L.A.; Nevzorova, Y.A.; Cubero, F.J. The Space of Disse: The Liver Hub in Health and Disease. Livers 2021, 1, 3–26. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.E.; Cárdenas, B.I.; Farran, N.; Fernandez, M. Metabolic Reprogramming of Liver Fibrosis. Cells 2021, 10, 3604. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Narumiya, S. Roles of hepatic stellate cells in liver inflammation: A new perspective. Inflamm. Regen. 2016, 36, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.; Serna-Salas, S.; Damba, T.; Borghesan, M.; Demaria, M.; Moshage, H. Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives. Mech. Ageing Dev. 2021, 199, 111572. [Google Scholar] [CrossRef]
- Wan, M.; Han, J.; Ding, L.; Hu, F.; Gao, P. Novel Immune Subsets and Related Cytokines: Emerging Players in the Progression of Liver Fibrosis. Front. Med. 2021, 8, 604894. [Google Scholar] [CrossRef]
- Kong, X.; Feng, D.; Wang, H.; Hong, F.; Bertola, A.; Wang, F.S.; Gao, B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012, 56, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Ma, H.; Jiang, S.; Zhong, Z.; Wang, X.; Li, C.; Yu, D.; Liu, L.; Xu, J.; Xia, C. Interleukin-9 blockage reduces early hepatic granuloma formation and fibrosis during Schistosoma japonicum infection in mice. Immunology 2019, 158, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, K.; Rombouts, K.; Saffioti, F.; Roccarina, D.; Rosselli, M.; Hall, A.; Luong, T.; Tsochatzis, E.A.; Thorburn, D.; Pinzani, M. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 2018, 68, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J. Immunol. 2013, 191, 1835–1844. [Google Scholar] [CrossRef]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012, 143, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Zhang, H.; Zou, Z.; Wang, F.; Jia, J. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J. Viral Hepat. 2012, 19, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kono, H.; Furuya, S.; Hirayama, K.; Tsuchiya, M.; Fujii, H. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J. Surg. Res. 2013, 183, 574–582. [Google Scholar] [CrossRef]
- Gu, L.; Deng, W.S.; Sun, X.F.; Zhou, H.; Xu, Q. Rapamycin ameliorates CCl4-induced liver fibrosis in mice through reciprocal regulation of the Th17/Treg cell balance. Mol. Med. Rep. 2016, 14, 1153–1161. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Han, P.; Lei, Y.; Xia, Y.; Tian, D.; Yan, W. The JAK inhibitor tofacitinib ameliorates immune-mediated liver injury in mice. Mol. Med. Rep. 2019, 20, 4883–4892. [Google Scholar] [CrossRef]
- Ussher, J.E.; Klenerman, P.; Willberg, C.B. Mucosal-associated invariant T-cells: New players in anti-bacterial immunity. Front. Immunol. 2014, 5, 450. [Google Scholar] [CrossRef]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood J. Am. Soc. Hematol. 2011, 117, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.; Weiss, E.; Paradis, V.; Wan, J.; Mabire, M.; Sukriti, S.; Rautou, P.-E.; Albuquerque, M.; Picq, O.; Gupta, A.C.; et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heydtmann, M.; Lalor, P.F.; Eksteen, J.A.; Hubscher, S.G.; Briskin, M.; Adams, D.H. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 2005, 174, 1055–1062. [Google Scholar] [CrossRef]
- Geissmann, F.; Cameron, T.O.; Sidobre, S.; Manlongat, N.; Kronenberg, M.; Briskin, M.J.; Dustin, M.L.; Littman, D.R. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005, 3, e113. [Google Scholar] [CrossRef]
- Shimaoka, T.; Kume, N.; Minami, M.; Hayashida, K.; Kataoka, H.; Kita, T.; Yonehara, S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J. Biol. Chem. 2000, 275, 40663–40666. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef]
- Issa, R.; Williams, E.; Trim, N.; Kendall, T.; Arthur, M.; Reichen, J.; Benyon, R.; Iredale, J. Apoptosis of hepatic stellate cells: Involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 2001, 48, 548–557. [Google Scholar] [CrossRef]
- Dienstag, J.L.; Goldin, R.D.; Heathcote, E.J.; Hann, H.; Woessner, M.; Stephenson, S.L.; Gardner, S.; Gray, D.F.; Schiff, E.R. Histological outcome during long-term lamivudine therapy. Gastroenterology 2003, 124, 105–117. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P. Reversibility of liver fibrosis. Ann. Hepatol. 2009, 8, 283–291. [Google Scholar] [CrossRef]
- Hammel, P.; Couvelard, A.; O’Toole, D.; Ratouis, A.; Sauvanet, A.; Fléjou, J.F.; Degott, C.; Belghiti, J.; Bernades, P.; Valla, D. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. New Engl. J. Med. 2001, 344, 418–423. [Google Scholar] [CrossRef]
- Dufour, J.-F.; DeLellis, R.; Kaplan, M.M. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann. Intern. Med. 1997, 127, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Parés, A.; Caballería, J.; Bruguera, M.; Torres, M.; Rodés, J. Histological course of alcoholic hepatitis: Influence of abstinence, sex and extent of hepatic damage. J. Hepatol. 1986, 2, 33–42. [Google Scholar] [CrossRef]
- Ismail, M.H.; Pinzani, M. Reversal of hepatic fibrosis: Pathophysiological basis of antifibrotic therapies. Hepatic Med. Evid. Res. 2011, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gea, V. Liver Fibrosis: What Is Reversible and What Not? How to Assess Regression. In Portal Hypertension VI; Springer: Cham, Switzerland, 2016; pp. 111–115. [Google Scholar]
- Kurokawa, T.; Ohkohchi, N. Platelets in liver disease, cancer and regeneration. World J. Gastroenterol. 2017, 23, 3228–3239. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Lal, P.; Birerdinc, A.; Younossi, Z.M. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.L.; Pavlides, M.; Moolla, A.; Ryan, J.D. Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front. Pharmacol. 2016, 7, 159. [Google Scholar] [CrossRef]
- Schuppan, D.; Kim, Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013, 123, 1887–1901. [Google Scholar] [CrossRef]
- Nallagangula, K.S.; Nagaraj, S.K.; Venkataswamy, L.; Chandrappa, M. Liver fibrosis: A compilation on the biomarkers status and their significance during disease progression. Future Sci. OA 2018, 4, FSO250. [Google Scholar] [CrossRef]
- Joseph, J. Serum Marker Panels for Predicting Liver Fibrosis–An Update. Clin. Biochem. Rev. 2020, 41, 67. [Google Scholar]
- Papastergiou, V.; Tsochatzis, E.; Burroughs, A.K. Non-invasive assessment of liver fibrosis. Ann. Gastroenterol. 2012, 25, 218. [Google Scholar]
- Bocsan, I.C.; Milaciu, M.V.; Pop, R.M.; Vesa, S.C.; Ciumarnean, L.; Matei, D.M.; Buzoianu, A.D. Cytokines genotype-phenotype correlation in nonalcoholic steatohepatitis. Oxidative Med. Cell. Longev. 2017, 2017, 4297206. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Liebe, R.; Hochrath, K.; Kazakov, A.; Alberts, R.; Laufs, U.; Bohm, M.; Fischer, H.P.; Williams, R.W.; Schughart, K.; et al. Systems genetics of liver fibrosis: Identification of fibrogenic and expression quantitative trait loci in the BXD murine reference population. PLoS ONE 2014, 9, e89279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Keogh, A.; Waldt, A.; Cuttat, R.; Neri, M.; Zhu, S.; Schuierer, S.; Ruchti, A.; Crochemore, C.; Knehr, J.; et al. Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis. Sci. Rep. 2021, 11, 19396. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Hundertmark, J.; Ritz, T.P.; Weiskirchen, R.; Tacke, F. Single Cell RNA Sequencing Identifies Subsets of Hepatic Stellate Cells and Myofibroblasts in Liver Fibrosis. Cells 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, M.; Sarfaraz, M.O.; Kroezen, Z.; Philbrick, H.; Poon, R.; Don-Wauchope, A.; Puglia, M.; Wishart, D.; Britz-McKibbin, P. A Cross-Platform Metabolomics Comparison Identifies Serum Metabolite Signatures of Liver Fibrosis Progression in Chronic Hepatitis C Patients. Front. Mol. Biosci. 2021, 8, 676349. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): Diagnostic, prognostic, and therapeutic applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Moran-Salvador, E.; Mann, J. Epigenetics and Liver Fibrosis. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 125–134. [Google Scholar] [CrossRef]
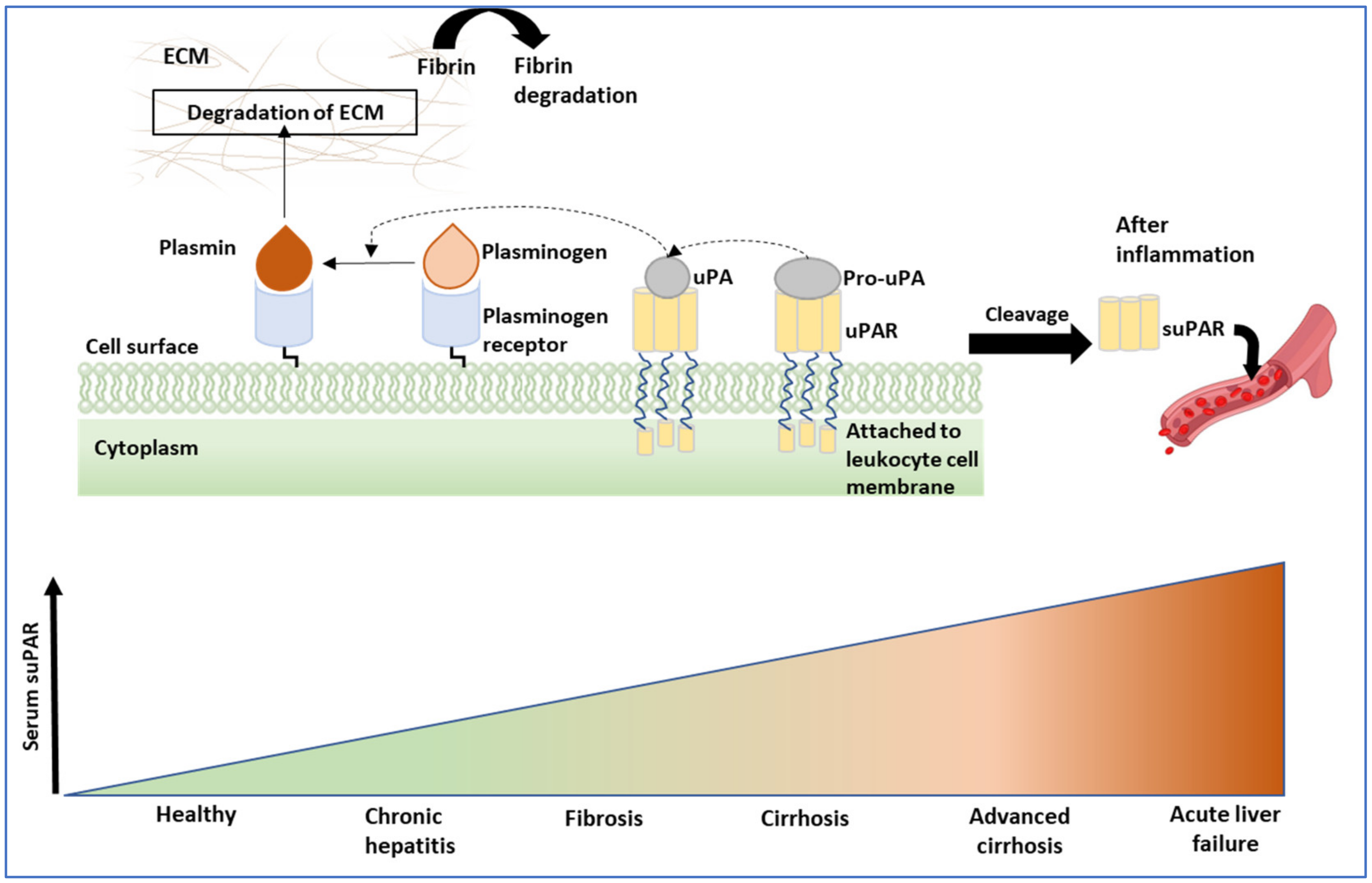
- Akdogan, O.; Atak Yucel, A.; Gok Sargin, Z.; Sonmez, C.; Esendagli Yilmaz, G.; Ozenirler, S. Evaluation of Plasma Urokinase-Type Plasminogen Activator Receptor (UPAR) in Patients With Chronic Hepatitis B, C and Non-Alcoholic Fatty Liver Disease (NAFLD) as Serological Fibrosis Marker. J. Clin. Exp. Hepatol. 2019, 9, 29–33. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Koch, A.; Seidler, S.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012, 32, 500–509. [Google Scholar] [CrossRef]
- Garnaes, E.; Mortensen, C.; Hobolth, L.; Andersen, O.; Nehlin, J.; Moller, S. Kinetics of the soluble urokinase plasminogen activator receptor (suPAR) in cirrhosis. PLoS ONE 2019, 14, e0220697. [Google Scholar] [CrossRef]
- Nikkola, A.; Aittoniemi, J.-J.; Huttunen, R.; Sand, J.; Laukkarinen, J. Mo1351 Systemic Levels of Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Predict the Severity of Acute Alcohol Pancreatitis. Gastroenterology 2015, 148, S-680. [Google Scholar] [CrossRef]
- Chounta, A.; Ellinas, C.; Tzanetakou, V.; Pliarhopoulou, F.; Mplani, V.; Oikonomou, A.; Leventogiannis, K.; Giamarellos-Bourboulis, E.J. Serum soluble urokinase plasminogen activator receptor as a screening test for the early diagnosis of hepatocellular carcinoma. Liver Int. 2015, 35, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Radu-Ionita, F.; Pyrsopoulos, N.T.; Jinga, M.; Tintoiu, I.C.; Sun, Z.; Bontas, E. Liver Diseases: A Multidisciplinary Textbook; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Eguchi, A.; Wree, A.; Feldstein, A.E. Biomarkers of liver cell death. J. Hepatol. 2014, 60, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Reuken, P.; Koch, A.; Bartneck, M.; Adams, D.; Trautwein, C.; Stallmach, A.; Tacke, F.; Bruns, T. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J. Intern. Med. 2013, 274, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, A.; Aittoniemi, J.; Huttunen, R.; Rajala, L.; Nordback, I.; Sand, J.; Laukkarinen, J. Plasma Level of Soluble Urokinase-type Plasminogen Activator Receptor Predicts the Severity of Acute Alcohol Pancreatitis. Pancreas 2017, 46, 77–82. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual role of Fas/FasL-mediated signal in peripheral immune tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Li, Z.; Ni, M.; Yu, H.; Wang, L.; Zhou, X.; Chen, T.; Liu, G.; Gao, Y. Gut Microbiota and Liver Fibrosis: One Potential Biomarker for Predicting Liver Fibrosis. Biomed. Res. Int. 2020, 2020, 3905130. [Google Scholar] [CrossRef]
- Oh, T.G.; Kim, S.M.; Caussy, C.; Fu, T.; Guo, J.; Bassirian, S.; Singh, S.; Madamba, E.V.; Bettencourt, R.; Richards, L.; et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020, 32, 878–888. [Google Scholar] [CrossRef]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease: Thematic review series: New lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lu, Y.; Li, X.-y. Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin. 2015, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. New Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef]
- Ghonem, N.S.; Assis, D.N.; Boyer, J.L. Fibrates and cholestasis. Hepatology 2015, 62, 635–643. [Google Scholar] [CrossRef]
- Guo, Y.-C.; Lu, L.-G. Antihepatic fibrosis drugs in clinical trials. J. Clin. Transl. Hepatol. 2020, 8, 304. [Google Scholar] [CrossRef]
- Wettstein, G.; Luccarini, J.M.; Poekes, L.; Faye, P.; Kupkowski, F.; Adarbes, V.; Defrêne, E.; Estivalet, C.; Gawronski, X.; Jantzen, I. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol. Commun. 2017, 1, 524–537. [Google Scholar] [CrossRef]
- University Hospital Southampton NHS Foundation Trust; National Institute for Health Research, UK. Treatment of Non Alcoholic Fatty Liver Disease With n-3 Fatty Acids. Available online: https://ClinicalTrials.gov/show/NCT00760513 (accessed on 25 January 2022).
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- The Effects of Resveratrol Supplement on Biochemical Factors and Hepatic Fibrosis in Patients With Nonalcoholic Steatohepatitis. Available online: https://ClinicalTrials.gov/show/NCT02030977 (accessed on 24 January 2022).
- Rinella, M.E.; Dufour, J.-F.; Anstee, Q.M.; Goodman, Z.; Younossi, Z.; Harrison, S.A.; Loomba, R.; Sanyal, A.J.; Bonacci, M.; Trylesinski, A. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. J. Hepatol. 2022, 76, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef]
- Flint, A.; Andersen, G.; Hockings, P.; Johansson, L.; Morsing, A.; Sundby Palle, M.; Vogl, T.; Loomba, R.; Plum-Mörschel, L. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2021, 54, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.Y.; Eom, Y.W.; Kim, M.Y.; Kang, S.H.; Baik, S.K. Role of the renin-angiotensin system in hepatic fibrosis and portal hypertension. Korean J. Intern. Med. 2018, 33, 453. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.; Bataller, R.; Sancho-Bru, P.; Domínguez, M.; Moreno, M.; Forns, X.; Bruguera, M.; Arroyo, V.; Brenner, D.A.; Ginès, P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am. J. Physiol. -Gastrointest. Liver Physiol. 2009, 297, G726–G734. [Google Scholar] [CrossRef]
- Effects of Losartan on Hepatic Fibrogenesis in Chronic Hepatitis C. Available online: https://ClinicalTrials.gov/show/NCT00298714 (accessed on 13 February 2022).
- Yonsei, U. Effect of Candesartan in Alcoholic Liver Fibrosis. Available online: https://ClinicalTrials.gov/show/NCT00990639 (accessed on 13 February 2022).
- Sherief, A.-E.; Tanta, U. Effect of Some Drugs on Liver Fibrosis. Available online: https://ClinicalTrials.gov/show/NCT03770936 (accessed on 13 February 2022).
- Evaluation of Irbesartan on Hepatic Fibrosis in Chronic Hepatitis C. Available online: https://ClinicalTrials.gov/show/NCT00265642 (accessed on 22 February 2022).
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Kim, S.H.; Cho, M.-Y.; Kim, K.S.; Park, K.-S.; Cha, S.-K.; Kim, M.Y.; Chang, S.J.; Baik, S.K. Synergistic effects of simvastatin and bone marrow-derived mesenchymal stem cells on hepatic fibrosis. Biochem. Biophys. Res. Commun. 2018, 497, 264–271. [Google Scholar] [CrossRef]
- Abraldes, J.G.; Albillos, A.; Bañares, R.; Turnes, J.; González, R.; García–Pagán, J.C.; Bosch, J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: A randomized controlled trial. Gastroenterology 2009, 136, 1651–1658. [Google Scholar] [CrossRef]
- Efficacy of Simvastatin in Alcoholic Liver Fibrosis. Available online: https://ClinicalTrials.gov/show/NCT04971577 (accessed on 13 February 2022).
- Merck, S.; Dohme, C. Prevention of Disease Progress in Chronic Hepatitis C Patients With Liver Fibrosis (Study P02570AM2)(COMPLETED). Available online: https://ClinicalTrials.gov/show/NCT00049842 (accessed on 13 February 2022).
- Merck, S.; Dohme, C. PEG-Intron Plus Rebetol Treatment of Chronic Hepatitis C Subjects Who Failed Response to Alpha-Interferon Plus Ribavirin (Study P02370). 2007. Available online: https://clinicaltrials.gov/ct2/show/NCT00039871 (accessed on 13 January 2022).
- RWTH Aachen University; Hannover Medical School. Induction of Fibrosis Regression on Patients With Chronic Hepatitis B Infection. Available online: https://ClinicalTrials.gov/show/NCT01341106 (accessed on 13 January 2022).
- Chang, T.T.; Liaw, Y.F.; Wu, S.S.; Schiff, E.; Han, K.H.; Lai, C.L.; Safadi, R.; Lee, S.S.; Halota, W.; Goodman, Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010, 52, 886–893. [Google Scholar] [CrossRef]
- Peking University People’s Hospital; RenJi Hospital; Peking University; Shanghai Zhongshan Hospital; Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine; Shanghai Public Health Clinical Center; Nanfang Hospital of Southern Medical University; Sir Run Run Shaw Hospital; Beijing YouAn Hospital; Peking University First Hospital; et al. Optimized Treatment and Regression of HBV-induced Liver Fibrosis. Available online: https://ClinicalTrials.gov/show/NCT01938781 (accessed on 13 February 2022).
- Judit Pich, Institut d’Investigacions Biomèdiques August Pi i Sunyer. Efficacy of the Combination of Simvastatin Plus Rifaximin in Patients With Decompensated Cirrhosis to Prevent ACLF Development. Available online: https://ClinicalTrials.gov/show/NCT03780673 (accessed on 22 February 2022).
- Popov, Y.; Schuppan, D. Targeting liver fibrosis: Strategies for development and validation of antifibrotic therapies. Hepatology 2009, 50, 1294–1306. [Google Scholar] [CrossRef]
- George, J.; Roulot, D.; Koteliansky, V.E.; Bissell, D.M. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor β type II receptor: A potential new therapy for hepatic fibrosis. Proc. Natl. Acad. Sci. USA 1999, 96, 12719–12724. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, Y.; Xiao, J.; Chai, Y.; Lei, J.; Huang, H.; Xiang, T.; Shen, W. A COL1A1 promoter-controlled expression of TGF-β soluble receptor inhibits hepatic fibrosis without triggering autoimmune responses. Dig. Dis. Sci. 2018, 63, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; Freeman, M.L.; Arteaga, C.L. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Investig. 2007, 117, 1305–1313. [Google Scholar] [CrossRef]
- Kemaladewi, D.U.; Pasteuning, S.; Van Der Meulen, J.W.; Van Heiningen, S.H.; van Ommen, G.-J.; Ten Dijke, P.; Aartsma-Rus, A.; Ac’t Hoen, P.; Hoogaars, W.M. Targeting TGF-β signaling by antisense oligonucleotide-mediated knockdown of TGF-β type I receptor. Mol. Ther. Nucleic Acids 2014, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Merkel, P.A.; Furst, D.E.; Khanna, D.; Emery, P.; Hsu, V.M.; Silliman, N.; Streisand, J.; Powell, J.; Åkesson, A. Recombinant human anti–transforming growth factor β1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007, 56, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yun, J.S.; Han, D.; Yook, J.I.; Kim, H.S.; Cho, E.S. TGF-β pathway in salivary gland fibrosis. Int. J. Mol. Sci. 2020, 21, 9138. [Google Scholar] [CrossRef] [PubMed]
- Muzzillo, D.; Imoto, M.; Fukuda, Y.; Koyama, Y.; Saga, S.; Nagai, Y.; Hayakawa, T. Clinical evaluation of serum tissue inhibitor of metalloproteinases-1 levels in patients with liver diseases. J. Gastroenterol. Hepatol. 1993, 8, 437–441. [Google Scholar] [CrossRef]
- Roderfeld, M.; Weiskirchen, R.; Wagner, S.; Berres, M.L.; Henkel, C.; Grötzinger, J.; Gressner, A.M.; Matern, S.; Roeb, E. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006, 20, 444–454. [Google Scholar] [CrossRef]
- Breuss, J.; Gallo, J.; DeLisser, H.; Klimanskaya, I.; Folkesson, H.; Pittet, J.; Nishimura, S.; Aldape, K.; Landers, D.; Carpenter, W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 1995, 108, 2241–2251. [Google Scholar] [CrossRef]
- Biogen STX-100 in Patients With Idiopathic Pulmonary Fibrosis (IPF). Available online: https://ClinicalTrials.gov/show/NCT01371305 (accessed on 22 February 2022).
- Patsenker, E.; Popov, Y.; Stickel, F.; Jonczyk, A.; Goodman, S.L.; Schuppan, D. Inhibition of integrin αvβ6 on cholangiocytes blocks transforming growth factor-β activation and retards biliary fibrosis progression. Gastroenterology 2008, 135, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, L.; Chen, L.; Wang, H.; Zhang, Y.; Bie, P. Role of integrin αvβ6 in the pathogenesis of ischemia-related biliary fibrosis after liver transplantation. Transplantation 2013, 95, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.-F.; Ding, J.; Yin, C.; Zhong, W.; Wu, K.; Zeng, X.; Yang, W.; Chen, Y.-X.; Zhang, J.-P.; Zhang, X. Hepatocyte nuclear factor 4α suppresses the development of hepatocellular carcinoma. Cancer Res. 2010, 70, 7640–7651. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Yin, C.; Hou, J.-L.; Zeng, X.; Chen, Y.-X.; Zhong, W.; Hu, P.-F.; Deng, X.; Tan, Y.; Zhang, J. Hepatocyte nuclear factor 4α attenuates hepatic fibrosis in rats. Gut 2010, 59, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.N.; Bohner, A.; Dapito, D.H.; Schwabe, R.F.; Lammert, F. TLR4 deficiency protects against hepatic fibrosis and diethylnitrosamine-induced pre-carcinogenic liver injury in fibrotic liver. PLoS ONE 2016, 11, e0158819. [Google Scholar] [CrossRef]
- Kanzler, H.; Barrat, F.J.; Hessel, E.M.; Coffman, R.L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 2007, 13, 552–559. [Google Scholar] [CrossRef]
- Yang, T.; Poenisch, M.; Khanal, R.; Hu, Q.; Dai, Z.; Li, R.; Song, G.; Yuan, Q.; Yao, Q.; Shen, X. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 2021, 75, 1420–1433. [Google Scholar] [CrossRef]
- Palmer, S.M.; Snyder, L.; Todd, J.L.; Soule, B.; Christian, R.; Anstrom, K.; Luo, Y.; Gagnon, R.; Rosen, G. Randomized, double-blind, placebo-controlled, phase 2 trial of BMS-986020, a lysophosphatidic acid receptor antagonist for the treatment of idiopathic pulmonary fibrosis. Chest 2018, 154, 1061–1069. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- Xiang, H.; Lu, Y.; Shao, M.; Wu, T. Lysophosphatidic acid receptors: Biochemical and clinical implications in different diseases. J. Cancer 2020, 11, 3519. [Google Scholar] [CrossRef]
- Swaney, J.; Chapman, C.; Correa, L.; Stebbins, K.; Bundey, R.; Prodanovich, P.; Fagan, P.; Baccei, C.; Santini, A.; Hutchinson, J. A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br. J. Pharmacol. 2010, 160, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Dituri, F.; Lupo, L.; Quaranta, M.; Antonaci, S.; Giannelli, G. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology 2011, 54, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Nakamura, T. HGF as a renotrophic and anti-fibrotic regulator in chronic renal disease. Front. Biosci. 2008, 13, 7072–7086. [Google Scholar] [CrossRef]
- Mizuno, S.; Nakamura, T. Hepatocyte growth factor: A regenerative drug for acute hepatitis and liver cirrhosis. Regen. Med. 2007, 2, 161–170. [Google Scholar] [CrossRef]
- Mizuno, S.; Nakamura, T. Suppressions of chronic glomerular injuries and TGF-β1 production by HGF in attenuation of murine diabetic nephropathy. Am. J. Physiol. -Ren. Physiol. 2004, 286, F134–F143. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumoto, K.; Mizuno, S.; Sawa, Y.; Matsuda, H.; Nakamura, T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am. J. Physiol. -Heart Circ. Physiol. 2005, 288, H2131–H2139. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Mizuno, S.; Matsumoto, K.; Li, M.-Y.; Nakamura, T. HGF reduces advancing lung fibrosis in mice: A potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005, 19, 1–18. [Google Scholar] [CrossRef]
- Kim, W.-H.; Matsumoto, K.; Bessho, K.; Nakamura, T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am. J. Pathol. 2005, 166, 1017–1028. [Google Scholar] [CrossRef]
- Ishikawa, T.; Factor, V.M.; Marquardt, J.U.; Raggi, C.; Seo, D.; Kitade, M.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-met signaling is required for stem-cell–mediated liver regeneration in mice. Hepatology 2012, 55, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Rachfal, A.W.; Brigstock, D.R. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol. Res. 2003, 26, 1–9. [Google Scholar] [CrossRef]
- Tong, Z.; Chen, R.; Alt, D.S.; Kemper, S.; Perbal, B.; Brigstock, D.R. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology 2009, 50, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Eitner, F.; Bücher, E.; van Roeyen, C.; Kunter, U.; Rong, S.; Seikrit, C.; Villa, L.; Boor, P.; Fredriksson, L.; Bäckström, G. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2008, 19, 281–289. [Google Scholar] [CrossRef]
- Pontén, A.; Li, X.; Thorén, P.; Aase, K.; Sjöblom, T.; Östman, A.; Eriksson, U. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am. J. Pathol. 2003, 163, 673–682. [Google Scholar] [CrossRef]
- Li, X.; Pontén, A.; Aase, K.; Karlsson, L.; Abramsson, A.; Uutela, M.; Bäckström, G.; Hellström, M.; Boström, H.; Li, H. PDGF-C is a new protease-activated ligand for the PDGF α-receptor. Nat. Cell Biol. 2000, 2, 302–309. [Google Scholar] [CrossRef]
- Campbell, J.S.; Hughes, S.D.; Gilbertson, D.G.; Palmer, T.E.; Holdren, M.S.; Haran, A.C.; Odell, M.M.; Bauer, R.L.; Ren, H.-P.; Haugen, H.S. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 3389–3394. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Y.-P.; Ying, W.; Yao, X.-X. Interleukin-1β upregulates matrix metalloproteinase-13 gene expression via c-Jun N-terminal kinase and p38 MAPK pathways in rat hepatic stellate cells. Mol. Med. Rep. 2013, 8, 1861–1865. [Google Scholar] [CrossRef]
- Jain, M.K.; Adams-Huet, B.; Terekhova, D.; Kushner, L.E.; Bedimo, R.; Li, X.; Holodniy, M. Acute and chronic immune biomarker changes during interferon/ribavirin treatment in HIV/HCV co-infected patients. J. Viral Hepat. 2015, 22, 25–36. [Google Scholar] [CrossRef]
- Okada, H.; Honda, M.; Campbell, J.S.; Sakai, Y.; Yamashita, T.; Takebuchi, Y.; Hada, K.; Shirasaki, T.; Takabatake, R.; Nakamura, M. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res. 2012, 72, 4459–4471. [Google Scholar] [CrossRef]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The immunology of fibrosis: Innate and adaptive responses. Trends Immunol. 2010, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- McQuitty, C.E.; Williams, R.; Chokshi, S.; Urbani, L. Immunomodulatory role of the extracellular matrix within the liver disease microenvironment. Front. Immunol. 2020, 11, 2903. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Lee, T.H.; Klampfer, L.; Shows, T.; Vilcek, J. Transcriptional regulation of TSG6, a tumor necrosis factor-and interleukin-1-inducible primary response gene coding for a secreted hyaluronan-binding protein. J. Biol. Chem. 1993, 268, 6154–6160. [Google Scholar] [CrossRef]
- Nentwich, H.A.; Mustafa, Z.; Rugg, M.S.; Marsden, B.D.; Cordell, M.R.; Mahoney, D.J.; Jenkins, S.C.; Dowling, B.; Fries, E.; Milner, C.M. A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module: Chromosomal localization, frequency analysis, modeling, and expression. J. Biol. Chem. 2002, 277, 15354–15362. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, G.W.; Ziff, E.B.; Vilcek, J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol. Cell. Biol. 1990, 10, 1982–1988. [Google Scholar]
- Lee, T.H.; Wisniewski, H.-G.; Vilcek, J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 1992, 116, 545–557. [Google Scholar] [CrossRef]
- Glant, T.T.; Kamath, R.V.; Bárdos, T.; Gál, I.; Szántó, S.; Murad, Y.M.; Sandy, J.D.; Mort, J.S.; Roughley, P.J.; Mikecz, K. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor α–stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002, 46, 2207–2218. [Google Scholar] [CrossRef]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, J.; Lee, C.; Oh, D.; Han, J.; Kim, T.-J.; Kim, S.-W.; Seo, Y.-S.; Oh, S.-h.; Jung, Y. Tumor necrosis factor-inducible gene 6 reprograms hepatic stellate cells into stem-like cells, which ameliorates liver damage in mouse. Biomaterials 2019, 219, 119375. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-M.; Wu, H.-M.; Li, Y.-H.; Xu, Z.-Y.; Yang, J.-H.; Liu, C.; He, Y.-F.; Wang, M.-J.; Wu, X.-N.; Zhang, Y. TSG-6 Inhibits Oxidative Stress and Induces M2 Polarization of Hepatic Macrophages in Mice With Alcoholic Hepatitis via Suppression of STAT3 Activation. Front. Pharmacol. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Noh, J.H.; Bodogai, M.; Martindale, J.L.; Yang, X.; Indig, F.E.; Basu, S.K.; Ohnuma, K.; Morimoto, C.; Johnson, P.F. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 2017, 31, 1529–1534. [Google Scholar] [CrossRef]
- Levi, N.; Papismadov, N.; Solomonov, I.; Sagi, I.; Krizhanovsky, V. The ECM path of senescence in aging: Components and modifiers. FEBS J. 2020, 287, 2636–2646. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Rossi, M.; Abdelmohsen, K. The Emergence of Senescent Surface Biomarkers as Senotherapeutic Targets. Cells 2021, 10, 1740. [Google Scholar] [CrossRef]
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Tacke, F. Interleukins in chronic liver disease: Lessons learned from experimental mouse models. Clin. Exp. Gastroenterol. 2014, 7, 297. [Google Scholar] [PubMed]
- Narayanan, S.; Surette, F.A.; Hahn, Y.S. The immune landscape in nonalcoholic steatohepatitis. Immune Netw. 2016, 16, 147–158. [Google Scholar] [CrossRef]
- Wang, K.S.; Ritz, J.; Frank, D.A. IL-2 induces STAT4 activation in primary NK cells and NK cell lines, but not in T cells. J. Immunol. 1999, 162, 299–304. [Google Scholar]
- Kovalovich, K.; DeAngelis, R.A.; Li, W.; Furth, E.E.; Ciliberto, G.; Taub, R. Increased toxin-induced liver injury and fibrosis in interleukin-6–deficient mice. Hepatology 2000, 31, 149–159. [Google Scholar] [CrossRef]
- Thompson, K.; Maltby, J.; Fallowfield, J.; McAulay, M.; Millward-Sadler, H.; Sheron, N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology 1998, 28, 1597–1606. [Google Scholar] [CrossRef]
- Louis, H.; Van Laethem, J.L.; Wu, W.; Quertinmont, E.; Degraef, C.; Van den Berg, K.; Demols, A.; Goldman, M.; Le Moine, O.; Geerts, A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology 1998, 28, 1607–1615. [Google Scholar] [CrossRef]
- Hoffmann, K.F.; Caspar, P.; Cheever, A.W.; Wynn, T.A. IFN-γ, IL-12, and TNF-α are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J. Immunol. 1998, 161, 4201–4210. [Google Scholar]
- Parihar, R.; Dierksheide, J.; Hu, Y.; Carson, W.E. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J. Clin. Investig. 2002, 110, 983–992. [Google Scholar] [CrossRef]
- Choi, S.S.; Chhabra, V.S.; Nguyen, Q.H.; Ank, B.J.; Stiehm, E.R.; Roberts, R.L. Interleukin-15 enhances cytotoxicity, receptor expression, and expansion of neonatal natural killer cells in long-term culture. Clin. Diagn. Lab. Immunol. 2004, 11, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.H.; Park, O.; Zheng, M.; Morales-Ibanez, O.; Kolls, J.K.; Bataller, R.; Gao, B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology 2010, 52, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, L.; Fan, F.; Zhu, L.; Li, Z.; Ruan, X.; Huang, H.; Wang, Z.; Huang, Z. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 2010, 53, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.-P.; Willems, B.; Villeneuve, J.-P.; Bilodeau, M.; Shoukry, N.H. Type 3 cytokines IL-17A and IL-22 drive TGF-β–dependent liver fibrosis. Sci. Immunol. 2018, 3, eaar7754. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Satelli, A.; Yan, J.; Xueqing, X.; Gagea, M.; Hunter, C.A.; Mishra, L.; Li, S. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology 2014, 60, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Pacocha, S.E.; Huang, S.-K.; Lichtenstein, L.M.; Essayan, D.M. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J. Pharmacol. Exp. Ther. 2000, 292, 988–994. [Google Scholar]
- McGee, H.M.; Schmidt, B.A.; Booth, C.J.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A.; Horsley, V. IL-22 promotes fibroblast-mediated wound repair in the skin. J. Investig. Dermatol. 2013, 133, 1321–1329. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Takeshita, I.; Maruoka, S.; Horie, T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase–dependent pathway. J. Allergy Clin. Immunol. 2001, 107, 1001–1008. [Google Scholar] [CrossRef]
- Theiss, A.L.; Simmons, J.G.; Jobin, C.; Lund, P.K. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J. Biol. Chem. 2005, 280, 36099–36109. [Google Scholar] [CrossRef]
- Motz, K.; Samad, I.; Yin, L.X.; Murphy, M.K.; Duvvuri, M.; Ding, D.; Hillel, A.T. Interferon-γ treatment of human laryngotracheal stenosis–derived fibroblasts. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1134–1140. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Feldman, G.; Freundlich, B.; Jimenez, S.A. Inhibition of excessive scleroderma fibroblast collagen production by recombinant γ-interferon: Association with a coordinate decrease in types I and III procollagen messenger RNA levels. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1986, 29, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Okazaki, H.; Sugawara, I.; Yamamoto, K.; Takizawa, H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 2003, 132, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; Bates, D.L.; Ring, A.M.; Krieg, C.; Lin, J.T.; Su, L.; Moraga, I.; Raeber, M.E.; Bowman, G.R.; Novick, P. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012, 484, 529–533. [Google Scholar] [CrossRef]
- Dubois, S.; Patel, H.J.; Zhang, M.; Waldmann, T.A.; Müller, J.R. Preassociation of IL-15 with IL-15Rα-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008, 180, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Spanholtz, J.; Sturtzel, C.; Tordoir, M.; Schlechta, B.; Groenewegen, D.; Hofer, E. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PLoS ONE 2014, 9, e87131. [Google Scholar] [CrossRef]
- Wasmuth, H. Chemokines as inflammatory mediators of fibrosis in liver fibrosis. J. Transl. Med. 2010, 8, I13. [Google Scholar] [CrossRef]
- Lacotte, S.; Brun, S.; Muller, S.; Dumortier, H. CXCR3, Inflammation, and autoimmune diseases. Contemp. Challenge Autoimmun. 2009, 1173, 310. [Google Scholar] [CrossRef]
- Groover, M.K.; Richmond, J.M. Potential therapeutic manipulations of the CXCR3 chemokine axis for the treatment of inflammatory fibrosing diseases. F1000Research 2020, 9, 1197. [Google Scholar] [CrossRef]
- Jiang, X.-P.; Ai, W.-B.; Wan, L.-Y.; Zhang, Y.-Q.; Wu, J.-F. The roles of microRNA families in hepatic fibrosis. Cell Biosci. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, G.; Wu, J.-H.; Jiang, C.-P. Diverse roles of miR-29 in cancer. Oncol. Rep. 2014, 31, 1509–1516. [Google Scholar] [CrossRef]
- Kitano, M.; Bloomston, P.M. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J. Clin. Med. 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Friedman, R.C.; Marquez, R.T.; Keck, K.; Kong, B.; Icardi, M.S.; Brown, K.E.; Burge, C.B.; Schmidt, W.N.; Wang, Y. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J. Infect. Dis. 2011, 203, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, M.; Noetel, A.; Elfimova, N.; Trebicka, J.; Schievenbusch, S.; Strack, I.; Molnar, L.; von Brandenstein, M.; Töx, U.; Nischt, R. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS ONE 2011, 6, e24568. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Iizuka, M.; Sekiya, Y.; Yoshizato, K.; Ikeda, K.; Kawada, N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem. Biophys. Res. Commun. 2010, 391, 316–321. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Tiao, M.-M.; Huang, L.-T.; Chuang, J.-H.; Kuo, K.-C.; Yang, Y.-L.; Wang, F.-S. Activation of Mir-29a in activated hepatic stellate cells modulates its profibrogenic phenotype through inhibition of histone deacetylases 4. PLoS ONE 2015, 10, e0136453. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghazwani, M.; Li, J.; Sun, M.; Stolz, D.B.; He, F.; Fan, J.; Xie, W.; Li, S. MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase. Biochem. Biophys. Res. Commun. 2014, 446, 940–944. [Google Scholar] [CrossRef]
- Mei, Z.; Su, T.; Ye, J.; Yang, C.; Zhang, S.; Xie, C. The miR-15 family enhances the radiosensitivity of breast cancer cells by targeting G2 checkpoints. Radiat. Res. 2015, 183, 196–207. [Google Scholar] [CrossRef]
- Zhu, K.; He, Y.; Xia, C.; Yan, J.; Hou, J.; Kong, D.; Yang, Y.; Zheng, G. MicroRNA-15a inhibits proliferation and induces apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol. Res. 2016, 24, 145. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Mackowiak, B.; Gao, B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Drummer IV, C.; Virtue, A.; Gao, T.; Wu, S.; Hernandez, M.; Singh, L.; Wang, H.; Yang, X.-F. Increased expression of resistin in microRNA-155-deficient white adipose tissues may be a possible driver of metabolically healthy obesity transition to classical obesity. Front. Physiol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, B.; Xin, X.; Xu, M.; Ji, G.; Yu, H. MicroRNA-34a promotes hepatic stellate cell activation via targeting ACSL1. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3008. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhang, X.; Lv, L.; Zhang, J.; Liang, W.; Wang, P. Fine-tuning the expression of microRNA-155 controls acetaminophen-induced liver inflammation. Int. Immunopharmacol. 2016, 40, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Charrier, A.; Zhou, Y.; Chen, R.; Yu, B.; Agarwal, K.; Tsukamoto, H.; Lee, L.J.; Paulaitis, M.E.; Brigstock, D.R. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 2014, 59, 1118–1129. [Google Scholar] [CrossRef]
- Wang, X.; Seo, W.; Park, S.H.; Fu, Y.; Hwang, S.; Rodrigues, R.M.; Feng, D.; Gao, B.; He, Y. MicroRNA-223 restricts liver fibrosis by inhibiting the TAZ-IHH-GLI2 and PDGF signaling pathways via the crosstalk of multiple liver cell types. Int. J. Biol. Sci. 2021, 17, 1153. [Google Scholar] [CrossRef]
- Bieback, K.; Kluter, H. Mesenchymal stromal cells from umbilical cord blood. Curr. Stem Cell Res. Ther. 2007, 2, 310–323. [Google Scholar] [CrossRef]
- Gasper, M.A.; Kunwar, P.; Itaya, G.; Lejarcegui, N.; Bosire, R.; Maleche-Obimbo, E.; Wamalwa, D.; Slyker, J.; Overbaugh, J.; Horton, H. Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection. AIDS 2014, 28, 1115. [Google Scholar] [CrossRef]
- Sarvaria, A.; Jawdat, D.; Madrigal, J.A.; Saudemont, A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front. Immunol. 2017, 8, 329. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Rabani, V.; Shahsavani, M.; Gharavi, M.; Piryaei, A.; Azhdari, Z.; Baharvand, H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol. Int. 2010, 34, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Kim, M.Y.; Cho, M.Y.; Baik, S.K.; Cho, Y.Z.; Kwon, S.O. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Okabe, M.; Saad Eldien, H.M.; Yoshida, T. AT-MSCs antifibrotic activity is improved by eugenol through modulation of TGF-β/Smad signaling pathway in rats. Molecules 2020, 25, 348. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, H.; Bai, Y.; Bai, M.; Gao, F.; Yu, J.; Wu, R.; Du, L.; Li, F. Ultrasound-targeted microbubble destruction optimized HGF-overexpressing bone marrow stem cells to repair fibrotic liver in rats. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, D.; Li, J.; Yan, X.; Zhu, J.; Xiao, P.; Chen, T.; Xie, X. Effects of bone marrow-derived mesenchymal stem cells on hypoxia and the transforming growth factor beta 1 (TGFβ-1) and SMADs pathway in a mouse model of cirrhosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Fang, X.Q.; Chen, Q.F.; Chen, H.; Xiao, P.; Peng, X.B.; Zhang, S.X.; Li, J.F.; Mao, X.R. Bone marrow-derived mesenchymal stem cells inhibit the proliferation of hepatic stellate cells by inhibiting the transforming growth factor β pathway. Mol. Med. Rep. 2015, 12, 7227–7232. [Google Scholar] [CrossRef]
- Stock, P.; Brückner, S.; Winkler, S.; Dollinger, M.M.; Christ, B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 2014, 15, 7004–7028. [Google Scholar] [CrossRef]
- Asian Institute of Gastroenterology, India. Combination of Autologous MSC and HSC Infusion in Patients With Decompensated Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT04243681 (accessed on 22 February 2022).
- Ukraine Association of Biobank. Long Term Follow up Mesenchymal Stem Cell Therapy for Patients Virus-related Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT05080465 (accessed on 22 February 2022).
- Sun Yat-Sen University; Third Affiliated Hospital, Sun Yat-Sen University. Autologous Bone Marrow Mesenchymal Stem Cells Transplantation Via Hepatic Artery in Patients With Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT00976287 (accessed on 13 January 2022).
- S-Evans Biosciences Co., Ltd.; Zhejiang University; Zhejiang General Hospital of Armed Police; Zhenjiang First People’s Hospital; Wuhan General Hospital of Guangzhou Military Command. Human Menstrual Blood-derived Mesenchymal Stem Cells for Patients With Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT01483248 (accessed on 20 January 2022).
- Alliancells Bioscience Corporation Limited. Safety and Efficacy Study of Umbilical Mesenchymal Stem Cells for Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT01573923 (accessed on 13 January 2022).
- Chaitanya Hospital, Pune. A Clinical Study to Evaluate the Safety and Efficacy of Mesenchymal Stem Cells in Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT01877759 (accessed on 23 February 2022).
- Shenzhen Beike Bio-Technology Co., Ltd.; No.85 Hospital, Changning, Shanghai, China. Human Umbilical Cord Mesenchymal Stem Cells Transplantation for Patients With Decompensated Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT01342250 (accessed on 23 February 2022).
- Rohto Pharmaceutical Co., Ltd. A Study of ADR-001 in Patients With Liver Cirrhosis. Available online: https://ClinicalTrials.gov/show/NCT03254758 (accessed on 23 February 2022).
- Shahid Beheshti University of Medical Sciences; Tarbiat Modarres University. Improvement of Liver Function in Liver Cirrhosis Patients After Autologous Mesenchymal Stem Cell Injection:a Phase I-II Clinical Trial. Available online: https://ClinicalTrials.gov/show/NCT00420134 (accessed on 27 December 2021).
- Royan, I. Transplantation of Autologous Mesenchymal Stem Cell in Decompensate Cirrhotic Patients With Pioglitazone. Available online: https://ClinicalTrials.gov/show/NCT01454336 (accessed on 20 January 2022).
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef]
- An, S.Y.; Jang, Y.J.; Lim, H.-J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.-Y.; Kim, J.H.; Do, B.-R. Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef]
- Fu, Q.; Ohnishi, S.; Sakamoto, N. Conditioned Medium from Human Amnion-Derived Mesenchymal Stem Cells Regulates Activation of Primary Hepatic Stellate Cells. Stem Cells Int. 2018, 2018, 4898152. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Cheng, X.; Wang, H.; Huang, W.; Wang, D.; Zhang, K.; Zhang, H.; Xue, Z.; Da, Y.; Zhang, N. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J. Transl. Med. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.J.; Domínguez, L.M.; Bayo, J.; García, M.G.; Mazzolini, G.D. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration: Cells and extracellular vesicles as therapeutic strategies. World J. Gastroenterol. 2018, 24, 2427. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Pu, Y.; Chen, X.; Qi, X.; Zhang, L.; Xu, L.; Li, W.; Ma, Y.; Zhou, S.; Zhu, J. hUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice. Stem Cell Res. Ther. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mardpour, S.; Hassani, S.N.; Mardpour, S.; Sayahpour, F.; Vosough, M.; Ai, J.; Aghdami, N.; Hamidieh, A.A.; Baharvand, H. Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. J. Cell. Physiol. 2018, 233, 9330–9344. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Huang, Y.; De Boer, W.B.; Adams, L.A.; MacQuillan, G.; Bulsara, M.K.; Jeffrey, G.P. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J. Hepatol. 2014, 61, 22–27. [Google Scholar] [CrossRef]
- Drenth, J.P.; Schattenberg, J.M. The nonalcoholic steatohepatitis (NASH) drug development graveyard: Established hurdles and planning for future success. Expert Opin. Investig. Drugs 2020, 29, 1365–1375. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]



| Cell Type | Functions | References | |
|---|---|---|---|
| 1 | Quiescent |
| [27] |
| 2 | Activated |
| [28] |
| 3 | Inactivated |
| [27] |
| 4 | Senescent |
| [29] |
| CT No. | Treatment/ Drug Name | Mechanism of Action | Target Diseases | Clinical Trial Design | Clinical Phase | Efficacy | Number of Patients | References |
|---|---|---|---|---|---|---|---|---|
| NCT00760513 | OMACOR | Reduce the synthesis of triglycerides (TGs) | NAFLD | Treatment with long-chain n-3 fatty acid for 18 months affects biomarkers of NAFLD. | Phase 4 | 20% decrease in liver fat | 103 participants | [92] |
| NCT02030977 | Resveratrol | Antioxidant | NAFLD, Liver Fibrosis | Effect of liver enzymes, inflammatory factors, and fibrosis in patients with NAFLD. Patients were steatosis grade 1. | Phase 3 | Study completed/ no results reported | 50 patients | [93,94] |
| NCT02548351 | Obeticholic acid REGENE-RATE | FXR agonist | NASH with fibrosis | Obeticholic acid treatment compared to placebo on histological improvement and liver-related clinical outcomes. | Phase 3 | Active | 2480 participants | [95,96] |
| NCT03008070 | IVA337 | PPAR agonist | NASH with fibrosis | A next-generation pan-PPAR agonist for the pathophysiology of NASH. | Phase 2 | Not worsening fibrosis at higher dose of 1200 mg | 247 participants | [91] |
| NCT02684591 | Aramchol | SCD1 inhibitor | NASH | To test the efficacy of 400 mg and 600 mg of Aramchol. | Phase 2 | No significant adverse effects, did not reduce hepatic fat | 247 participants | [88] |
| NCT03357380 | Semaglutide | reduces HbA1c, | NAFLD | Comparing changes in early-stage scar tissue and fat deposition in the liver. Participants self-inject medicine once daily for 72 weeks. | Phase 1 | No significant adverse Effect, did not reduce hepatic fat | 67 participants | [97] |
| NCT No. | Treatment/Drug Name | Mechanismof Action | Target Diseases | Clinical Trial Design | Clinical Phase | Efficacy | Number of Patients | References |
|---|---|---|---|---|---|---|---|---|
| NCT00049842 | Peginterferon alph-2b (SCH 54031) | Type 1 interferon activator | Liver fibrosis, chronic hepatitis C | Evaluate safety and efficacy of PEG-Intron vs. no treatment | Phase 3 | Lower fibrosis progression | 540 participants | [108,109] |
| NCT01938781 | Entecavir, Peg-IFN | Inhibits HBV DNA polymerase | Liver fibrosis | For patients with F2/F3, one arm is entecavir for 2 years, and the other is entecavir for 0.5 years and entecavir plus peg-IFN for 1 year | Phase 4 | Study completed/ no results reported | 400 participants | [110,111,112] |
| NCT00298714 | Losartan | Angiotensin II type 1 receptor antagonist | Liver fibrosis, chronic hepatitis C | Administration of angiotensin II type 1 (AT1) receptor antagonists in HSCs (fibrosis F2-F3) | Phase 4 | Study completed/ no results reported | 20 participants | [99,100] |
| NCT00990639 | Candesartan andramipril | Angiotensin II type 1 receptor antagonist | Liver fibrosis extent with chronichepatitis C | Evaluating drug action and changes in FibroScan recording | Phase 3 | Pending | 45 participants | [101,102] |
| NCT00265642 | Irbesartan | Angiotensin II type 1 receptor antagonist | Liver fibrosis, chronic hepatitis C | AT1 receptor antagonists of angiotensin II have inhibitory effects on TGF-beta 1 production, and can limit the progression of liver fibrosis | Phase 3 | No results reported | 200 participants | [103] |
| NCT04971577 | Simvastatin | HMG-CoA reductase inhibitors (statins) | Liver fibrosis | Simvastatin for reducing liver fibrosis in patients with advanced fibrosis due to alcohol | Phase 2/3 | Active | 90 participants | [107,113] |
| Interleukin Type | Produced by | Response Cell | Function | References |
|---|---|---|---|---|
| IL-2 | CD4+ T cells, CD8+ T cells, dendritic cells, and thymic cells | T cells and NK cells | Enhances cytotoxicity in NK cells; activates STAT1, STAT3, and STAT5. | [180] |
| IL-6 | Lymphocytes, monocytes, fibroblasts, vascular smooth muscle cells, and endothelial cells | Non-parenchymal cells | Deletion of IL-6 increases hepatocyte injury and apoptosis. | [181] |
| IL-10 | Hepatic stellate cells, liver sinusoidal endothelial cells, Kupffer cells, lymphocytes, and Th cells | HSCs | IL-10 inhibits HSC activation. | [182,183] |
| IL-12 | Macrophages, dendritic cells, and B lymphocytes | Th1 |
| [35,184,185] |
| IL-15 | Monocytes | NK cells |
| [186] |
| IL-22 | αβ T-cell classes Th1, Th22, and Th17, along with γδ T cells, NKTs, ILC3, neutrophils, and macrophages | HSCs |
| [31,187,188,189] |
| IL-30 | Th2 cells upon activation | NKT and HSCs | Attenuates liver fibrosis through inducing NKG2D–rae1 interaction. | [190] |
| NCT No. | Sponsor | Target Diseases | Clinical Trial Design | Clinical Phase | Status | Number of Patients | References |
|---|---|---|---|---|---|---|---|
| NCT04243681 | Asian Institute of Gastroenterology, India | Liver cirrhosis | Combination of autologous mesenchymal and hematopoietic stem cells infused in patients | Phase 4 | No results reported | 5 participants | [233] |
| NCT05080465 | Ukraine Association of Biobank | Liver cirrhosis | Long-term follow-up autologous MSC therapy for patients with virus-related liver cirrhosis | Phase 3 | Active | 700 participants | [234] |
| NCT00976287 | Sun Yat-Sen University | Liver fibrosis, chronic hepatitis C | Liver function was monitored by serum examination. The levels of serum alanine aminotransferase (ALT), total bilirubin (TB), prothrombin time (PT), and albumin (ALB) were examined at pre-transplantation, and 3 days to 2 years post-transplantation | Phase 2 | Results not posted | 50 participants | [235] |
| NCT01483248 | Zhejiang University, China | Liver cirrhosis, fibrosis | Menstrual blood-derived stem cells can improve the disease conditions in patients with liver cirrhosis. | Phase 1/2 | No results posted | 50 participants | [236] |
| NCT01573923 | Allian cells Bioscience Corporation Limited | Liver cirrhosis | Intravenous administration of umbilical MSCs for the treatment of patients with liver cirrhosis in the next three years. | Phase 1/2 | No results reported | 320 participants | [237] |
| NCT01877759 | Chaitanya Hospital, India | Liver cirrhosis | Bone-marrow-derived autologous stem cells + human umbilical-cord-derived MSCs | Phase 1/2 | No results reported | 20 participants | [238] |
| NCT01342250 | Shenzhen Beike Bio-Technology Co., Ltd. | Liver cirrhosis | Safety and efficacy of human umbilical cord (hUC)-MSC transplantation for patients with decompensated liver cirrhosis | Phase 1/2 | No results reported | 20 participants | [239] |
| NCT03254758 | Rohto Pharmaceutical Co., Ltd., Japan | Decompensated liver cirrhosis | First-in-human study of ADR-001, adipose-derived mesenchymal stem cells (AD-MSCs) | Phase 1/2 | Recruiting | 27 participants | [240] |
| NCT00420134 | Shahid Beheshti University of Medical Sciences, Iran | Liver failure, cirrhosis | Investigators try to separate MSCs from end-stage liver disease, and then these cells are differentiated into the progenitors of hepatocytes; finally, the investigators inject these cells into the portal vein under ultrasound guidance. | Phase 1/2 | No results reported | 30 Participants | [241] |
| NCT01454336 | Royan Institute | Liver fibrosis | Pioglitazone and autologous bone marrow MSC transplantation. | Phase 1 | Completed | 3 participants | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jangra, A.; Kothari, A.; Sarma, P.; Medhi, B.; Omar, B.J.; Kaushal, K. Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis. Cells 2022, 11, 1500. https://doi.org/10.3390/cells11091500
Jangra A, Kothari A, Sarma P, Medhi B, Omar BJ, Kaushal K. Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis. Cells. 2022; 11(9):1500. https://doi.org/10.3390/cells11091500
Chicago/Turabian StyleJangra, Anshika, Ashish Kothari, Phulen Sarma, Bikash Medhi, Balram Ji Omar, and Karanvir Kaushal. 2022. "Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis" Cells 11, no. 9: 1500. https://doi.org/10.3390/cells11091500
APA StyleJangra, A., Kothari, A., Sarma, P., Medhi, B., Omar, B. J., & Kaushal, K. (2022). Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis. Cells, 11(9), 1500. https://doi.org/10.3390/cells11091500







