Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biopsies and Donors
2.2. Cell Culture Condition
2.3. Isolation and Activation of T Cells
2.4. Skin Substitutes Production Using the Self-Assembly Method
2.5. Histology and Immunofluorescence Analyses
2.6. Western Blot Analysis
2.7. Gas Chromatography (GC-FID) Analysis
2.8. Cytokine Array
2.9. ELISA Assays
2.10. Statistical Analysis
3. Results
3.1. ALA Regulates the Hyperproliferation of Psoriatic Keratinocytes in the Presence of T Cells
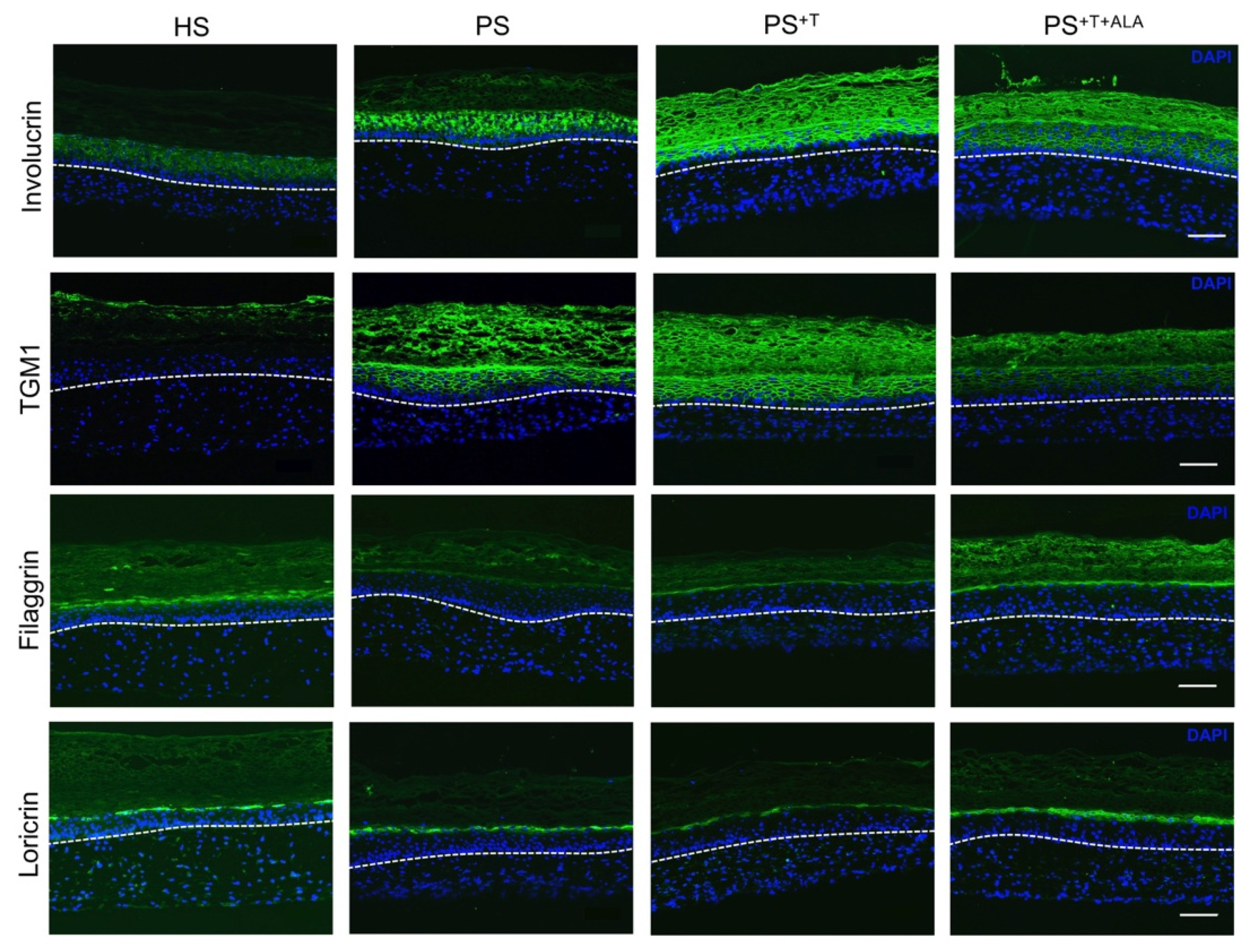
3.2. Restoration of Epidermal Cell Differentiation Proteins following ALA Supplementation in Psoriatic Substitutes
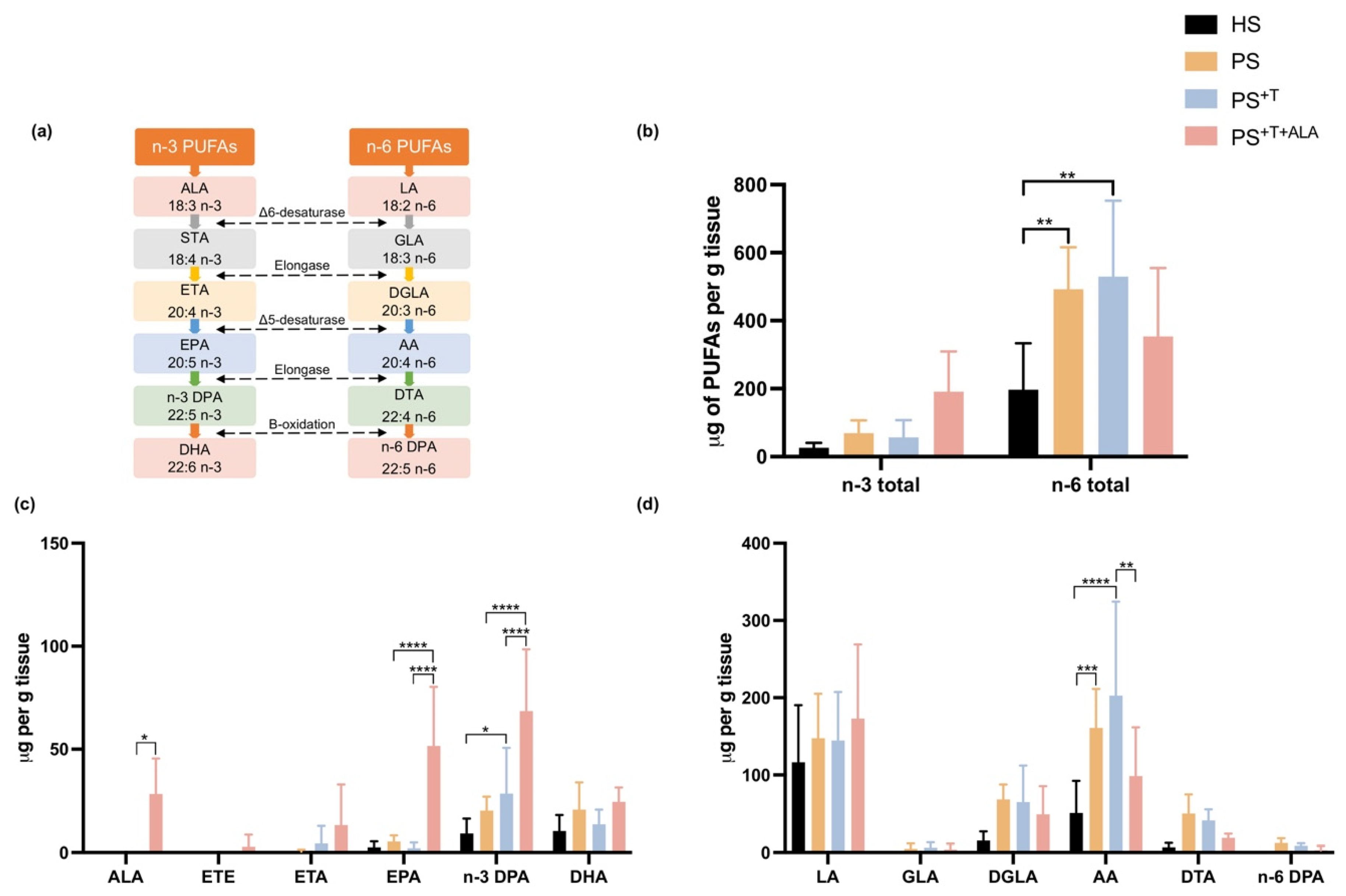
3.3. Effects of ALA Supplementation on the Epidermal n-3 and n-6 Phospholipids of the Skin Substitutes
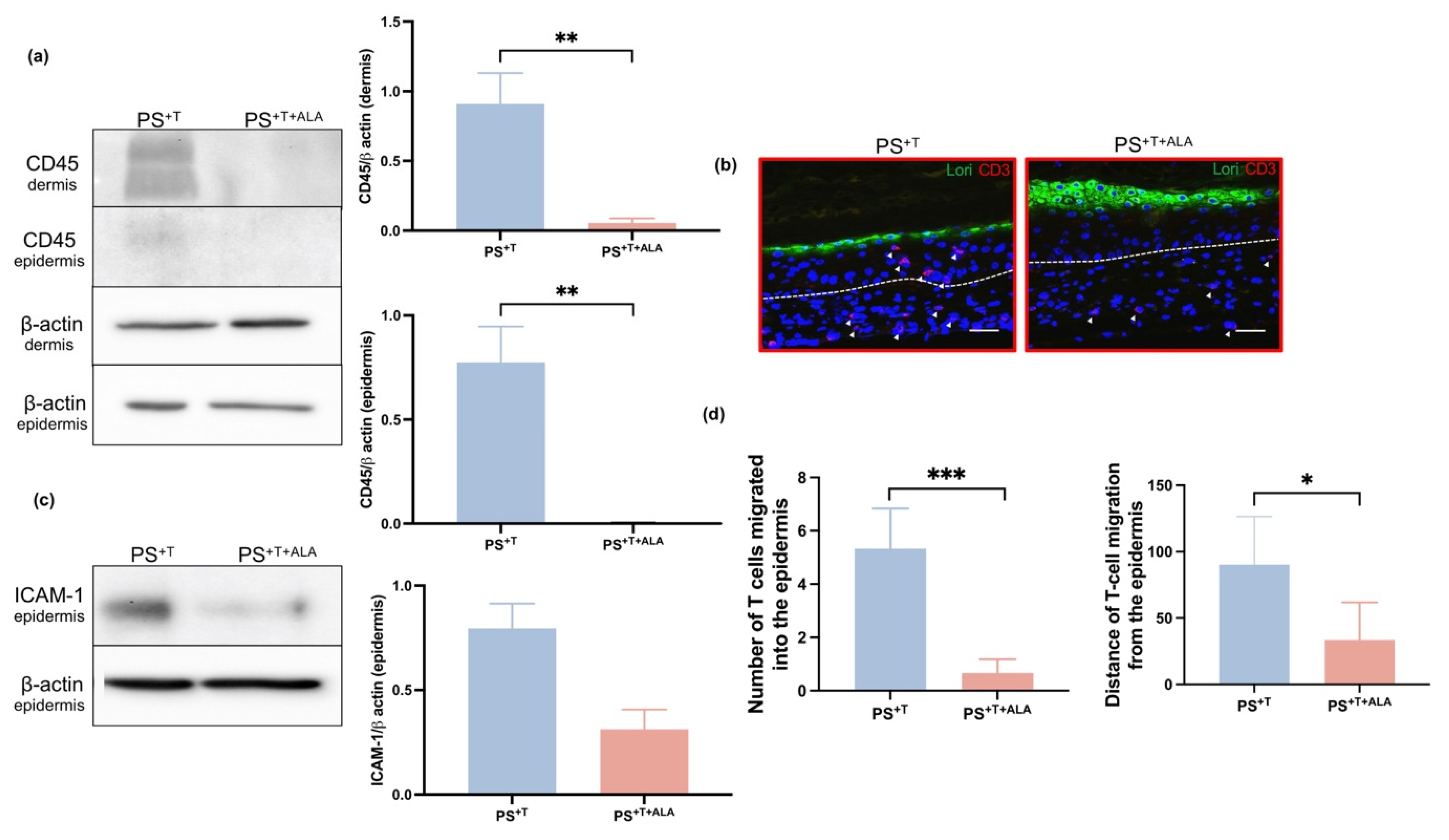
3.4. Impact of ALA on the Addition of T Cells to Psoriatic Skin Substitutes
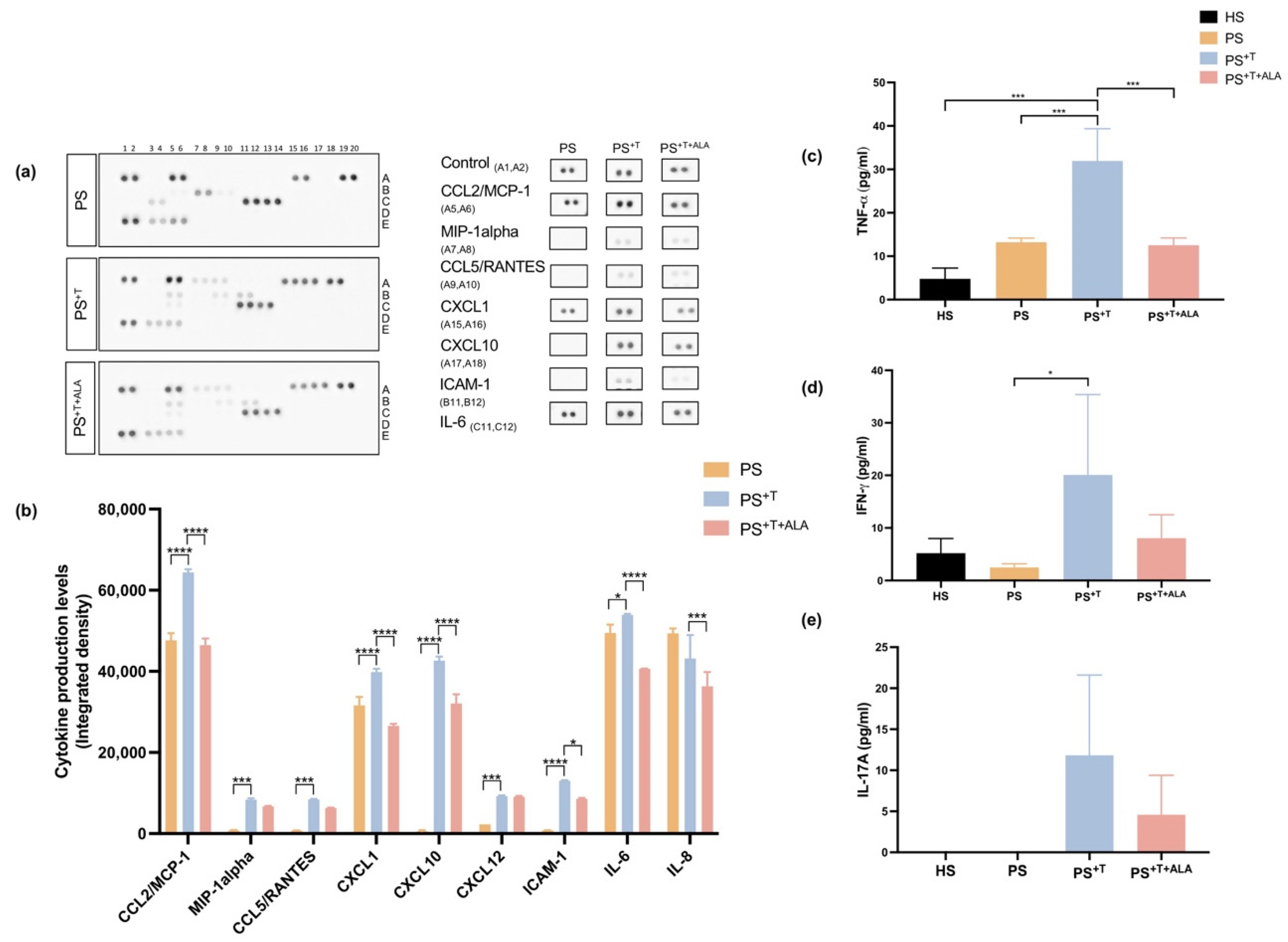
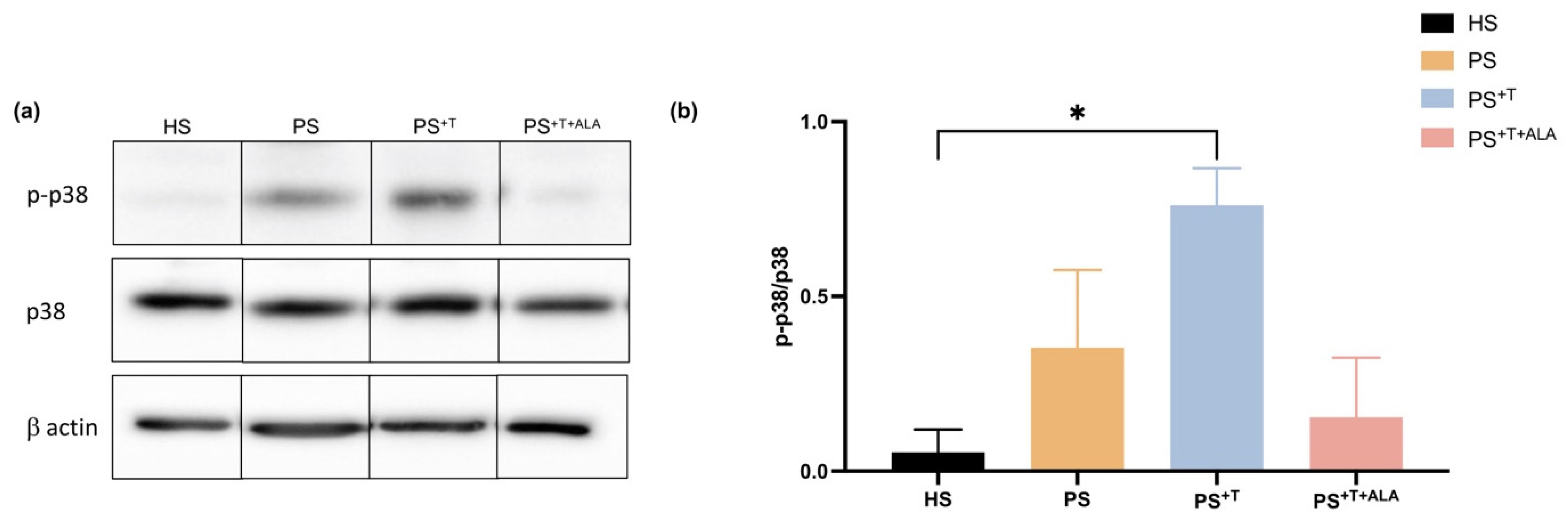
3.5. Impact of ALA Supplementation on Inflammatory Cytokine Secretion by T Cells and Downstream Signaling in Psoriatic Substitutes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lebwohl, M. Psoriasis. Lancet 2003, 361, 1197–1204. [Google Scholar] [CrossRef]
- Martinez-Ortega, J.M.; Nogueras, P.; Munoz-Negro, J.E.; Gutierrez-Rojas, L.; Gonzalez-Domenech, P.; Gurpegui, M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: A case-control study. J. Psychosom. Res. 2019, 124, 109780. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Qin, J.Z.; Nestle, F.O. Immunopathogenesis of psoriasis. Clin. Rev. Allergy Immunol. 2007, 33, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T cells in the peripheral circulation in psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkhurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, T.; Honma, M.; Iinuma, S.; Iwasaki, T.; Takahashi, H.; Ishida-Yamamoto, A. Alteration of serum thymus and activation-regulated chemokine level during biologic therapy for psoriasis: Possibility as a marker reflecting favorable response to anti-interleukin-17A agents. J. Dermatol. 2018, 45, 710–714. [Google Scholar] [CrossRef]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Norris, P.C.; English, J.T.; Dey, A.K.; Chaturvedi, A.; Baumer, Y.; Silverman, J.; Playford, M.P.; Serhan, C.N.; Mehta, N.N. Identification of proresolving and inflammatory lipid mediators in human psoriasis. J. Clin. Lipidol. 2018, 12, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Kabashima, K. Current understanding of the role of dietary lipids in the pathophysiology of psoriasis. J. Dermatol. Sci. 2019, 94, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upala, S.; Yong, W.C.; Theparee, T.; Sanguankeo, A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: A systematic review. Int. J. Rheum. Dis. 2017, 20, 442–450. [Google Scholar] [CrossRef]
- Qin, S.; Wen, J.; Bai, X.C.; Chen, T.Y.; Zheng, R.C.; Zhou, G.B.; Ma, J.; Feng, J.Y.; Zhong, B.L.; Li, Y.M. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Mol. Med. Rep. 2014, 9, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Radzikowska, U.; Rinaldi, A.O.; Celebi Sozener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef]
- Jeffery, N.M.; Sanderson, P.; Sherrington, E.J.; Newsholme, E.A.; Calder, P.C. The ratio of n-6 to n-3 polyunsaturated fatty acids in the rat diet alters serum lipid levels and lymphocyte functions. Lipids 1996, 31, 737–745. [Google Scholar] [CrossRef]
- Endres, S.; Meydani, S.N.; Ghorbani, R.; Schindler, R.; Dinarello, C.A. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J. Leukoc. Biol. 1993, 54, 599–603. [Google Scholar] [CrossRef]
- Rossetti, R.G.; Seiler, C.M.; DeLuca, P.; Laposata, M.; Zurier, R.B. Oral administration of unsaturated fatty acids: Effects on human peripheral blood T lymphocyte proliferation. J. Leukoc. Biol. 1997, 62, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.; Allsopp, P.J.; Nyland, J.F.; Magee, P.J.; Strain, J.J.; Doherty, L.C.; Watson, G.E.; Ball, E.; Riddell, C.; Armstrong, D.J.; et al. Inflammatory response following in vitro exposure to methylmercury with and without n-3 long chain polyunsaturated fatty acids in peripheral blood mononuclear cells from systemic lupus erythematosus patients compared to healthy controls. Toxicol. Vitr. 2018, 52, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balic, A.; Vlasic, D.; Zuzul, K.; Marinovic, B.; Bukvic Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, J.A.; Wold, A.E.; Sandberg, A.S.; Ostman, S.M. The Polyunsaturated Fatty Acids Arachidonic Acid and Docosahexaenoic Acid Induce Mouse Dendritic Cells Maturation but Reduce T-Cell Responses In Vitro. PLoS ONE 2015, 10, e0143741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.Y.; Arita, M.; Kim, K.; Li, X.; Zhang, H.; Kang, J.X. An omega-3 polyunsaturated fatty acid derivative, 18-HEPE, protects against CXCR4-associated melanoma metastasis. Carcinogenesis 2018, 39, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, H.; Mahmoudi, M.; Banihashemi, M.; Rastin, M.; Azad, F.J. Investigation of dietary supplements prevalence as complementary therapy: Comparison between hospitalized psoriasis patients and non-psoriasis patients, correlation with disease severity and quality of life. Complement. Ther. Med. 2017, 33, 65–71. [Google Scholar] [CrossRef]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guérin, S.S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Rioux, G.; Simard, M.; Morin, S.; Lorthois, I.; Guerin, S.L.; Pouliot, R. Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: Focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 2021, 136, 210–222. [Google Scholar] [CrossRef]
- Germain, L.; Rouabhia, M.; Guignard, R.; Carrier, L.; Bouvard, V.; Auger, F.A. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993, 19, 99–104. [Google Scholar] [CrossRef]
- Simard, M.; Julien, P.; Fradette, J.; Pouliot, R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells 2019, 8, 1142. [Google Scholar] [CrossRef] [Green Version]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gevariya, N.; Besancon, M.; Robitaille, K.; Picard, V.; Diabate, L.; Alesawi, A.; Julien, P.; Fradet, Y.; Bergeron, A.; Fradet, V. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate 2019, 79, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2021, 140, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, W.; Li, B.; Wang, G. Keratin 17 in psoriasis: Current understanding and future perspectives. Semin. Cell Dev. Biol. 2021. [Google Scholar] [CrossRef]
- Bernard, B.A.; Robinson, S.M.; Vandaele, S.; Mansbridge, J.N.; Darmon, M. Abnormal maturation pathway of keratinocytes in psoriatic skin. Br. J. Dermatol. 1985, 112, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Guerard, S.; Fortin, M.M.; Rusu, D.; Soucy, J.; Poubelle, P.E.; Pouliot, R. Pathological crosstalk in vitro between T lymphocytes and lesional keratinocytes in psoriasis: Necessity of direct cell-to-cell contact. Lab. Investig. 2012, 92, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H. Crosstalk between keratinocytes and T cells in a 3D microenvironment: A model to study inflammatory skin diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Altin, J.G.; Sloan, E.K. The role of CD45 and CD45-associated molecules in T cell activation. Immunol. Cell. Biol. 1997, 75, 430–445. [Google Scholar] [CrossRef]
- Courtney, A.H.; Shvets, A.A.; Lu, W.; Griffante, G.; Mollenauer, M.; Horkova, V.; Lo, W.L.; Yu, S.; Stepanek, O.; Chakraborty, A.K.; et al. CD45 functions as a signaling gatekeeper in T cells. Sci. Signal. 2019, 12, eaaw8151. [Google Scholar] [CrossRef]
- Symington, F.W.; Santos, E.B. Lysis of human keratinocytes by allogeneic HLA class I-specific cytotoxic T cells. Keratinocyte ICAM-1 (CD54) and T cell LFA-1 (CD11a/CD18) mediate enhanced lysis of IFN-gamma-treated keratinocytes. J. Immunol. 1991, 146, 2169–2175. [Google Scholar]
- Juffermans, N.P.; Dekkers, P.E.; Peppelenbosch, M.P.; Speelman, P.; van Deventer, S.J.; van Der Poll, T. Expression of the chemokine receptors CXCR1 and CXCR2 on granulocytes in human endotoxemia and tuberculosis: Involvement of the p38 mitogen-activated protein kinase pathway. J. Infect. Dis. 2000, 182, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Tang, X.; Wang, F.; Han, J. Association Between Daily Dietary Eicosatetraenoic Acid Intake and the Lower Risk of Psoriasis in American Adults. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Niehues, H.; van den Bogaard, E.H. Past, present and future of in vitro 3D reconstructed inflammatory skin models to study psoriasis. Exp. Dermatol. 2018, 27, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzicka, T.; Simmet, T.; Peskar, B.A.; Ring, J. Skin levels of arachidonic acid-derived inflammatory mediators and histamine in atopic dermatitis and psoriasis. J. Investig. Dermatol. 1986, 86, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.Y.; Kim, W.; Callaway, E.; Smith, R.; Jia, Q.; Zhou, L.; McMurray, D.N.; Chapkin, R.S. fat-1 transgene expression prevents cell culture-induced loss of membrane n-3 fatty acids in activated CD4+ T-cells. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldon, S.E.; O’Loughlin, C.W.; Ray, D.M.; Landskroner-Eiger, S.; Seweryniak, K.E.; Phipps, R.P. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am. J. Pathol. 2006, 169, 1183–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijeira, A.; Hunter, M.C.; Russo, E.; Proulx, S.T.; Frei, T.; Debes, G.F.; Coles, M.; Melero, I.; Detmar, M.; Rouzaut, A.; et al. T Cell Migration from Inflamed Skin to Draining Lymph Nodes Requires Intralymphatic Crawling Supported by ICAM-1/LFA-1 Interactions. Cell Rep. 2017, 18, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Orlik, C.; Deibel, D.; Kublbeck, J.; Balta, E.; Ganskih, S.; Habicht, J.; Niesler, B.; Schroder-Braunstein, J.; Schakel, K.; Wabnitz, G.; et al. Keratinocytes costimulate naive human T cells v.via CD2: A potential target to prevent the development of proinflammatory Th1 cells in the skin. Cell Mol. Immunol. 2020, 17, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Schurer, N.Y.; Rippke, F.; Vogelsang, K.; Schliep, V.; Ruzicka, T. Fatty acid uptake by cultured human keratinocytes grown in medium deficient in or supplemented with essential fatty acids. Arch. Dermatol. Res. 1999, 291, 47–53. [Google Scholar] [CrossRef]
- Hou, T.Y.; Barhoumi, R.; Fan, Y.Y.; Rivera, G.M.; Hannoush, R.N.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress CD4+ T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim. Biophys. Acta 2016, 1858, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.; Kim, S.; Kim, S.; Kim, D.I.; Kang, K.W.; Hong, S.H.; Lee, S.M.; Koh, H.R.; Seo, Y.J. n-3 Polyunsaturated Fatty Acids Impede the TCR Mobility and the TCR-pMHC Interaction of Anti-Viral CD8+ T Cells. Viruses 2020, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Libby, P. Control of endothelial leukocyte adhesion molecules by fatty acids. Lipids 1996, 31 (Suppl. S1), S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Ward, R.; Pestel, J.; Robert, M.; Pesenti, S.; Bendridi, N.; Michalski, M.C.; Laville, M.; Vidal, H.; Eljaafari, A. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-17A Secretion through Decreased ICAM-1 Expression in T Cells Co-Cultured with Adipose-Derived Stem Cells Harvested from Adipose Tissues of Obese Subjects. Mol. Nutr. Food Res. 2019, 63, e1801148. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Farinas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.; Krueger, J.G. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J. Investig. Dermatol. 2012, 132, 2552–2564. [Google Scholar] [CrossRef] [Green Version]
- Carabelli, J.; Prato, C.A.; Sanmarco, L.M.; Aoki, M.P.; Campetella, O.; Tribulatti, M.V. Interleukin-6 signalling mediates Galectin-8 co-stimulatory activity of antigen-specific CD4 T-cell response. Immunology 2018, 155, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Kolobaric, N.; Drenjancevic, I.; Matic, A.; Susnjara, P.; Mihaljevic, Z.; Mihalj, M. Dietary Intake of n-3 PUFA-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults-A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef]
- Jaudszus, A.; Gruen, M.; Watzl, B.; Ness, C.; Roth, A.A.; Lochner, A.; Barz, D.; Gabriel, H.; Rothe, M.; Jahreis, G. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J. Lipid. Res. 2013, 54, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Stando, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Avina, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF alpha, IL-1 beta, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Li, Y.; Fischer, M.J.M.; Steinhoff, M.; Chen, W.; Wang, J. Th2 Modulation of Transient Receptor Potential Channels: An Unmet Therapeutic Intervention for Atopic Dermatitis. Front. Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef] [PubMed]
- Hammerberg, C.; Bata-Csorgo, Z.; Voorhees, J.J.; Cooper, K.D. IL-1 and IL-1 receptor antagonist regulation during keratinocyte cell cycle and differentiation in normal and psoriatic epidermis. Arch. Dermatol. Res. 1998, 290, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurchiu, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, T.; Kabashima, K. Prostanoids and leukotrienes in the pathophysiology of atopic dermatitis and psoriasis. Int. Immunol. 2019, 31, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.Y.; Pathak, H.B.; Godwin, A.K.; Kwon, Y. Epithelial-stromal communication via CXCL1-CXCR2 interaction stimulates growth of ovarian cancer cells through p38 activation. Cell Oncol. 2021, 44, 77–92. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [Green Version]
- Rincon, M.; Pedraza-Alva, G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 2003, 192, 131–142. [Google Scholar] [CrossRef]
- Wang, X.; Breeze, A.; Kulka, M. N-3 polyunsaturated fatty acids inhibit IFN-gamma-induced IL-18 binding protein production by prostate cancer cells. Cancer Immunol. Immunother. 2015, 64, 249–258. [Google Scholar] [CrossRef]
- Kim, H.H.; Cho, S.; Lee, S.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J. Lipid Res. 2006, 47, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loft, N.D.; Vaengebjerg, S.; Halling, A.S.; Skov, L.; Egeberg, A. Adverse events with IL-17 and IL-23 inhibitors for psoriasis and psoriatic arthritis: A systematic review and meta-analysis of phase III studies. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1151–1160. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, S.; Simard, M.; Rioux, G.; Julien, P.; Pouliot, R. Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model. Cells 2022, 11, 1513. https://doi.org/10.3390/cells11091513
Morin S, Simard M, Rioux G, Julien P, Pouliot R. Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model. Cells. 2022; 11(9):1513. https://doi.org/10.3390/cells11091513
Chicago/Turabian StyleMorin, Sophie, Mélissa Simard, Geneviève Rioux, Pierre Julien, and Roxane Pouliot. 2022. "Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model" Cells 11, no. 9: 1513. https://doi.org/10.3390/cells11091513
APA StyleMorin, S., Simard, M., Rioux, G., Julien, P., & Pouliot, R. (2022). Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model. Cells, 11(9), 1513. https://doi.org/10.3390/cells11091513








