A Comparative Study of the Role of Formins in Drosophila Embryonic Dorsal Closure
Abstract
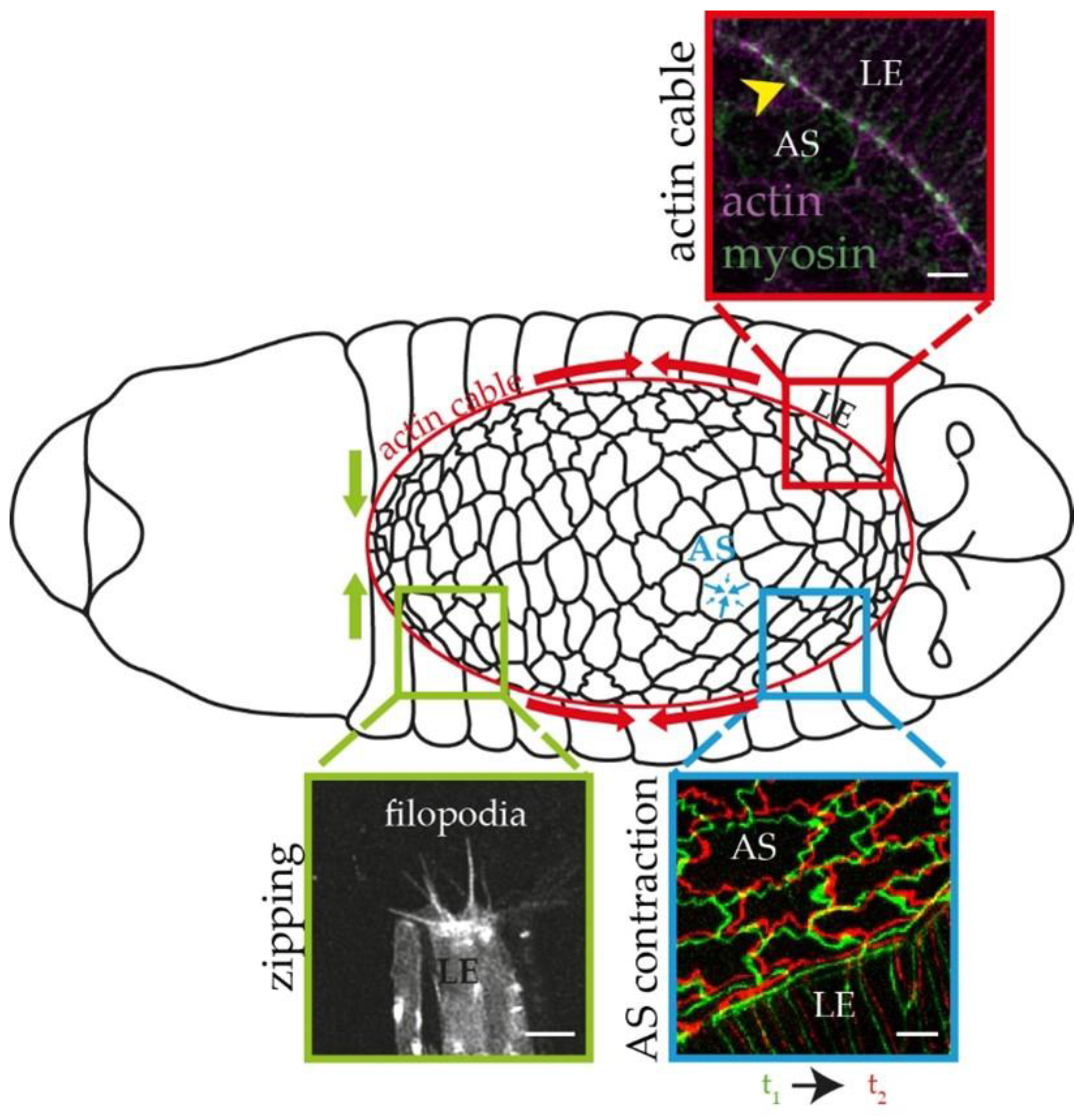
:1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks and Genetics
2.2. Antibody Generation
2.3. Immunohistochemistry
2.4. Western Blot Analysis
2.5. Live Imaging and Image Analysis
2.5.1. Trachea Analysis
2.5.2. AS Cell Dynamics
2.5.3. AS Cell Shape
2.5.4. AS Cell Area
2.5.5. Filopodia Number and Length
2.5.6. Dorsal Closure Parameters
2.6. Statistics and Figures
3. Results
3.1. Form3, DAAM, Dia, and Frl Localize to AS and Epidermal Cells during Dorsal Closure
3.2. Formin LOF Mutations Used during Our Studies
3.3. Comparison of the DC Phenotypes of the Formin Mutants
3.4. Form3, DAAM, and Frl Contribute to the Zipping of the Epithelial Sheets
3.5. Formins Differently Regulate the Shape and Contraction Dynamics of AS the Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, P.; Solon, J. Drosophila dorsal closure: An orchestra of forces to zip shut the embryo. Mech. Dev. 2017, 144, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, A.; Woolner, S.; Martin, P. Dynamic analysis of dorsal closure in Drosophila: From genetics to cell biology. Dev. Cell 2002, 3, 9–19. [Google Scholar] [CrossRef]
- Kiehart, D.P.; Galbraith, C.G.; Edwards, K.A.; Rickoll, W.L.; Montague, R.A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 2000, 149, 471–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutson, M.S.; Tokutake, Y.; Chang, M.S.; Bloor, J.W.; Venakides, S.; Kiehart, D.P.; Edwards, G.S. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 2003, 300, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasakarnis, L.; Frei, E.; Caussinus, E.; Affolter, M.; Brunner, D. Amnioserosa cell constriction but not epidermal actin cable tension autonomously drives dorsal closure. Nat. Cell Biol. 2016, 18, 1161–1172. [Google Scholar] [CrossRef]
- Ducuing, A.; Vincent, S. The actin cable is dispensable in directing dorsal closure dynamics but neutralizes mechanical stress to prevent scarring in the Drosophila embryo. Nat. Cell Biol. 2016, 18, 1149–1160. [Google Scholar] [CrossRef]
- Martin, P.; Parkhurst, S.M. Parallels between tissue repair and embryo morphogenesis. Development 2004, 131, 3021–3034. [Google Scholar] [CrossRef] [Green Version]
- Jankovics, F.; Brunner, D. Transiently reorganized microtubules are essential for zippering during dorsal closure in Drosophila melanogaster. Dev. Cell 2006, 11, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.S.; Goldstein, E.S.; Perrimon, N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes Dev. 1997, 11, 1728–1737. [Google Scholar] [CrossRef] [Green Version]
- Kockel, L.; Vorbrüggen, G.; Jäckle, H.; Mlodzik, M.; Bohmann, D. Requirement for Drosophila 14-3-3 zeta in Raf-dependent photoreceptor development. Genes Dev. 1997, 11, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Riesgo-Escovar, J.R.; Hafen, E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev. 1997, 11, 1717–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, D.J.; Tishkina, A.; Harris, T.J. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 2010, 137, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, G.B.; Murugesu, S.; Adams, R.J.; Martinez-Arias, A.; Gorfinkiel, N. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development 2010, 137, 2743–2752. [Google Scholar] [CrossRef] [Green Version]
- Homem, C.C.; Peifer, M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development 2008, 135, 1005–1018. [Google Scholar] [CrossRef] [Green Version]
- Dehapiot, B.; Clement, R.; Alegot, H.; Gazso-Gerhat, G.; Philippe, J.M.; Lecuit, T. Assembly of a persistent apical actin network by the formin Frl/Fmnl tunes epithelial cell deformability. Nat. Cell Biol. 2020, 22, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Kovar, D.R. Molecular details of formin-mediated actin assembly. Curr. Opin. Cell Biol. 2006, 18, 11–17. [Google Scholar] [CrossRef]
- Zhou, F.; Leder, P.; Martin, S.S. Formin-1 protein associates with microtubules through a peptide domain encoded by exon-2. Exp. Cell Res. 2006, 312, 1119–1126. [Google Scholar] [CrossRef]
- Bartolini, F.; Moseley, J.B.; Schmoranzer, J.; Cassimeris, L.; Goode, B.L.; Gundersen, G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008, 181, 523–536. [Google Scholar] [CrossRef] [Green Version]
- Roth-Johnson, E.A.; Vizcarra, C.L.; Bois, J.S.; Quinlan, M.E. Interaction between microtubules and the Drosophila formin Cappuccino and its effect on actin assembly. J Biol. Chem. 2014, 289, 4395–4404. [Google Scholar] [CrossRef] [Green Version]
- Szikora, S.; Földi, I.; Tóth, K.; Migh, E.; Vig, A.; Bugyi, B.; Maléth, J.; Hegyi, P.; Kaltenecker, P.; Sanchez-Soriano, N.; et al. The formin DAAM is required for coordination of the actin and microtubule cytoskeleton in axonal growth cones. J. Cell Sci. 2017, 130, 2506–2519. [Google Scholar] [CrossRef] [Green Version]
- Young, K.G.; Thurston, S.F.; Copeland, S.; Smallwood, C.; Copeland, J.W. INF1 Is a Novel Microtubule-associated Formin. Mol. Biol. Cell 2008, 19, 5168–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, J.; Ramabhadran, V.; Neumanne, E.; Gurel, P.; Blanchoin, L.; Vantard, M.; Higgs, H.N. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol. Biol. Cell 2011, 22, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Foldi, I.; Szikora, S.; Mihály, J. Formin’ bridges between microtubules and actin filaments in axonal growth cones. Neural Regen. Res. 2017, 12, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Linardopoulou, E.V.; Osborn, G.E.; Parkhurst, S.M. Formins in development: Orchestrating body plan origami. Biochim. Biophys. Acta 2010, 1803, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Castrillon, D.H.; Wasserman, S.A. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 1994, 120, 3367–3377. [Google Scholar] [CrossRef]
- Afshar, K.; Stuart, B.; Wasserman, S.A. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 2000, 127, 1887–1897. [Google Scholar] [CrossRef]
- Grosshans, J.; Wenzl, C.; Herz, H.M.; Bartoszewski, S.; Schnorrer, F.; Vogt, N.; Schwarz, H.; Müller, H.A. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 2005, 132, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Homem, C.C.; Peifer, M. Exploring the roles of diaphanous and enabled activity in shaping the balance between filopodia and lamellipodia. Mol. Biol. Cell 2009, 20, 5138–5155. [Google Scholar] [CrossRef] [Green Version]
- Nowotarski, S.H.; McKeon, N.; Moser, R.J.; Peifer, M. The actin regulators Enabled and Diaphanous direct distinct protrusive behaviors in different tissues during Drosophila development. Mol. Biol. Cell 2014, 25, 3147–3165. [Google Scholar] [CrossRef]
- Gombos, R.; Migh, E.; Antal, O.; Mukherjee, A.; Jenny, A.; Mihaly, J. The Formin DAAM Functions as Molecular Effector of the Planar Cell Polarity Pathway during Axonal Development in Drosophila. J. Neurosci. 2015, 35, 10154–10167. [Google Scholar] [CrossRef] [Green Version]
- Jankovics, F.; Henn, L.; Bujna, A.; Vilmos, P.; Kiss, N.; Erdelyi, M. A functional genomic screen combined with time-lapse microscopy uncovers a novel set of genes involved in dorsal closure of Drosophila embryos. PLoS ONE 2011, 6, e22229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrillon, D.H.; Gönczy, P.; Alexander, S.; Rawson, R.; Eberhart, C.G.; Viswanathan, S.; DiNardo, S.; Wasserman, S.A. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: Characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 1993, 135, 489–505. [Google Scholar] [CrossRef]
- Larkin, A.; Marygold, S.J.; Antonazzo, G.; Attrill, H.; dos Santos, G.; Garapati, P.V.; Goodman, J.L.; Gramates, L.S.; Millburn, G.; Strelets, V.B.; et al. FlyBase: Updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 2020, 49, D899–D907. [Google Scholar] [CrossRef]
- Chougule, A.B.; Hastert, M.C.; Thomas, J.H. Drak Is Required for Actomyosin Organization During Drosophila Cellularization. G3 (Bethesda) 2016, 6, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwartz, A.; Dhanyasi, N.; Schejter, E.D.; Shilo, B.Z. The Drosophila formin Fhos is a primary mediator of sarcomeric thin-filament array assembly. Elife 2016, 5, e16540. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Benitez, D.; Knust, E. Crumbs is an essential regulator of cytoskeletal dynamics and cell-cell adhesion during dorsal closure in Drosophila. Elife 2015, 4, e07398. [Google Scholar] [CrossRef]
- Tanaka, H.; Takasu, E.; Aigaki, T.; Kato, K.; Hayashi, S.; Nose, A. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev. Biol. 2004, 274, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Takacs, Z.; Jankovics, F.; Vilmos, P.; Lenart, P.; Roper, K.; Erdelyi, M. The spectraplakin Short stop is an essential microtubule regulator involved in epithelial closure in Drosophila. J. Cell Sci. 2017, 130, 712–724. [Google Scholar] [CrossRef] [Green Version]
- Mellor, H. The role of formins in filopodia formation. Biochim. Biophys. Acta 2010, 1803, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Wells, A.R.; Zou, R.S.; Tulu, U.S.; Sokolow, A.C.; Crawford, J.M.; Edwards, G.S.; Kiehart, D.P. Complete canthi removal reveals that forces from the amnioserosa alone are sufficient to drive dorsal closure in Drosophila. Mol. Biol. Cell 2014, 25, 3552–3568. [Google Scholar] [CrossRef] [PubMed]
- Heisenberg, C.P. Dorsal closure in Drosophila: Cells cannot get out of the tight spot. Bioessays 2009, 31, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Heisenberg, C.P.; Bellaïche, Y. Forces in tissue morphogenesis and patterning. Cell 2013, 153, 948–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiehart, D.P.; Crawford, J.M.; Aristotelous, A.; Venakides, S.; Edwards, G.S. Cell Sheet Morphogenesis: Dorsal Closure in Drosophila melanogaster as a Model System. Annu. Rev. Cell Dev. Biol. 2017, 33, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Aristotelous, A.C.; Crawford, J.M.; Edwards, G.S.; Kiehart, D.P.; Venakides, S. Mathematical models of dorsal closure. Prog. Biophys. Mol. Biol. 2018, 137, 111–131. [Google Scholar] [CrossRef]
- Zimmermann, D.; Kovar, D.R. Feeling the force: Formin’s role in mechanotransduction. Curr. Opin. Cell Biol. 2019, 56, 130–140. [Google Scholar] [CrossRef]
- Harden, N.; Ricos, M.; Ong, Y.M.; Chia, W.; Lim, L. Participation of small GTPases in dorsal closure of the Drosophila embryo: Distinct roles for Rho subfamily proteins in epithelial morphogenesis. J. Cell Sci. 1999, 112 (Pt 3), 273–284. [Google Scholar] [CrossRef]
- Magie, C.R.; Meyer, M.R.; Gorsuch, M.S.; Parkhurst, S.M. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development 1999, 126, 5353–5364. [Google Scholar] [CrossRef]
- Magie, C.R.; Pinto-Santini, D.; Parkhurst, S.M. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 2002, 129, 3771–3782. [Google Scholar] [CrossRef]
- Abreu-Blanco, M.T.; Verboon, J.M.; Parkhurst, S.M. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr. Biol. 2014, 24, 144–155. [Google Scholar] [CrossRef] [Green Version]





| WT | DAAMEx4 | frl59 | form31 | dia1 | ||
|---|---|---|---|---|---|---|
| Dorsal closure | Closure phenotype | n = 53 embryos | p < 0.0001 n = 44 | p = 0.0196 n = 48 | p < 0.0001 n = 54 | p < 0.0001 n = 42 |
| DC duration | n = 38 embryos | ns n = 37 | ns n = 40 | p = 0.0002 n = 46 | p < 0.0001 n = 44 | |
| LE convergence speed | n = 23 embryos | ns n = 17 | p = 0.0019 n = 14 | ns n = 17 | p = 0.0247 n = 8 | |
| zipping speed | n = 23 embryos | p = 0.0075 n = 17 | p < 0.0001 n = 14 | p = 0.0065 n = 17 | p < 0.0001 n = 8 | |
| length/width ratio | n = 40 embryos | ns n = 39 | p = 0.0104 n = 39 | ns n = 47 | ns n = 42 | |
| Amnioserosa | AS cell area | n = 54 cells | p = 0.0387 n = 54 | p < 0.0001 n = 54 | p < 0.0001 n = 54 | p < 0.0001 n = 54 |
| AS cell perimeter | n = 54 cells | ns n = 54 | p < 0.0001 n = 54 | ns n = 54 | p = 0.018 n = 54 | |
| AS cell contraction | n = 54 cells | p < 0.0001 n = 54 | p = 0.0002 n = 54 | p < 0.0001 n = 54 | p < 0.0001 n = 54 | |
| AS cell shape | n = 54 cells | p < 0.0001 n = 54 | ns n = 54 | p < 0.0001 n = 54 | p < 0.0001 n = 54 | |
| AS cell pulsation | n = 54 cells | ns n = 54 | ns n = 54 | ns n = 54 | ns n = 54 | |
| Filopodia | filopodia number | n = 72 embryos | ns n = 64 | ns n = 75 | ns n = 64 | n.d. |
| filopodia length | n = 69 embryos | ns n = 64 | ns n = 75 | ns n = 64 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, K.; Földi, I.; Mihály, J. A Comparative Study of the Role of Formins in Drosophila Embryonic Dorsal Closure. Cells 2022, 11, 1539. https://doi.org/10.3390/cells11091539
Tóth K, Földi I, Mihály J. A Comparative Study of the Role of Formins in Drosophila Embryonic Dorsal Closure. Cells. 2022; 11(9):1539. https://doi.org/10.3390/cells11091539
Chicago/Turabian StyleTóth, Krisztina, István Földi, and József Mihály. 2022. "A Comparative Study of the Role of Formins in Drosophila Embryonic Dorsal Closure" Cells 11, no. 9: 1539. https://doi.org/10.3390/cells11091539
APA StyleTóth, K., Földi, I., & Mihály, J. (2022). A Comparative Study of the Role of Formins in Drosophila Embryonic Dorsal Closure. Cells, 11(9), 1539. https://doi.org/10.3390/cells11091539







