Consequences of Chromosome Loss: Why Do Cells Need Each Chromosome Twice?
Abstract
:1. Introduction
2. Monosomy Is Detrimental in Most Organisms, but Can Be Tolerated
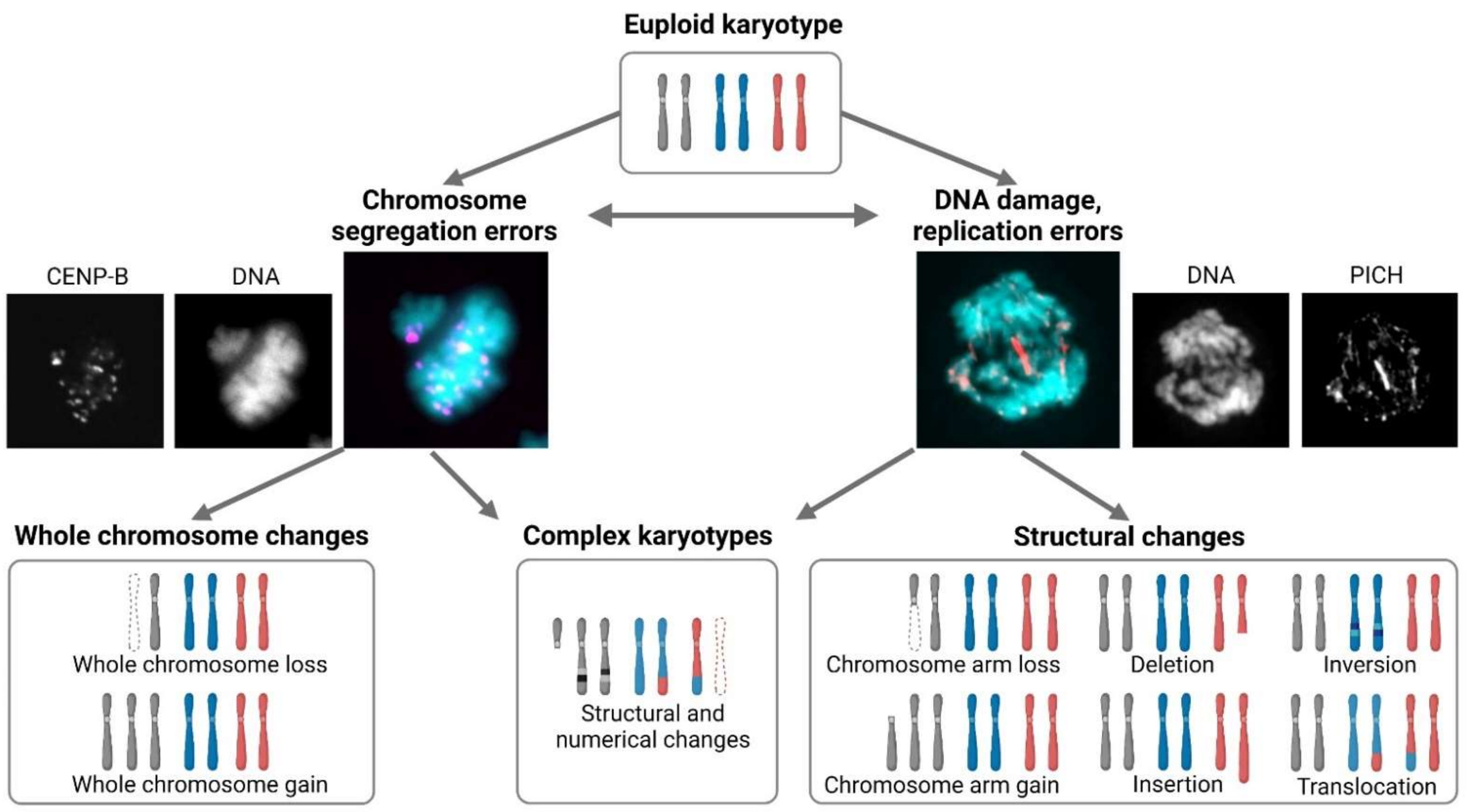
3. Model Systems for the Analysis of the Consequences of Chromosome Loss
4. Reduced Proliferation and Impaired Genomic Stability Due to Monosomy
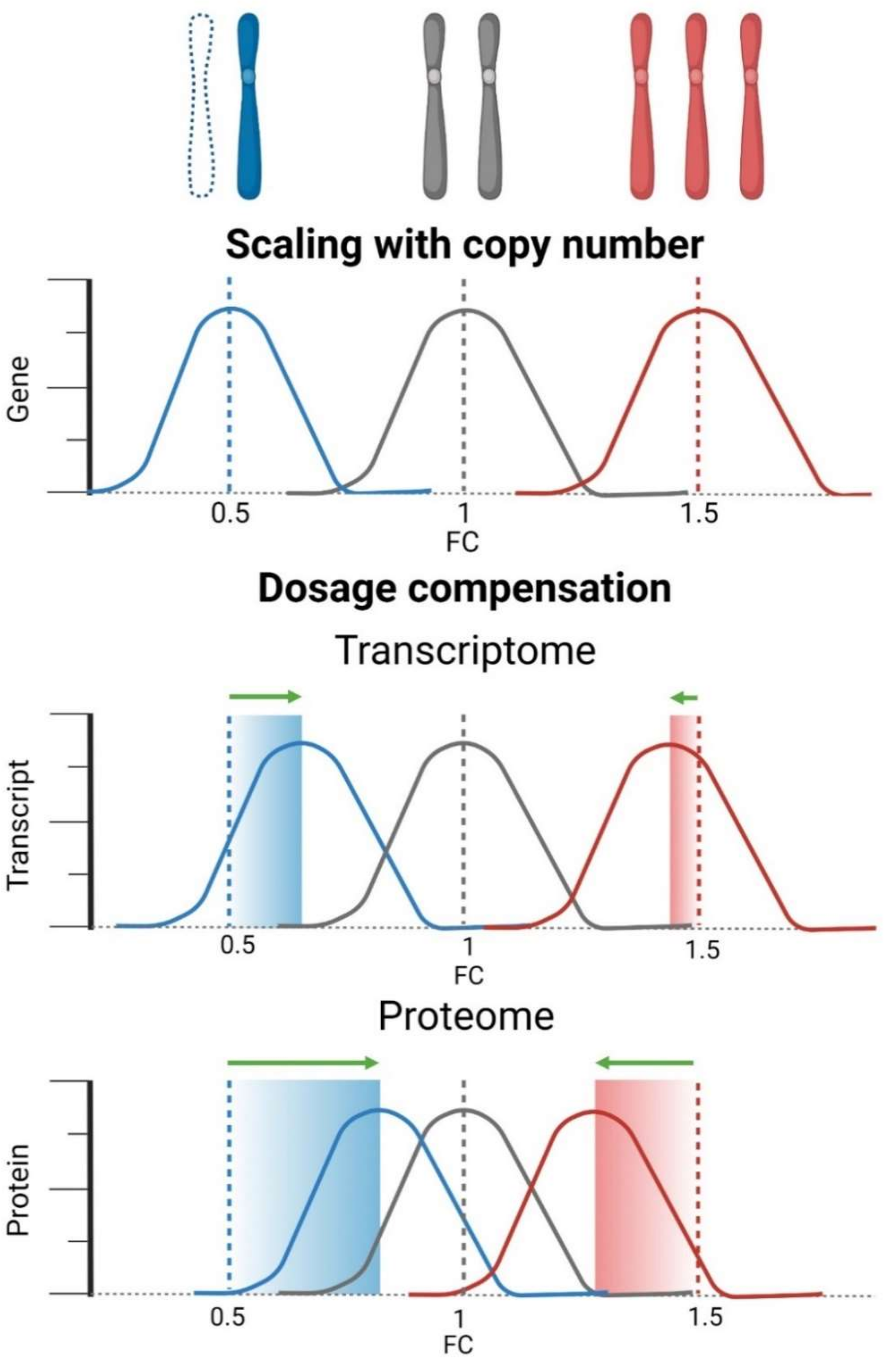
5. Loss of a Chromosome Leads to a Reduced Expression of the Monosomic Genes
6. Genome-Wide Expression Changes in Response to Chromosome Loss
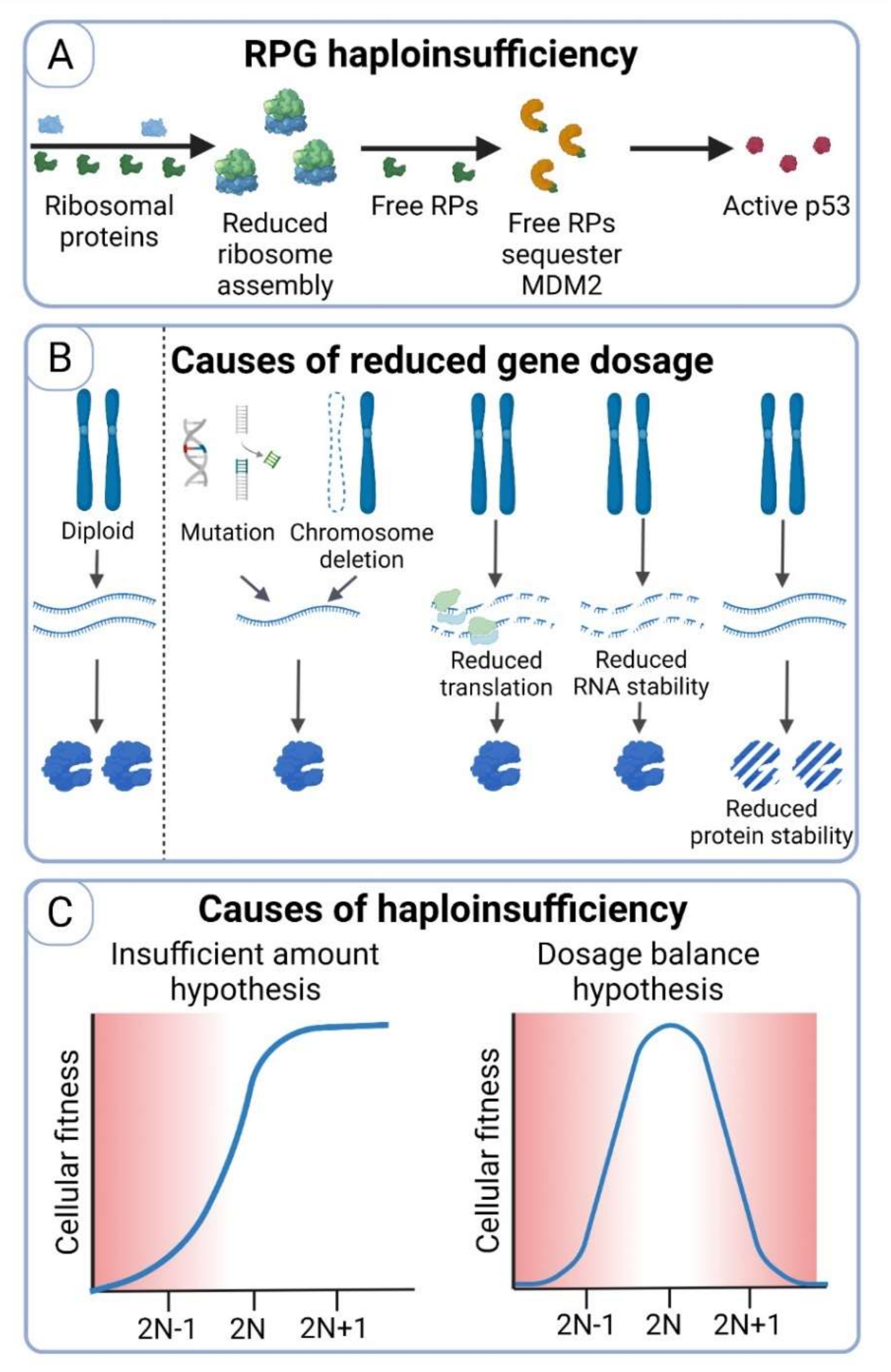
7. Haploinsufficiency as the Main Detrimental Consequence of Chromosome Loss
8. Cellular Response to Monosomy Differs from the Response to Trisomy
9. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sansregret, L.; Swanton, C. The Role of Aneuploidy in Cancer Evolution. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, R.C.; Demko, Z.P.; Ryan, A.; Banjevic, M.; Hill, M.; Sigurjonsson, S.; Rabinowitz, M.; Petrov, D.A. Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genet. 2015, 11, e1005601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, J.J.; Amon, A. New Insights into the Troubles of Aneuploidy. Annu. Rev. Cell Dev. Biol. 2012, 28, 189–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, E.; Minasi, M.G.; Fiorentino, F. Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts. N. Engl. J. Med. 2015, 373, 2089–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, T.; Debrot, S.; Dimpfl, J.; Zweig, A.; Zamora, T.; Epstein, C.J. The early lethality of autosomal monosomy in the mouse. J. Exp. Zool 1985, 236, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Knouse, K.A.; Wu, J.; Whittaker, C.A.; Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 13409–13414. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, H.; Sun, C.; Zhang, J.; Wang, J.; Du, M.; Li, J.; Di, L.; Shen, J.; Geng, S. Low-frequency somatic copy number alterations in normal human lymphocytes revealed by large-scale single-cell whole-genome profiling. Genome Res. 2022, 32, 44–54. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Chunduri, N.K.; Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019, 21, 54–62. [Google Scholar] [CrossRef]
- Zhu, J.; Tsai, H.-J.; Gordon, M.R.; Li, R. Cellular Stress Associated with Aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Santaguida, S.; Richardson, A.; Iyer, D.R.; M'Saad, O.; Zasadil, L.; Knouse, K.A.; Wong, Y.L.; Rhind, N.; Desai, A.; Amon, A. Chromosome Mis-segregation Generates Cell-Cycle-Arrested Cells with Complex Karyotypes that Are Eliminated by the Immune System. Dev. Cell 2017, 41, 638–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigano, C.; von Schubert, C.; Ahrne, E.; Schmidt, A.; Lorber, T.; Bubendorf, L.; De Vetter, J.R.F.; Zaman, G.J.R.; Storchova, Z.; Nigg, E.A. Quantitative proteomic and phosphoproteomic comparison of human colon cancer DLD-1 cells differing in ploidy and chromosome stability. Mol. Biol. Cell 2018, 29, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Krivega, M.; Stiefel, C.M.; Karbassi, S.; Andersen, L.L.; Chunduri, N.K.; Donnelly, N.; Pichlmair, A.; Storchová, Z. Genotoxic stress in constitutive trisomies induces autophagy and the innate immune response via the cGAS-STING pathway. Commun. Biol. 2021, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Stingele, S.; Stoehr, G.; Peplowska, K.; Cox, J.; Mann, M.; Storchova, Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 2012, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Oromendia, A.B.; Dodgson, S.E.; Amon, A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012, 26, 2696–2708. [Google Scholar] [CrossRef] [Green Version]
- Santaguida, S.; Vasile, E.; White, E.; Amon, A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 2015, 29, 2010–2021. [Google Scholar] [CrossRef] [Green Version]
- Santaguida, S.; Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 2015, 16, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, A.; Ohori, M.; Iwai, K.; Nakayama, Y.; Nambu, T.; Morishita, D.; Kawamoto, T.; Miyamoto, M.; Hirayama, T.; Okaniwa, M.; et al. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat. Commun. 2015, 6, 7668. [Google Scholar] [CrossRef] [Green Version]
- Sheltzer, J.M.; Torres, E.M.; Dunham, M.J.; Amon, A. Transcriptional consequences of aneuploidy. Proc. Natl. Acad. Sci. USA 2012, 109, 12644–12649. [Google Scholar] [CrossRef] [Green Version]
- Durrbaum, M.; Kuznetsova, A.Y.; Passerini, V.; Stingele, S.; Stoehr, G.; Storchova, Z. Unique features of the transcriptional response to model aneuploidy in human cells. BMC Genom. 2014, 15, 139. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.M.; Sokolsky, T.; Tucker, C.M.; Chan, L.Y.; Boselli, M.; Dunham, M.J.; Amon, A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 2007, 317, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhang, C.-Z.; Wala, J.; Mermel, C.H. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, M.N.; Wang, T.; Tao, X.; Weatherbee, B.A.T.; Sun, L.; Zhan, Y.; Keller, L.; Smith, G.D.; Pellicer, A.; Scott, R.T., Jr.; et al. Developmental potential of aneuploid human embryos cultured beyond implantation. Nat. Commun. 2020, 11, 3987. [Google Scholar] [CrossRef]
- Biancotti, J.C.; Narwani, K.; Mandefro, B.; Golan-Lev, T.; Buehler, N.; Hill, D.; Svendsen, C.N.; Benvenisty, N. The in vitro survival of human monosomies and trisomies as embryonic stem cells. Stem Cell Res. 2012, 9, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Bellott, D.W.; Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Cho, T.-J.; Koutseva, N.; Zaghlul, S.; Graves, T.; Rock, S. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 2014, 508, 494–499. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Nelliat, A. A double-edged sword: Aneuploidy is a prevalent strategy in fungal adaptation. Genes 2019, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Alvaro, D.; Sunjevaric, I.; Reid, R.J.; Lisby, M.; Stillman, D.J.; Rothstein, R. Systematic hybrid LOH: A new method to reduce false positives and negatives during screening of yeast gene deletion libraries. Yeast 2006, 23, 1097–1106. [Google Scholar] [CrossRef]
- Beach, R.R.; Ricci-Tam, C.; Brennan, C.M.; Moomau, C.A.; Hsu, P.H.; Hua, B.; Silberman, R.E.; Springer, M.; Amon, A. Aneuploidy Causes Non-genetic Individuality. Cell 2017, 169, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Darrah, L.; Coe, E. Dosage effects on morphological and quantitative traits in maize aneuploids. Genome 1996, 39, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Guan, J.; Luo, J.; Zhao, L.; Li, Y.; Chen, W.; Zhang, L.; Ning, S.; Yuan, Z.; Li, A. A transcriptomic view of the ability of nascent hexaploid wheat to tolerate aneuploidy. BMC Plant. Biol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Li, N.; Gong, L.; Gou, X.; Wang, B.; Deng, X.; Li, C.; Dong, Q.; Zhang, H.; Liu, B. Global Analysis of Gene Expression in Response to Whole-Chromosome Aneuploidy in Hexaploid Wheat. Plant. Physiol. 2017, 175, 828–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knouse, K.A.; Davoli, T.; Elledge, S.J.; Amon, A. Aneuploidy in Cancer: Seq-ing Answers to Old Questions. Annu. Rev. Cancer Biol. 2017, 1, 335–354. [Google Scholar] [CrossRef]
- Breems, D.A.; Van Putten, W.L.; De Greef, G.E.; Van Zelderen-Bhola, S.L.; Gerssen-Schoorl, K.B.; Mellink, C.H.; Nieuwint, A.; Jotterand, M.; Hagemeijer, A.; Beverloo, H.B. Monosomal karyotype in acute myeloid leukemia: A better indicator of poor prognosis than a complex karyotype. J. Clin. Oncol. 2008, 26, 4791–4797. [Google Scholar] [CrossRef]
- Ahn, H.K.; Kim, D.H.D.; Park, S.; Jang, J.H.; Kim, K.; Kim, H.-J.; Kim, S.-H.; Jung, C.W. Monosomy Karyotype in Acute Myeloid Leukemia Predicts Adverse Treatment Outcome with Low Complete Remission Rate and Worse Event-Free and Overall Survival, and Associates with High Functional Activity of Multidrug Resistance (MDR). Blood 2009, 114, 3112. [Google Scholar] [CrossRef]
- Yona, A.H.; Manor, Y.S.; Herbst, R.H.; Romano, G.H.; Mitchell, A.; Kupiec, M.; Pilpel, Y.; Dahan, O. Chromosomal duplication is a transient evolutionary solution to stress. Proc. Natl. Acad. Sci. USA 2012, 109, 21010–21015. [Google Scholar] [CrossRef] [Green Version]
- Selmecki, A.M.; Dulmage, K.; Cowen, L.E.; Anderson, J.B.; Berman, J. Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet. 2009, 5, e1000705. [Google Scholar] [CrossRef]
- Kaya, A.; Mariotti, M.; Tyshkovskiy, A.; Zhou, X.; Hulke, M.L.; Ma, S.; Gerashchenko, M.V.; Koren, A.; Gladyshev, V.N. Molecular signatures of aneuploidy-driven adaptive evolution. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lukow, D.A.; Sausville, E.L.; Suri, P.; Chunduri, N.K.; Wieland, A.; Leu, J.; Smith, J.C.; Girish, V.; Kumar, A.A.; Kendall, J. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 2021, 56, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.R.; Martis, V.; Martin, S.; Tijhuis, A.E.; Hong, C.; Wardenaar, R.; Dumont, M.; Zerbib, J.; Spierings, D.C.; Fachinetti, D. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 2021, 56, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Barney, J.B.; Chandrashekarappa, D.G.; Soncini, S.R.; Schmidt, M.C. Drug resistance in diploid yeast is acquired through dominant alleles, haploinsufficiency, gene duplication and aneuploidy. PLoS Genet. 2021, 17, e1009800. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kravets, A.; Bethlendy, G.; Welle, S.; Rustchenko, E. Chromosome 5 monosomy of Candida albicans controls susceptibility to various toxic agents, including major antifungals. Antimicrob. Agents Chemother. 2013, 57, 5026–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, F.; Balaban, G.; Moorhead, P.; Bianchi, D.; Schlesinger, H. Abnormalities of chromosome 1p in human neuroblastoma tumors and cell lines. Cancer Genet. Cytogen. 1982, 7, 33–42. [Google Scholar] [CrossRef]
- Caron, H.; van Sluis, P.; de Kraker, J.; Bökkerink, J.; Egeler, M.; Laureys, G.; Slater, R.; Westerveld, A.; Voute, P.; Versteeg, R. Allelic loss of chromosome 1p as a predictor of unfavorable outcome in patients with neuroblastoma. N. Engl. J. Med. 1996, 334, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Hasle, H.; Alonzo, T.A.; Auvrignon, A.; Behar, C.; Chang, M.; Creutzig, U.; Fischer, A.; Forestier, E.; Fynn, A.; Haas, O.A.; et al. Monosomy 7 and deletion 7q in children and adolescents with acute myeloid leukemia: An international retrospective study. Blood 2007, 109, 4641–4647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosono, N.; Makishima, H.; Jerez, A.; Yoshida, K.; Przychodzen, B.; McMahon, S.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Miyano, S.; et al. Recurrent genetic defects on chromosome 7q in myeloid neoplasms. Leukemia 2014, 28, 1348–1351. [Google Scholar] [CrossRef] [Green Version]
- Honda, H.; Nagamachi, A.; Inaba, T. -7/7q- syndrome in myeloid-lineage hematopoietic malignancies: Attempts to understand this complex disease entity. Oncogene 2015, 34, 2413–2425. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Rappaport, A.R.; Kitzing, T.; Schultz, N.; Zhao, Z.; Shroff, A.S.; Dickins, R.A.; Vakoc, C.R.; Bradner, J.E. MLL3 is a haploinsufficient 7q tumor suppressor in acute myeloid leukemia. Cancer Cell 2014, 25, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, C.; Xu, Z.; Scuoppo, C.; Rillahan, C.D.; Gao, J.; Spitzer, B.; Bosbach, B.; Kastenhuber, E.R.; Baslan, T.; et al. Deletions linked to TP53 loss drive cancer through p53-independent mechanisms. Nature 2016, 531, 471–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- William, W.N.; Zhao, X.; Bianchi, J.J.; Lin, H.Y.; Cheng, P.; Lee, J.J.; Carter, H.; Alexandrov, L.B.; Abraham, J.P.; Spetzler, D.B. Immune evasion in HPV− head and neck precancer–cancer transition is driven by an aneuploid switch involving chromosome 9p loss. Proc. Natl. Acad. Sci. USA 2021, 118, 5313. [Google Scholar] [CrossRef]
- Mora, J.; Cheung, N.-K.V.; Kushner, B.H.; LaQuaglia, M.P.; Kramer, K.; Fazzari, M.; Heller, G.; Chen, L.; Gerald, W.L. Clinical categories of neuroblastoma are associated with different patterns of loss of heterozygosity on chromosome arm 1p. J. Mol. Diagn. 2000, 2, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Kushner, B.H.; Cheung, N.K. Allelic loss of chromosome 1p in neuroblastoma. N. Engl. J. Med. 1996, 334, 1608–1609. [Google Scholar] [CrossRef]
- Ball, M.K.; Kollmeyer, T.M.; Praska, C.E.; McKenna, M.L.; Giannini, C.; Raghunathan, A.; Jentoft, M.E.; Lachance, D.H.; Kipp, B.R.; Jenkins, R.B. Frequency of false-positive FISH 1p/19q codeletion in adult diffuse astrocytic gliomas. Neuro-Oncol. Adv. 2020, 2, vdaa109. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, G.; Jenkins, R. Gliomas with 1p/19q codeletion: Aka oligodendroglioma. Cancer J. 2008, 14, 352–357. [Google Scholar] [CrossRef]
- Wahl, M.; Phillips, J.J.; Molinaro, A.M.; Lin, Y.; Perry, A.; Haas-Kogan, D.A.; Costello, J.F.; Dayal, M.; Butowski, N.; Clarke, J.L.; et al. Chemotherapy for adult low-grade gliomas: Clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro-Oncol. 2016, 19, 242–251. [Google Scholar] [CrossRef]
- Weller, M.; Stupp, R.; Hegi, M.E.; Van Den Bent, M.; Tonn, J.C.; Sanson, M.; Wick, W.; Reifenberger, G. Personalized care in neuro-oncology coming of age: Why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro-Oncol. 2012, 14, iv100–iv108. [Google Scholar] [CrossRef] [Green Version]
- Zabarovsky, E.R.; Lerman, M.I.; Minna, J.D. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene 2002, 21, 6915–6935. [Google Scholar] [CrossRef] [Green Version]
- Whang-Peng, J.; Kao-Shan, C.; Lee, E.; Bunn, P.; Carney, D.; Gazdar, A.; Minna, J. Specific chromosome defect associated with human small-cell lung cancer: Deletion 3p (14–23). Science 1982, 215, 181–182. [Google Scholar] [CrossRef]
- Falor, W.H.; Ward-Skinner, R.; Wegryn, S. A 3p deletion in small cell lung carcinoma. Cancer Genet. Cytogenet. 1985, 16, 175–177. [Google Scholar] [CrossRef]
- Kilic, E.; Naus, N.C.; van Gils, W.; Klaver, C.C.; van Til, M.E.; Verbiest, M.M.; Stijnen, T.; Mooy, C.M.; Paridaens, D.; Beverloo, H.B. Concurrent loss of chromosome arm 1p and chromosome 3 predicts a decreased disease-free survival in uveal melanoma patients. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Gambrelle, J.; Labialle, S.; Dayan, G.; Gayet, L.; Barakat, S.; Grange, J.; Baggetto, L. Toward monosomy 3 as the main prognosis factor of uveal melanoma: Current cytogenetic data. J. Fr. D'ophtalmologie 2004, 27, 1061–1067. [Google Scholar] [CrossRef]
- Scholes, A.G.; Damato, B.E.; Nunn, J.; Hiscott, P.; Grierson, I.; Field, J.K. Monosomy 3 in uveal melanoma: Correlation with clinical and histologic predictors of survival. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1008–1011. [Google Scholar] [CrossRef] [Green Version]
- Kawankar, N.; Rao Vundinti, B. Cytogenetic abnormalities in myelodysplastic syndrome: An overview. Hematology 2011, 16, 131–138. [Google Scholar] [CrossRef]
- Le Beau, M.M.; Espinosa, R.; Neuman, W.L.; Stock, W.; Roulston, D.; Larson, R.A.; Keinanen, M.; Westbrook, C.A. Cytogenetic and molecular delineation of the smallest commonly deleted region of chromosome 5 in malignant myeloid diseases. Proc. Natl. Acad. Sci. USA 1993, 90, 5484–5488. [Google Scholar] [CrossRef] [Green Version]
- McNerney, M.E.; Brown, C.D.; Wang, X.; Bartom, E.T.; Karmakar, S.; Bandlamudi, C.; Yu, S.; Ko, J.; Sandall, B.P.; Stricker, T. CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2013, 121, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.; TaherNazerHussain, F.; Patnaik, M.; Knudson, R.; Van Dyke, D.; Tefferi, A. Autosomal monosomies among 24,262 consecutive cytogenetic studies: Prevalence, chromosomal distribution and clinicopathologic correlates of sole abnormalities. Am. J. Hematol. 2011, 86, 353–356. [Google Scholar] [CrossRef]
- Mantadakis, E.; Shannon, K.M.; Singer, D.A.; Finklestein, J.; Chan, K.W.; Hilden, J.M.; Sandler, E.S. Transient monosomy 7: A case series in children and review of the literature. Cancer 1999, 85, 2655–2661. [Google Scholar] [CrossRef]
- Van Der Bosch, K.; Becker, I.; Savelyeva, L.; Brüderlein, S.; Schlag, P.; Schwab, M. Deletions in the short arm of chromosome 8 are present in up to 90% of human colorectal cancer cell lines. Genes Chromosomes Cancer 1992, 5, 91–95. [Google Scholar] [CrossRef]
- El Gammal, A.T.; Brüchmann, M.; Zustin, J.; Isbarn, H.; Hellwinkel, O.J.; Köllermann, J.; Sauter, G.; Simon, R.; Wilczak, W.; Schwarz, J. Chromosome 8p deletions and 8q gains are associated with tumor progression and poor prognosis in prostate cancer. Clin. Cancer Res. 2010, 16, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Crowther, J.; Pastor, T.; Asbagh, L.A.; Baietti, M.F.; De Troyer, M.; Vazquez, I.; Talebi, A.; Renzi, F.; Dehairs, J. Loss of chromosome 8p governs tumor progression and drug response by altering lipid metabolism. Cancer Cell 2016, 29, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Matsuyama, H.; Oba, K.; Yoshihiro, S.; Takahashi, M.; Naito, K. Numerical aberrations of chromosome 9 in bladder cancer.: A possible prognostic marker for early tumor recurrence. Cancer Genet. Cytogenet. 2002, 134, 41–45. [Google Scholar] [CrossRef]
- Stoehr, R.; Zietz, S.; Burger, M.; Filbeck, T.; Denzinger, S.; Obermann, E.C.; Hammerschmied, C.; Wieland, W.F.; Knuechel, R.; Hartmann, A. Deletions of chromosomes 9 and 8p in histologically normal urothelium of patients with bladder cancer. Eur. Urol. 2005, 47, 58–63. [Google Scholar] [CrossRef]
- Pratt, D.; Abdullaev, Z.; Papanicolau-Sengos, A.; Ketchum, C.; Panneer Selvam, P.; Chung, H.-J.; Lee, I.; Raffeld, M.; Gilbert, M.R.; Armstrong, T.S. High-grade glioma with pleomorphic and pseudopapillary features (HPAP): A proposed type of circumscribed glioma in adults harboring frequent TP53 mutations and recurrent monosomy 13. Acta Neuropathol. 2022, 143, 403–414. [Google Scholar] [CrossRef]
- Batra, S.K.; McLendon, R.E.; Koo, J.S.; Castelino-Prabhu, S.; Fuchs, H.E.; Krischer, J.P.; Friedman, H.S.; Bigner, D.D.; Bigner, S.H. Prognostic implications of chromosome 17p deletions in human medulloblastomas. J. Neuro-Oncol. 1995, 24, 39–45. [Google Scholar] [CrossRef]
- Shaikh, N.; Dixit, K.; Raizer, J. Recent advances in managing/understanding meningioma. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Ragel, B.T.; Jensen, R.L. Molecular genetics of meningiomas. Neurosurg. Focus 2005, 19, 1–8. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Rouleau, G.A.; Nordenskjöld, M.; Collins, V.P. Molecular genetic analysis of chromosome 22 in 81 cases of meningioma. Cancer Res. 1990, 50, 5863–5867. [Google Scholar]
- Licciardi, F.; Lhakhang, T.; Kramer, Y.G.; Zhang, Y.; Heguy, A.; Tsirigos, A. Human blastocysts of normal and abnormal karyotypes display distinct transcriptome profiles. Sci. Rep. 2018, 8, 14906. [Google Scholar] [CrossRef] [Green Version]
- McCallie, B.R.; Parks, J.C.; Patton, A.L.; Griffin, D.K.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Hypomethylation and Genetic Instability in Monosomy Blastocysts May Contribute to Decreased Implantation Potential. PLoS ONE 2016, 11, e0159507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Solis, R.; Liu, P.; Bradley, A. Chromosome engineering in mice. Nature 1995, 378, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Lewandoski, M.; Martin, G.R. Cre-mediated chromosome loss in mice. Nat. Genet. 1997, 17, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Li, L.B.; Chang, K.H.; Wang, P.R.; Hirata, R.K.; Papayannopoulou, T.; Russell, D.W. Trisomy correction in Down syndrome induced pluripotent stem cells. Cell Stem Cell 2012, 11, 615–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Suzuki, Y.; Ikeya, T.; Hirota, K. Targeting chromosome trisomy for chromosome editing. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Proudfoot, C.; Mileham, A.J.; McLaren, D.G.; Whitelaw, C.B.; Lillico, S.G. Highly efficient targeted chromosome deletions using CRISPR/Cas9. Biotechnol Bioeng 2015, 112, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Zuo, E.; Huo, X.; Yao, X.; Hu, X.; Sun, Y.; Yin, J.; He, B.; Wang, X.; Shi, L.; Ping, J.; et al. CRISPR/Cas9-mediated targeted chromosome elimination. Genome Biol. 2017, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, M.V.; Xu, J.; Mitchell, C.; Marin, D.; Zimmerman, R.; Rana, B.; Weinstein, E.; King, R.T.; Palmerola, K.L.; Smith, M.E.; et al. Allele-Specific Chromosome Removal after Cas9 Cleavage in Human Embryos. Cell 2020, 183, 1650–1664. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, S.N.; Fujita, K.; Tomioka, K.; Miyamoto, T.; Matsuura, S. Applications of genome editing technology in research on chromosome aneuploidy disorders. Cells 2020, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.; Raaijmakers, J.A.; Bakker, B.; Spierings, D.C.J.; Lansdorp, P.M.; Foijer, F.; Medema, R.H. p53 Prohibits Propagation of Chromosome Segregation Errors that Produce Structural Aneuploidies. Cell. Rep. 2017, 19, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Chunduri, N.K.; Menges, P.; Zhang, X.; Wieland, A.; Gotsmann, V.L.; Mardin, B.R.; Buccitelli, C.; Korbel, J.O.; Willmund, F.; Kschischo, M. Systems approaches identify the consequences of monosomy in somatic human cells. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, D.C.; Soto, M.; Schubert, M.; Bakker, B.; Spierings, D.C.; Szuhai, K.; Lansdorp, P.M.; Foijer, F.; Medema, R.H.; Raaijmakers, J.A. Monosomies, trisomies and segmental aneuploidies differentially affect chromosomal stability. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bunz, F.; Fauth, C.; Speicher, M.R.; Dutriaux, A.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B.; Lengauer, C. Targeted inactivation of p53 in human cells does not result in aneuploidy. Cancer Res. 2002, 62, 1129–1133. [Google Scholar] [PubMed]
- Lopez-Garcia, C.; Sansregret, L.; Domingo, E.; McGranahan, N.; Hobor, S.; Birkbak, N.J.; Horswell, S.; Gronroos, E.; Favero, F.; Rowan, A.J.; et al. BCL9L Dysfunction Impairs Caspase-2 Expression Permitting Aneuploidy Tolerance in Colorectal Cancer. Cancer Cell. 2017, 31, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Redman-Rivera, L.N.; Shaver, T.M.; Jin, H.; Marshall, C.B.; Schafer, J.M.; Sheng, Q.; Hongo, R.A.; Beckermann, K.E.; Wheeler, F.C.; Lehmann, B.D.; et al. Acquisition of aneuploidy drives mutant p53-associated gain-of-function phenotypes. Nat. Commun. 2021, 12, 5184. [Google Scholar] [CrossRef]
- Leung, G.M.K.; Zhang, C.; Ng, N.K.L.; Yang, N.; Lam, S.S.Y.; Au, C.H.; Chan, T.L.; Ma, E.S.K.; Tsui, S.P.; Ip, H.W.; et al. Distinct mutation spectrum, clinical outcome and therapeutic responses of typical complex/monosomy karyotype acute myeloid leukemia carrying TP53 mutations. Am. J. Hematol. 2019, 94, 650–657. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, S.; Wu, Z.; Kajigaya, S.; Feng, X.; Liu, Q.; Townsley, D.M.; Cooper, J.; Chen, J.; Keyvanfar, K.; et al. Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells. Blood 2017, 130, 2762–2773. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.Q.; Ouyang, M.; Brandmaier, A.; Hao, H.; Shen, W.H. PTEN in the maintenance of genome integrity: From DNA replication to chromosome segregation. Bioessays 2017, 39. [Google Scholar] [CrossRef]
- van Harn, T.; Foijer, F.; van Vugt, M.; Banerjee, R.; Yang, F.; Oostra, A.; Joenje, H.; te Riele, H. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 2010, 24, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Tutt, A.; Gabriel, A.; Bertwistle, D.; Connor, F.; Paterson, H.; Peacock, J.; Ross, G.; Ashworth, A. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr. Biol. 1999, 9, 1107–1110. [Google Scholar] [CrossRef] [Green Version]
- Kravets, A.; Qin, H.; Ahmad, A.; Bethlendy, G.; Gao, Q.; Rustchenko, E. Widespread occurrence of dosage compensation in Candida albicans. PLoS ONE 2010, 5, e10856. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Yang, H.; Chen, C.; Hou, J.; Hanson, K.M.; Albert, P.S.; Ji, T.; Cheng, J.; Birchler, J.A. Genomic imbalance determines positive and negative modulation of gene expression in diploid maize. Plant Cell 2021, 33, 917–939. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, P.; Lundberg, L.E.; Johansson, A.M.; Ryden, P.; Svensson, M.J.; Larsson, J. Buffering of segmental and chromosomal aneuploidies in Drosophila melanogaster. PLoS Genet. 2009, 5, e1000465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, J.H.; Cho, D.Y.; Mattiuzzo, N.R.; Artieri, C.G.; Jiang, L.; Dale, R.K.; Smith, H.E.; McDaniel, J.; Munro, S.; Salit, M.; et al. Mediation of Drosophila autosomal dosage effects and compensation by network interactions. Genome Biol. 2012, 13, r28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, L.E.; Figueiredo, M.L.; Stenberg, P.; Larsson, J. Buffering and proteolysis are induced by segmental monosomy in Drosophila melanogaster. Nucleic Acids Res. 2012, 40, 5926–5937. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Presgraves, D.C. Translational compensation of gene copy number alterations by aneuploidy in Drosophila melanogaster. Nucleic Acids Res. 2017, 45, 2986–2993. [Google Scholar] [CrossRef] [Green Version]
- Dephoure, N.; Hwang, S.; O'Sullivan, C.; Dodgson, S.E.; Gygi, S.P.; Amon, A.; Torres, E.M. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. Elife 2014, 3, e03023. [Google Scholar] [CrossRef]
- Schukken, K.M.; Sheltzer, J.M. Extensive protein dosage compensation in aneuploid human cancers. bioRxiv 2021. [Google Scholar]
- Cheng, P.; Zhao, X.; Katsnelson, L.; Moya, R.; Shwetar, J.; Fenyö, D.; Davoli, T. Proteogenomic analysis of aneuploidy reveals divergent types of gene expression regulation across cellular pathways. bioRxiv 2012. [Google Scholar] [CrossRef]
- Li, J.J.; Bickel, P.J.; Biggin, M.D. System wide analyses have underestimated protein abundances and the importance of transcription in mammals. PeerJ. 2014, 2, e270. [Google Scholar] [CrossRef] [Green Version]
- Schwanhausser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, M.; Rooney, M.S.; Mertins, P.; Przybylski, D.; Chevrier, N.; Satija, R.; Rodriguez, E.H.; Fields, A.P.; Schwartz, S.; Raychowdhury, R.; et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science 2015, 347, 1259038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Teo, G.; Krueger, S.; Rock, T.M.; Koh, H.W.; Choi, H.; Vogel, C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol. Syst. Biol. 2016, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- McShane, E.; Sin, C.; Zauber, H.; Wells, J.N.; Donnelly, N.; Wang, X.; Hou, J.; Chen, W.; Storchova, Z.; Marsh, J.A.; et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell 2016, 167, 803–815. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.J.; Khatiwada, S.; Cui, Y.; Reineke, L.C.; Dooling, S.W.; Kim, J.J.; Li, W.; Walter, P.; Costa-Mattioli, M. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science 2019, 366, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Uechi, T.; Tanaka, T.; Kenmochi, N. A complete map of the human ribosomal protein genes: Assignment of 80 genes to the cytogenetic map and implications for human disorders. Genomics 2001, 72, 223–230. [Google Scholar] [CrossRef]
- Boria, I.; Garelli, E.; Gazda, H.T.; Aspesi, A.; Quarello, P.; Pavesi, E.; Ferrante, D.; Meerpohl, J.J.; Kartal, M.; Da Costa, L.; et al. The ribosomal basis of Diamond-Blackfan Anemia: Mutation and database update. Hum. Mutat. 2010, 31, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Ebert, B.L.; Pretz, J.; Bosco, J.; Chang, C.Y.; Tamayo, P.; Galili, N.; Raza, A.; Root, D.E.; Attar, E.; Ellis, S.R.; et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature 2008, 451, 335–339. [Google Scholar] [CrossRef]
- Bursać, S.; Prodan, Y.; Pullen, N.; Bartek, J.; Volarević, S. Dysregulated ribosome biogenesis reveals therapeutic liabilities in cancer. Trends Cancer 2021, 7, 57–76. [Google Scholar] [CrossRef]
- Deutschbauer, A.M.; Jaramillo, D.F.; Proctor, M.; Kumm, J.; Hillenmeyer, M.E.; Davis, R.W.; Nislow, C.; Giaever, G. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 2005, 169, 1915–1925. [Google Scholar] [CrossRef] [Green Version]
- Ohnuki, S.; Ohya, Y. High-dimensional single-cell phenotyping reveals extensive haploinsufficiency. PLoS Biol. 2018, 16, e2005130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, V.T.; Kassahn, K.S.; Marcos, A.E.; Ragan, M.A. Identification of human haploinsufficient genes and their genomic proximity to segmental duplications. Eur. J. Hum. Genet. 2008, 16, 1350–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidman, J.G.; Seidman, C. Transcription factor haploinsufficiency: When half a loaf is not enough. J. Clin. Investig. 2002, 109, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lee, I.; Marcotte, E.M.; Hurles, M.E. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010, 6, e1001154. [Google Scholar] [CrossRef] [Green Version]
- Veitia, R.A. Exploring the etiology of haploinsufficiency. Bioessays 2002, 24, 175–184. [Google Scholar] [CrossRef]
- Pehlivan, T.; Pober, B.R.; Brueckner, M.; Garrett, S.; Slaugh, R.; Van Rheeden, R.; Wilson, D.B.; Watson, M.S.; Hing, A.V. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am. J. Med. Genet. 1999, 83, 201–206. [Google Scholar] [CrossRef]
- Papp, B.; Pal, C.; Hurst, L.D. Dosage sensitivity and the evolution of gene families in yeast. Nature 2003, 424, 194–197. [Google Scholar] [CrossRef]
- Cheng, Z.; Mugler, C.F.; Keskin, A.; Hodapp, S.; Chan, L.Y.; Weis, K.; Mertins, P.; Regev, A.; Jovanovic, M.; Brar, G.A. Small and Large Ribosomal Subunit Deficiencies Lead to Distinct Gene Expression Signatures that Reflect Cellular Growth Rate. Mol. Cell 2019, 73, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Morrill, S.A.; Amon, A. Why haploinsufficiency persists. Proc. Natl. Acad. Sci. USA 2019, 116, 11866–11871. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Fry, E.A. Haploinsufficient tumor suppressor genes. Adv. Med. Biol. 2017, 118, 83. [Google Scholar]
- Boultwood, J.; Pellagatti, A.; McKenzie, A.N.; Wainscoat, J.S. Advances in the 5q- syndrome. Blood 2010, 116, 5803–5811. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Kitzing, T.; Roessler, S.; Zuber, J.; Krasnitz, A.; Schultz, N.; Revill, K.; Weissmueller, S.; Rappaport, A.R.; Simon, J.; et al. A cluster of cooperating tumor-suppressor gene candidates in chromosomal deletions. Proc. Natl. Acad. Sci. USA 2012, 109, 8212–8217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagi, I.; Benvenisty, N. Haploidy in Humans: An Evolutionary and Developmental Perspective. Dev. Cell 2017, 41, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olbrich, T.; Mayor-Ruiz, C.; Vega-Sendino, M.; Gomez, C.; Ortega, S.; Ruiz, S.; Fernandez-Capetillo, O. A p53-dependent response limits the viability of mammalian haploid cells. Proc. Natl. Acad. Sci. USA 2017, 114, 9367–9372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passerini, V.; Ozeri-Galai, E.; de Pagter, M.S.; Donnelly, N.; Schmalbrock, S.; Kloosterman, W.P.; Kerem, B.; Storchova, Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016, 7, 10754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheltzer, J.M.; Blank, H.M.; Pfau, S.J.; Tange, Y.; George, B.M.; Humpton, T.J.; Brito, I.L.; Hiraoka, Y.; Niwa, O.; Amon, A. Aneuploidy drives genomic instability in yeast. Science 2011, 333, 1026–1030. [Google Scholar] [CrossRef] [Green Version]

| Affected Chromosome | Cancer Type | Putative Effector Gene | Frequency of Deletion | Reference |
|---|---|---|---|---|
| 1p | Neuroblastoma | MYCN | 5–52% [53] | [45,54] |
| 1p, 19q | OligodendrogliomaAstrocytic gliomas, Glioblastoma | 70% [55] 2–25%; 4–12% [56] | [57,58] | |
| 3p | Lung carcinoma, Lung squamous cell carcinoma | Multiple, reviewed in: [59] | 80% [24] | [60,61] |
| 3 | Uveal melanoma | BAP1 | 50% [62] | [63,64] |
| 5/5q | Myeloblastic syndrome | EGR1, APC, DIAPH1, NPM1 | n/a | [65,66] |
| 7/7q | Myeloblastic syndrome, Myeloid leukemia | CUX1 [67], MLL3 [50] | 12–70% | [65,68,69] |
| 8p | Prostate carcinoma Renal clear cell carcinoma | ~13% | [70,71,72] | |
| 9 | Bladder cancer | ARF, TGFβ | [73,74] | |
| 13 | High-grade glioma | BRCA2 | 90% | [75] |
| 17p | Multiple myeloma, Lymphocytic leukemia, Colorectal cancer, Medulloblastoma | TP53, BRCA1 | n/a | [76] |
| 22 | Meningioma | NF2 | 40–75% [77] | [78,79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunduri, N.K.; Barthel, K.; Storchova, Z. Consequences of Chromosome Loss: Why Do Cells Need Each Chromosome Twice? Cells 2022, 11, 1530. https://doi.org/10.3390/cells11091530
Chunduri NK, Barthel K, Storchova Z. Consequences of Chromosome Loss: Why Do Cells Need Each Chromosome Twice? Cells. 2022; 11(9):1530. https://doi.org/10.3390/cells11091530
Chicago/Turabian StyleChunduri, Narendra Kumar, Karen Barthel, and Zuzana Storchova. 2022. "Consequences of Chromosome Loss: Why Do Cells Need Each Chromosome Twice?" Cells 11, no. 9: 1530. https://doi.org/10.3390/cells11091530
APA StyleChunduri, N. K., Barthel, K., & Storchova, Z. (2022). Consequences of Chromosome Loss: Why Do Cells Need Each Chromosome Twice? Cells, 11(9), 1530. https://doi.org/10.3390/cells11091530






